Abstract
Background
Legg-Calvé-Perthes disease (LCPD) is a childhood hip disorder thought to be caused by disruption of blood supply to the developing femoral head. There is potential for imaging to help assess revascularization of the femoral head.Purpose
To investigate whether quantitative susceptibility mapping (QSM) can detect neovascularization in the epiphyseal cartilage following ischemic injury to the developing femoral head.Study type
Prospective.Animal model
Right femoral head ischemia was surgically induced in 6-week-old male piglets. The animals were sacrificed 48 hours (n = 3) or 4 weeks (n = 7) following surgery, and the operated and contralateral control femoral heads were harvested for ex vivo MRI.Field strength/sequence
Preclinical 9.4T MRI to acquire susceptibility-weighted 3D gradient echo (GRE) images with 0.1 mm isotropic spatial resolution.Assessment
The 3D GRE images were used to manually segment the cartilage overlying the femoral head and were subsequently postprocessed using QSM. Vessel volume, cartilage volume, and vessel density were measured and compared between operated and control femoral heads at each timepoint. Maximum intensity projections of the QSM images were subjectively assessed to identity differences in cartilage canal appearance, location, and density.Statistical tests
Paired t-tests with Bonferroni correction were used (P < 0.008 considered significant).Results
Increased vascularity of the epiphyseal cartilage following ischemic injury was clearly identified using QSM. No changes were detected 48 hours after surgery. Vessel volume, cartilage volume, and vessel density were all increased in the operated vs. control femoral heads 4 weeks after surgery (P = 0.001, 0.002, and 0.001, respectively). Qualitatively, the increase in vessel density at 4 weeks was due to the formation of new vessels that were organized in a brush-like orientation in the epiphyseal cartilage, consistent with the histological appearance of neovascularization.Data conclusion
QSM can detect neovascularization in the epiphyseal cartilage following ischemic injury to the femoral head.Level of evidence
1 Technical Efficacy: Stage 1 J. Magn. Reson. Imaging 2019;50:106-113.Free full text

Quantitative Susceptibility Mapping Detects Neovascularization of the Epiphyseal Cartilage After Ischemic Injury in a Piglet Model of Legg-Calvé-Perthes Disease
Associated Data
Abstract
Background:
Legg-Calvé-Perthes disease (LCPD) is a childhood hip disorder thought to be caused by disruption of blood supply to the developing femoral head. There is potential for imaging to help assess revascularization of the femoral head.
Purpose:
To investigate whether quantitative susceptibility mapping (QSM) can detect neovascularization in the epiphyseal cartilage following ischemic injury to the developing femoral head.
Study Type:
Prospective.
Animal Model:
Right femoral head ischemia was surgically induced in 6-week-old male piglets. The animals were sacrificed 48 hours (n = 3) or 4 weeks (n = 7) following surgery, and the operated and contralateral control femoral heads were harvested for ex vivo MRI.
Field Strength/Sequence:
Preclinical 9.4T MRI to acquire susceptibility-weighted 3D gradient echo (GRE) images with 0.1 mm isotropic spatial resolution.
Assessment:
The 3D GRE images were used to manually segment the cartilage overlying the femoral head and were sub-sequently postprocessed using QSM. Vessel volume, cartilage volume, and vessel density were measured and compared between operated and control femoral heads at each timepoint. Maximum intensity projections of the QSM images were subjectively assessed to identity differences in cartilage canal appearance, location, and density.
Statistical Tests:
Paired t-tests with Bonferroni correction were used (P < 0.008 considered significant).
Results:
Increased vascularity of the epiphyseal cartilage following ischemic injury was clearly identified using QSM. No changes were detected 48 hours after surgery. Vessel volume, cartilage volume, and vessel density were all increased in the operated vs. control femoral heads 4 weeks after surgery (P = 0.001, 0.002, and 0.001, respectively). Qualitatively, the increase in vessel density at 4 weeks was due to the formation of new vessels that were organized in a brush-like orientation in the epiphyseal cartilage, consistent with the histological appearance of neovascularization.
Data Conclusion:
QSM can detect neovascularization in the epiphyseal cartilage following ischemic injury to the femoral head.
