Abstract
Free full text

Cellular differentiation: Potential insight into butyrate paradox?
ABSTRACT
We recently demonstrated that cellular responses to butyrate depend on the differentiation status of the colonic epithelium. Here, we apply the implications of these findings to cancer biology and discuss discrepancies in the effects of butyrate on cancer progression.
The gut microbiota affects host physiology and pathophysiologic processes such as cancer progression. Increasingly, studies highlight functional mechanisms driven by host–microbiota interactions in which small molecules such as microbial metabolites often act as mediators. Butyrate, a short-chain fatty acid, is an abundant metabolite (present in mM concentrations) in the lumen of mouse and human colons. It is the product of a subset of microbes including Firmicutes that reside at the surface colonic epithelium and ferment host diet-derived carbohydrates (Fig. 1). Multiple studies have demonstrated that butyrate has an immunoregulatory function2 in addition to a role in energy homeostasis in colonic epithelium.3
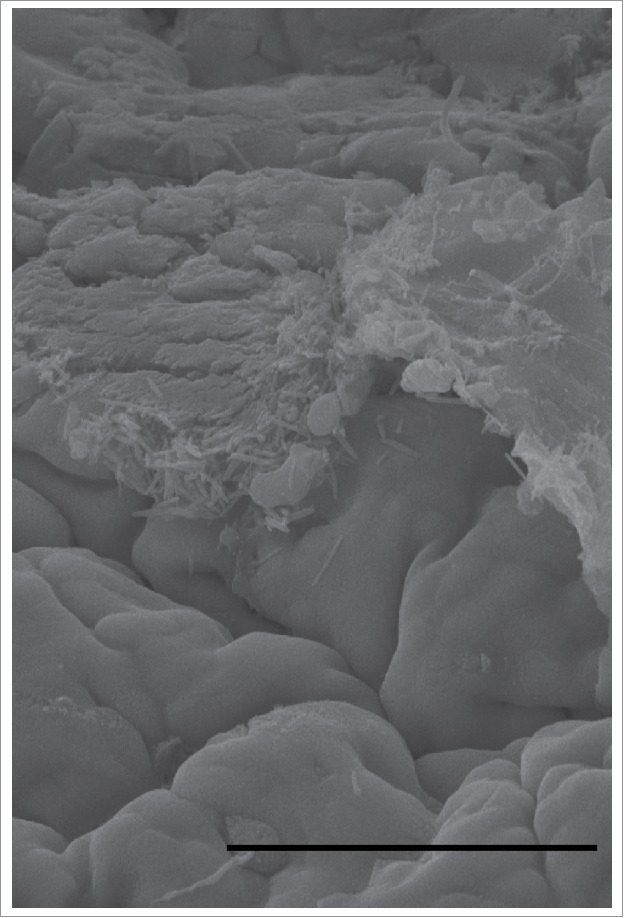
Apical surface of the mouse cecum on scanning electron microscopy. Rod-shaped bacteria are associated with the apical surface of colonocytes. The size and morphology of these bacteria are consistent with Lachnospiraceae-Ruminococcaceae bacteria known to produce butyrate. This demonstrates the close association of microbes that produce butyrate and the colonocytes that can metabolize it. Scale bar = 50 µm.
In colon cancer cells, butyrate has been proposed to modulate a wide array of processes including proliferation, differentiation, and apoptosis. The overall conclusion of many investigators is that butyrate inhibits growth of cancer cells and promotes that of normal colonic epithelium, a situation termed the “butyrate paradox.” In addition, discrepancies exist regarding the reported effects of butyrate on cancer development and progression. For example, in separate studies butyrate suppressed or promoted cancer cell growth.4,5 Specific tumor responses to butyrate may depend on specific mutations, tissue source, and tumor microenvironment. However, the key dictating factor(s) responsible for the heterogeneous response of tumors to butyrate of remain unknown.
In primary epithelial cell culture, we observed differential effects of butyrate on colonic stem cells versus colonocytes.1 We performed an unbiased screen of normal mouse colonic microbial metabolites and identified butyrate as a potent suppressor of stem cell proliferation. Butyrate inhibited histone deacetylase (HDAC) in stem cells, resulting in chromatin remodeling and extensive changes in gene expression. Interestingly, in contrast to stem cells, colonocytes were resistant to butyrate-mediated HDAC inhibition with no significant changes in gene expression. Colonocytes also tolerated higher, toxic, doses of butyrate compared to stem cells. Together, our data demonstrate that cellular differentiation status is a key determinant of cellular responses to butyrate in primary colonic epithelial cells.
One potential feature that may differentiate the responses to butyrate in stem cells versus colonocytes is their metabolic profiles. Gene expression and metabolic analyses demonstrated that colonocytes are enriched for mRNAs encoded by genes involved in lipid metabolism including fatty acid oxidation and the tricarboxylic acid (TCA) cycle. In contrast, stem cells (like cancer cells) rely more on glycolysis. Metabolic profiling and radiolabeling showed that colonocytes, but not stem cells, can readily metabolize butyrate through oxidation.