LEGG-CALVÉ-PERTHES DISEASE (LCPD) is a serious childhood hip disorder that can result in femoral head deformity and early-onset osteoarthritis.1 LCPD is caused by the interruption of blood supply to the developing femoral head, which can lead to extensive ischemic injury to the secondary ossification center (SOC) and the overlying subarticular epiphyseal cartilage (ie, growth cartilage).2 Over time, the affected femoral head spontaneously revascularizes, which initiates reparative and resorptive processes that weaken the femoral head and make it susceptible to deformation and collapse.2 The timing and rate of revascularization are important factors in determining the progression of femoral head healing and to predict clinical outcome. However, there is a lack of imaging technologies to noninvasively and serially assess revascularization patterns in both the SOC and the epiphyseal cartilage following ischemic injury.3
The vascular supply to the developing femoral head is provided by blood vessels passing through cartilage canals in the epiphyseal cartilage.3,4 Unlike articular cartilage, which is an avascular tissue, epiphyseal cartilage is only present during childhood and is extensively vascularized via a network of cartilage canal vessels.5 Epiphyseal cartilage serves as a growth cartilage responsible for the volumetric growth of the SOC. As the femoral head grows, the epiphyseal cartilage is gradually replaced by the advancing SOC until the calcified layer of the cartilage is formed and only the articular cartilage remains.5 In LCPD, there is a growth arrest of the SOC due to the cessation of blood flow and necrosis of the deep layer of the epiphyseal cartilage.1 It has been shown in a piglet model of LCPD, using micro-computed tomography (CT) angiography (μCTA), that revascularization of the femoral head following ischemic injury is initiated by invasion of new vessels (neovascularization) from the femoral neck through the epiphyseal cartilage.6 These vessels express high levels of hypoxia-inducible factor-1 and vascular endothelial growth factor.6 The pattern of neovascularization observed in the piglet model is consistent with the findings from a serial perfusion study in patients with LCPD using contrast-enhanced magnetic resonance imaging (MRI), which demonstrated that revascularization in the SOC is first observed at the periphery of the femoral head.3 Interestingly, younger children with LCPD tend to have better clinical outcomes than older children,7,8 which may be related to their greater epiphyseal cartilage volume and, thus, greater capacity to repair and remodel the femoral head following ischemic injury.6 Clinically, it would be useful to image the vascularity of the epiphyseal cartilage in LCPD to better understand the recovery of the epiphyseal cartilage from ischemic injury, to identify restoration of epiphyseal growth, and to assess the efficacy of new angiogenic treatments. Currently, contrast-enhanced MRI is the only available in vivo imaging technique to visualize vascularity in the developing femoral head.9,10 However, recent findings of gadolinium deposition in the brain has renewed concerns regarding its potential long-term effects in the pediatric patient population.11
Recently, an MRI technique based on susceptibility-weighted imaging (SWI) was introduced12 and further developed13–19 to image vascularity in the epiphyseal cartilage without the administration of exogenous contrast agents. SWI has been applied to visualize epiphyseal cartilage vascularity in the distal femur ex vivo12–17 and, more recently, in vivo in children,18,19 demonstrating its potential for clinical use. SWI is sensitive to magnetic susceptibility differences between the cartilage canals and the surrounding cartilage matrix.12 SWI images can be further processed using quantitative susceptibility mapping (QSM) to improve visualization of the cartilage canals and enable quantification.14,17 QSM of cartilage canals has been extensively validated histologically and provides detailed visualization of the 3D vascular architecture of epiphyseal cartilage in the distal femur.13,16,17
The purpose of this study was to investigate whether QSM can detect neovascularization following ischemic injury to the developing femoral head in a piglet model of LCPD. We hypothesized that neovascularization in the epiphyseal cartilage can be detected in the ischemic femoral head during the early repair phase.
Materials and Methods
Animals
Our prospective study was conducted from November 2015 to February 2018 in accordance with approved Institutional Animal Care and Use Committee protocols. Eleven 6-week-old male piglets were purchased from one of two commercial providers (Change of Pace in Aubrey, TX; or Manthei Hog Farm in Elk River, MN) and underwent surgery to induce whole right femoral head ischemia. Male piglets were chosen because LCPD is five times more common in boys than girls,20 and the size of the femoral head of a 6-week-old piglet is similar to that of a 4- to 6-year-old child, which is a typical age of LCPD onset.21 The animal model is well established and involves placing a tight ligature about the femoral neck and transecting the ligamentum teres to interrupt all blood flow to the developing femoral head.22,23 The surgeries were performed by either a board-certified pediatric orthopedic surgeon (H.K.W.K.) or a board-certified large animal orthopedic surgeon (F.T.) with respectively 20 years and 1 year of experience using the model. The piglets were then euthanized either 48 hours (n = 3) or 4 weeks (n = 8) after surgery, and the operated (right) and contralateral control (left) femoral heads were harvested and frozen at −20°C for later imaging. The 48-hour timepoint corresponds to acute ischemic damage to the femoral head, whereas the 4-week timepoint corresponds to the early repair stage including revascularization of the epiphyseal cartilage.6 One of the surgeries at the 4-week postsurgical timepoint failed to induce ischemic injury to the femoral head, as confirmed by histological assessment and was excluded. Thus, a total of n = 7 femoral head pairs were evaluated at the 4-week timepoint.
MRI
Femoral head specimens were individually thawed at 4°C and imaged using a preclinical 9.4T MRI system (Agilent Technologies; Santa Clara, CA) equipped with a Varian console and millipede radiofrequency coil (Varian NMR Systems; Palo Alto, CA). To avoid air-tissue interface artifacts at the cartilage surface of the femoral head, the specimens were immersed in Fomblin (Specialty Fluids; Castaic, CA). Imaging included a high-spatial-resolution 3D gradient echo (GRE) sequence with magnetic susceptibility weighting to provide detailed visualization of the epiphyseal cartilage vascularity. Typical sequence parameters were: field-of-view = 38.4 × 38.4 × 38.4 mm3; sampling matrix = 384 × 384 × 384; acquired spatial resolution = 0.1 × 0.1 × 0.1 mm3; repetition time / echo time (TR/TE) = 40/15 msec; flip angle = 15°; bandwidth (BW) = 37 Hz/px; and scan time = 100 minutes. Fat saturation was not applied. These scan parameters were chosen to match those used in prior studies.12,14,17 Imaging was performed by MRI scientists (C.P.J. and L.W.) with 12 and 10 years of experience, respectively.
Histology
Immediately after imaging, the specimens were bisected with a bone saw in a plane including the insertion point of the ligamentum teres and the apex of the greater trochanter and fixed in 10% neutral buffered formalin. The specimens were then decalcified in 10% EDTA, processed in paraffin, sectioned at 5 μm in one location oriented along the bisection plane, and stained with hematoxylin and eosin. Selected sections were additionally stained with toluidine blue and safranin O. Histological analysis was performed by a board-certified orthopedic veterinary pathologist (C.S.C.) with 30 years of experience to qualitatively evaluate the morphology of sites from both the operated and control femoral heads. The pathologist was blinded to the surgery to validate that ischemic injury was induced in the operated femoral heads. Additionally, regions of reparative changes were noted, including evidence of neovascularization in the epiphyseal cartilage.
Data Analysis