The metabolic switch to aerobic glycolysis during malignant transformation has been extensively characterized and is known as the “Warburg effect.” Previous work suggests that cancer cells that have undergone this metabolic transformation are unable to efficiently oxidize butyrate, resulting in accumulation of intracellular butyrate that inhibits HDAC and cellular proliferation.6 Genetic manipulation to inhibit the Warburg effect prevented the effect of butyrate on histone acetylation and restored cell proliferation, suggesting a mechanistic link between cellular metabolism and response to butyrate.6 In addition, dichotomous effects of butyrate on cell proliferation were largely dose-dependent; a low dose (micromolar) of butyrate stimulated proliferation whereas a high dose (millimolar) inhibited it. This dose dependency has also been reported using an in vivo model of intestinal polyposis,7 in which low-dose, but not high-dose, butyrate enhanced intestinal tumor size in Apcmin/+Msh2−/− mice. These studies suggest a cellular threshold for butyrate metabolism that limits its intracellular accumulation. Together, these data provide a potential explanation for previous reports of impaired butyrate metabolism in cancer cells and suggest that the butyrate paradox can be explained by the differentiation status of the cancer cells linked to their metabolic profiles. Interestingly, our preliminary examination of HDAC expression revealed a significant downregulation in several HDAC enzymes in colonocytes compared to stem cells (unpublished). This suggests that colonocytes may be resistant to the effects of butyrate as a result of decreased HDAC targets, in addition to their ability to metabolize butyrate. It remains to be explored whether colonocytes exhibit intrinsic mechanisms to resist the effects of butyrate or whether modulating butyrate metabolism in stem cells and colonocytes is sufficient to alter their responses to butyrate.
The effect of butyrate on intestinal stem cell proliferation is mediated by forkhead box O3 (Foxo3) through its increased binding to the promoters of cell cycle arrest genes. Integration of chromatin immunoprecipitation sequencing (ChIP-seq) and microarray data revealed increased histone acetylation and expression of genes containing Foxo3-binding sites in stem cells after butyrate treatment, and inhibition of Foxo3 reversed the butyrate effect in vitro and in vivo. Foxo family transcription factors regulate various fundamental biological processes including proliferation, apoptosis, and maintenance of pluripotency in hematopoietic, neural, and embryonic stem cells.8 Foxo3 is regulated by phosphoinositide 3-kinase (PI3k)-Akt and extracellular signal-regulated kinase (Erk) pathways that are often dysregulated during carcinogenesis and is therefore implicated as a tumor suppressor.9 Drugs with effects that include activation of Foxo3 induce apoptosis in leukemic cells from patients resistant to conventional therapy, suggesting that Foxo3 may be a therapeutic target.9 We speculate that Foxo3 might play a role in intestinal tumorigenesis, highlighting the therapeutic potential of targeting Foxo3 either directly or by modulating butyrate responses in intestinal cancers. Interestingly, Foxo3 regulates mitochondrial metabolism in hematopoietic stem cells,10 suggesting that it may be a linking factor for cellular differentiation and metabolism in intestinal epithelial cells.
Our study suggests that differentiation status dictates the cellular responses to butyrate, providing a potential mechanism to explain the butyrate paradox. Compared with primary colonocytes, cancer cells exhibit a poorly differentiated phenotype such as immature brush borders. Studies have reported distinct mutations associated with more or less differentiated phenotypes of cancer cells, suggesting that cancer cells fall within the spectrum of differentiation. Our data suggest that the discrepancy observed in the effect of butyrate observed in cancer cells may be explained by the differentiation status of the cancer cells used in the study, potentially through their metabolic capacity to oxidize and tolerate butyrate.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
Articles from Molecular & Cellular Oncology are provided here courtesy of Taylor & Francis
Full text links
Read article at publisher's site: https://doi.org/10.1080/23723556.2016.1212685
Read article for free, from open access legal sources, via Unpaywall:
https://www.tandfonline.com/doi/pdf/10.1080/23723556.2016.1212685?needAccess=true
Citations & impact
Impact metrics
Citations of article over time
Article citations
Potential antitumor effects of short-chain fatty acids in breast cancer models.
Am J Cancer Res, 14(5):1999-2019, 15 May 2024
Cited by: 0 articles | PMID: 38859825
Short-chain fatty acids induced lung tumor cell death and increased peripheral blood CD4+ T cells in NSCLC and control patients ex vivo.
Front Immunol, 15:1328263, 08 Apr 2024
Cited by: 1 article | PMID: 38650948 | PMCID: PMC11033355
Epigenetic effects of short-chain fatty acids from the large intestine on host cells.
Microlife, 4:uqad032, 16 Jun 2023
Cited by: 7 articles | PMID: 37441522 | PMCID: PMC10335734
Review Free full text in Europe PMC
Differential gene expression in iPSC-derived human intestinal epithelial cell layers following exposure to two concentrations of butyrate, propionate and acetate.
Sci Rep, 12(1):13988, 17 Aug 2022
Cited by: 12 articles | PMID: 35977967 | PMCID: PMC9385623
Colon mucus in colorectal neoplasia and beyond.
World J Gastroenterol, 28(32):4475-4492, 01 Aug 2022
Cited by: 4 articles | PMID: 36157924 | PMCID: PMC9476883
Review Free full text in Europe PMC
Go to all (10) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Butyrate-induced differentiation of colon cancer cells is PKC and JNK dependent.
Dig Dis Sci, 50(3):490-498, 01 Mar 2005
Cited by: 50 articles | PMID: 15810631
Butyrate and the colonocyte. Implications for neoplasia.
Dig Dis Sci, 41(4):727-739, 01 Apr 1996
Cited by: 82 articles | PMID: 8674394
Review
Caveolin-1 is transcribed from a hypermethylated promoter to mediate colonocyte differentiation and apoptosis.
Exp Cell Res, 334(2):323-336, 01 Apr 2015
Cited by: 12 articles | PMID: 25842166