Each 3D susceptibility-weighted GRE MRI image was postprocessed using QSM to better visualize and quantify the vascularity of the epiphyseal cartilage.14,17 The workflow is summarized in Fig. 1. QSM postprocessing was performed in MatLab (R2013b; Math-Works; Natick, MA) using a script and methods provided by Bilgic et al,24 which included Laplacian phase unwrapping, sophisticated harmonic artifact reduction on phase (SHARP) filtering, and calculation of magnetic susceptibility values. First, the 3D GRE magnitude image was used to manually segment the cartilage overlying the femoral head with ITK-SNAP software,25 yielding a binary cartilage mask. Second, the cartilage mask was applied to the 3D GRE phase image, and the phase was unwrapped using a Laplacian filter.26 Third, SHARP filtering was applied to remove the background field.27 The SHARP filtering used an adaptive kernel size (9 × 9 × 9 voxels to 3 × 3 × 3 voxels, corresponding to filter lengths of ~0.9–0.3 mm) and the cartilage mask was eroded by two pixels (0.2 mm) to reduce artifacts at the edge of the cartilage.24 A truncation factor of 0.25 was selected based on visual quality. Finally, QSM values were calculated using a closed-form L2-regularized solution, which is one of four algorithms provided by Bilgic et al.24
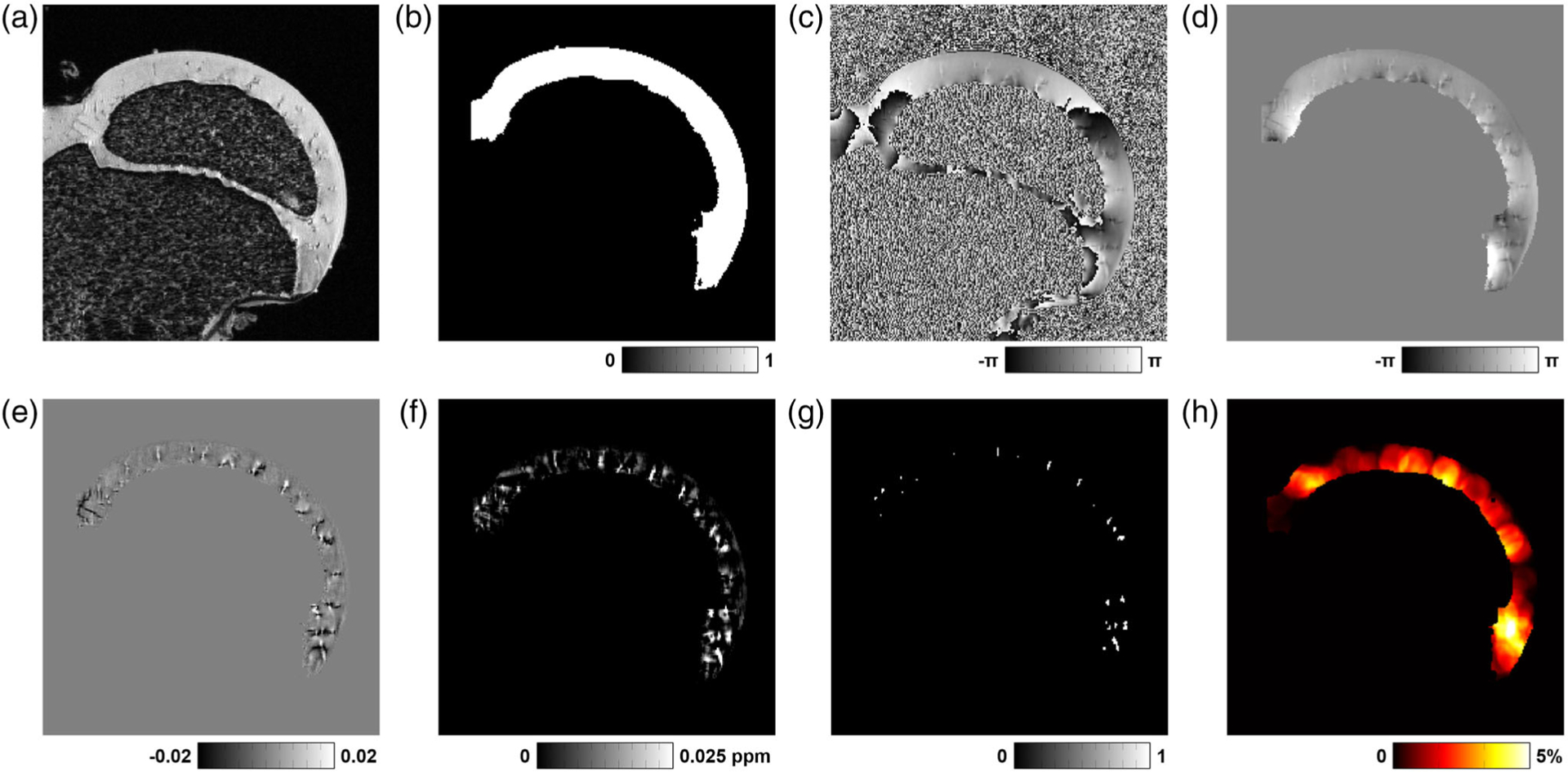
Overview of QSM postprocessing steps to visualize and quantify cartilage canals of the femoral head. (a) Original 3D GRE magnitude image (single slice, cropped to a field-of-view of 23.4 × 23.4 mm2 for display). (b) Cartilage mask generated after segmentation of the 3D GRE magnitude image using ITK-SNAP. This mask was used to measure cartilage volume. (c) Original 3D GRE phase image. (d) Laplacian unwrapped phase of the segmented cartilage. (e) Field map after SHARP filtering. (f) QSM map generated after magnetic susceptibility calculation using an L2-regularized algorithm. (g) Cartilage canal vessel mask generated after applying a threshold value of 0.025 ppm to the QSM map. This mask was used to measure vessel volume and vessel density. (h) Vessel density map generated by calculating the local density of vessels in a sphere with a 2.1 mm diameter.
Cartilage volume, vessel volume, and vessel density were then quantitatively measured. Cartilage volume was measured as the total number of segmented cartilage voxels prior to QSM postprocessing (ie, using the cartilage mask). Vessel volume was measured as the total number of voxels remaining after thresholding the postprocessed QSM images; all voxels with a QSM value ≥0.025 ppm were assumed to belong to cartilage canals. This threshold value was visually found to robustly separate the cartilage canal voxels (which have high QSM values on the order of 0.040 ppm17) from the surrounding cartilage matrix. Vessel density was then calculated by dividing the vessel volume by the total cartilage volume in the QSM postprocessed image.
3D maps of vessel density were generated for each specimen by counting the number of cartilage canal voxels in the postprocessed QSM image (ie, those voxels with a QSM value ≥0.025 ppm) within a sphere of diameter 21 voxels (ie, volume = 4.9 mm3). Each voxel of the vessel density map was assigned the ratio of the number of cartilage canal voxels to the total number of voxels in the sphere (ie, 4945 voxels) centered at the same location. In cases where the sphere extended outside of the cartilage volume, the density calculation only included those voxels within the cartilage volume.
Lastly, the QSM images were subjectively assessed by two independent observers (C.P.J., an MRI scientist with 12 years of experience; and J.M.E., a board-certified musculoskeletal radiologist and MRI scientist with 30 years of experience) to identify changes in cartilage canal architecture. To visualize the cartilage canals, maximum intensity projections (MIPs) of the 3D QSM images were generated at multiple projection angles. The general appearance, location, and density of the cartilage canals were noted.
Statistical Analysis
Measurements of vessel volume, cartilage volume, and vessel density were compared between the operated and control femoral heads at the 48-hour and 4-week timepoints using two-tailed paired t-tests conducted using R statistical software (v. 3.3.1; R Foundation for Statistical Computing; Vienna, Austria). Given a target Type I error rate of α = 0.05 and six hypotheses (three measures × two timepoints), P < 0.008 was considered statistically significant after conservative Bonferroni correction for multiple comparisons. Secondary post-hoc two-tailed Student’s t-tests were also performed to compare measurements between the 48-hour and 4-week timepoints, with P < 0.05 (uncorrected) considered hypothesis generating.
Results
At the 48-hour timepoint, no differences in vessel volume (P = 0.479), cartilage volume (P = 0.503), or vessel density (P = 0.147) were detected between the operated and control femoral heads (Table 1 and Fig. 2). The paired specimens had very similar vessel volume (mean difference [Δ] = 1.0 ± 2.0 mm3), cartilage volume (Δ = −46 ± 99 mm3), and vessel density (Δ = 0.12 ± 0.09%). GRE and QSM images and vessel density maps from a representative pair of control and operated femoral heads at the 48-hour timepoint are shown in Fig. 3. 3D rotating MIPs of the QSM images for each femoral head are shown in Supplemental Movie 1. In both the control and operated femoral heads, the cartilage canals were oriented linearly and were evenly distributed across the extent of the epiphyseal cartilage. These observations were consistent across all three pairs of femoral heads at the 48-hour timepoint (see Supplemental Fig. S1).
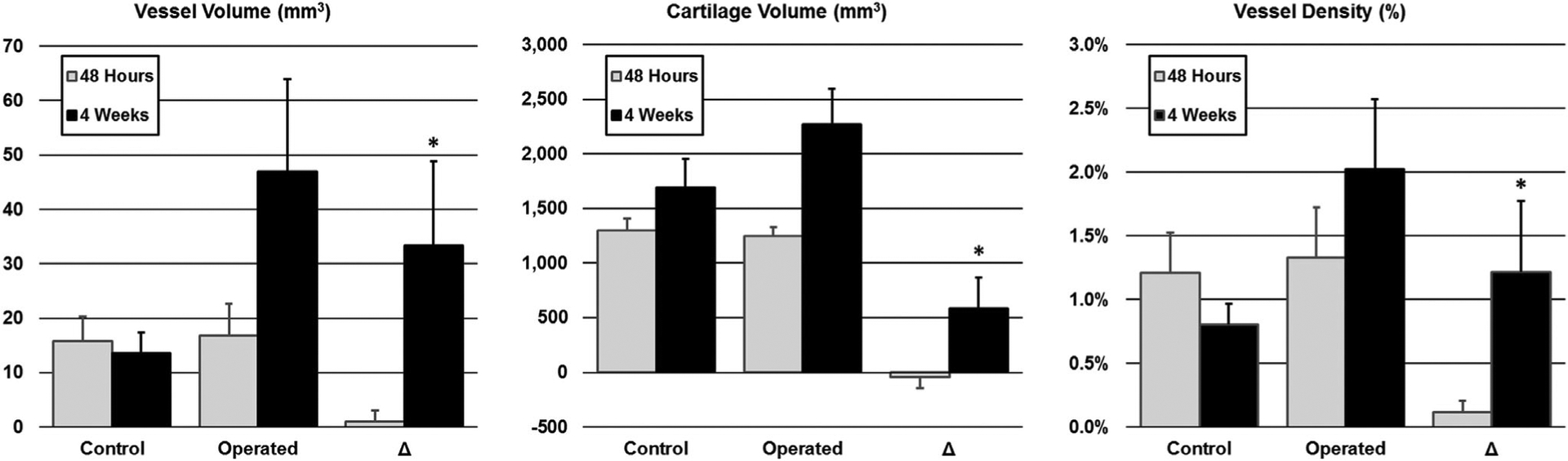
Quantitative analysis of vessel volume, cartilage volume, and vessel density of paired control and operated femoral heads. Plotted are the means and standard deviations for the three measurements at both the 48-hour (gray bars) and 4-week (black bar) timepoints. Results are shown for the control femoral heads, operated femoral heads, and the paired difference (Δ) between the operated and control femoral heads. No differences were detected between the operated and control femoral heads 48 hours following surgical induction of complete femoral head ischemia. In contrast, 4 weeks following surgery, all three measures were significantly increased in the operated vs. control femoral heads (*P < 0.008 for the paired differences).
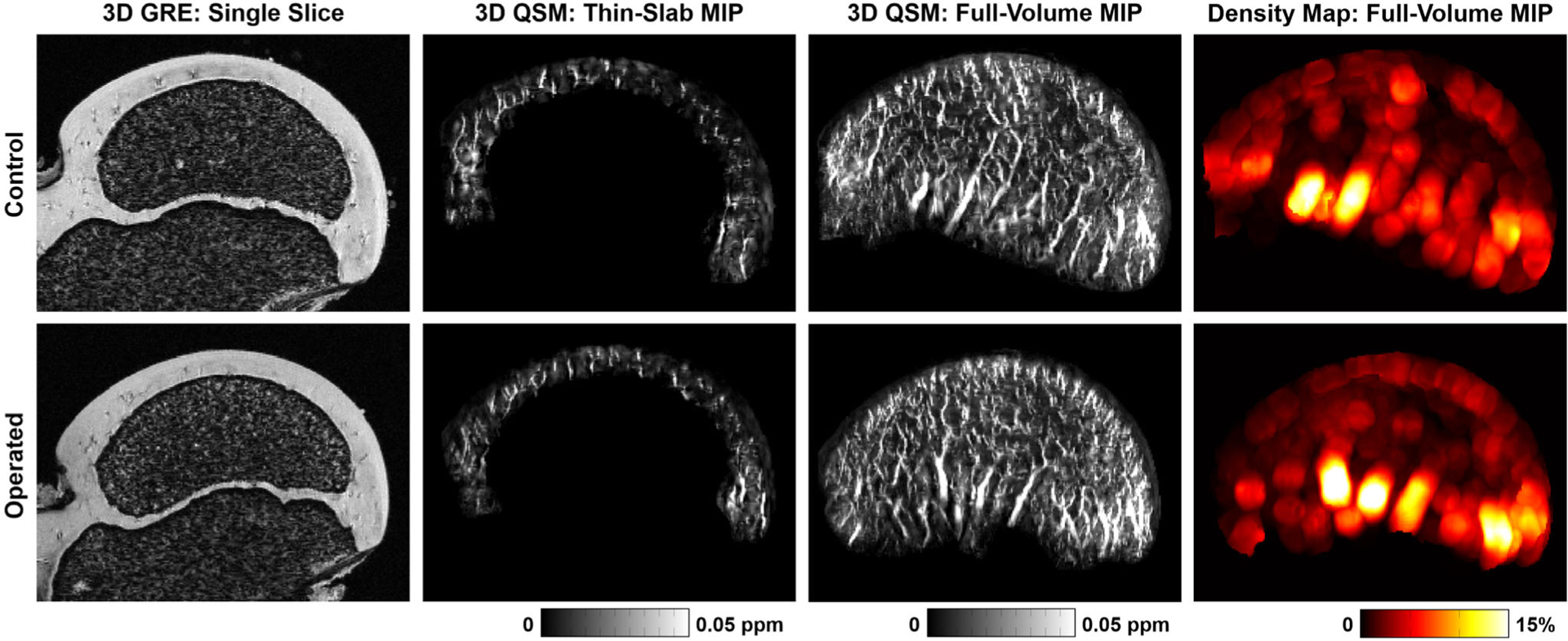
Images of a representative pair of control and operated femoral heads 48 hours after surgical induction of ischemia. Shown are: (i) single slices of the 3D GRE magnitude images (cropped to a field-of-view of 23.9 × 17.8 mm2 for display), on which the cartilage canals appear dark against a bright cartilage background; (ii) thin-slab MIPs (2.0 mm) of the 3D QSM images at the same location as the GRE images, on which the cartilage canals appear bright (ie, higher magnetic susceptibility values compared to the cartilage background); (iii) full-volume MIPs of the 3D QSM images, which show the 3D architecture of the cartilage canals; and (iv) full-volume MIPs of 3D vessel density maps derived from the QSM images, which show the regional vessel density across the femoral head. The two femoral heads have a similar appearance on all images, including similar size of the secondary ossification center, thickness of the cartilage, and vessel architecture and density, which demonstrates that the QSM method is not sensitive to the acute effects of ischemia. Also see Supplemental Movie 1 and Supplemental Figure S1.
TABLE 1.
Quantitative Measurements of Epiphyseal Cartilage Vascularity in Paired Control and Operated Femoral Heads 48 Hours and 4 Weeks Following Surgical Induction of Complete Femoral Head Ischemia
| 48 Hours | 4 Weeks | |||||
|---|---|---|---|---|---|---|
| Control | Operated | Paired Diff. | Control | Operated | Paired Diff. | |
| Vessel Volume (mm3) | 15.8 ± 4.5 | 16.8 ± 5.9 | 1.0 ± 2.0 | 13.6 ± 3.8 | 46.9 ± 17.0 | 33.3 ± 15.5 * |
| Cartilage Volume (mm3) | 1296 ± 114 | 1250 ± 80 | −46 ± 99 | 1689 ± 266 | 2271 ± 323 | 582 ± 289 * |
| Vessel Density (%) | 1.21 ± 0.31 | 1.33 ± 0.40 | 0.12 ± 0.09 | 0.80 ± 0.16 | 2.02 ± 0.55 | 1.22 ± 0.55 * |
Values are reported as mean ± standard deviation.
In contrast, significant differences between the operated and control femoral heads were detected at the 4-week timepoint. Quantitatively, the operated femoral heads had significantly greater vessel volume (P = 0.001; Δ = 33.3 ± 15.5 mm3), cartilage volume (P = 0.002; Δ = 582 ± 289 mm3), and vessel density (P = 0.001; Δ = 1.22 ± 0.55%) than their paired controls (Table 1 and Fig. 2). Compared to the collective operated and control findings at the 48-hour timepoint, the operated femoral heads at the 4-week timepoint had greater vessel volume (P = 0.001; Δ = 30.6 ± 17.6 mm3), cartilage volume (P < 0.001; Δ = 998 ± 336 mm3), and vessel density (P = 0.014; Δ = 0.75 ± 0.64%). By comparison, the control femoral heads at the 4-week timepoint had similar vessel volume to the collective findings at 48 hours (P = 0.273; Δ = −2.7 ± 6.1 mm3) but greater cartilage volume (P = 0.004; Δ = 416 ± 282 mm3), resulting in reduced vessel density (P = 0.006; Δ = −0.47 ± 0.36%). Thus, whereas the control femoral heads had reduced vessel density over time, the operated femoral heads had increased vessel density.
Qualitatively, increased vessel density in the operated femoral heads at the 4-week timepoint was primarily localized to the epiphyseal cartilage near the metaphyseal physis. GRE and QSM images and vessel density maps from a representative pair of operated and control femoral heads at the 4-week timepoint are shown in Fig. 4. Comparative rotating MIPs of their 3D QSM images are shown in Supplemental Movie 2. Clusters of brush-like vessels were seen near the metaphyseal physis in the operated femoral head, whereas the control femoral head had the same linear architecture observed at the 48-hour timepoint. Additionally, the operated femoral head had thicker cartilage and smaller SOC due to the cessation of blood flow, which produces a growth arrest of the SOC. The increased density of vessels near the metaphyseal physis was further evident in the vessel density map. This pattern of increased vessel density was consistent across all pairs of femoral heads at 4-week timepoint (see Supplemental Fig. S2). The histological appearance of cartilage canals for a pair of control and operated femoral heads are shown in Fig. 5a. While the cartilage canals of the control femoral head appeared singly in cross-section on histology, the cartilage canals of the operated femoral head were composed of a large central vessel surrounded by many smaller vessels, which is characteristic of neovascularization. For comparison, targeted MIPs of 3D QSM images from control and operated femoral heads are shown in Fig. 5b, which have a cartilage canal architecture consistent with the histological findings.
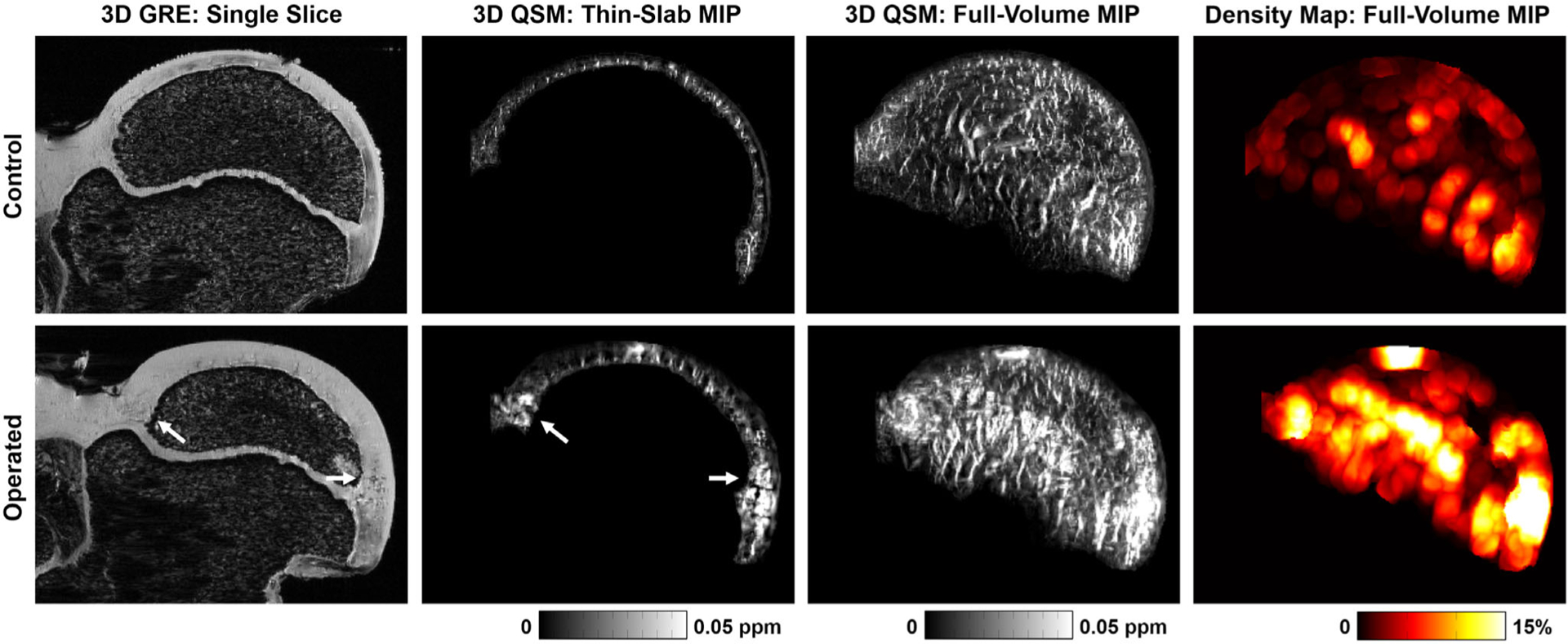
Images of a representative pair of control and operated femoral head 4 weeks after surgical induction of ischemia. Images are the same as described in Fig. 2. The field-of-view of the images was cropped to 32.2 × 24.0 mm2 for display. In contrast to the results at 48 hours, the control and operated femoral heads have significant differences at the 4-week timepoint. In addition to thicker cartilage and a smaller secondary ossification center in the operated femoral head (due to lack of development), increased vessel density is evident in the epiphyseal cartilage near the metaphyseal physis (arrows). On the 3D QSM full-volume MIP, this dense vasculature can be seen to have a brush-like architecture that is different from that seen in the control femoral head or at the 48-hour timepoint (cf. Fig. 2). The vessel density map shows regional density near the metaphyseal physis exceeding 15%, which is much greater than that seen in the control femoral head or the results at 48 hours (cf. Fig. 2). Also see Supplemental Movie 2 and Supplemental Figure S2.
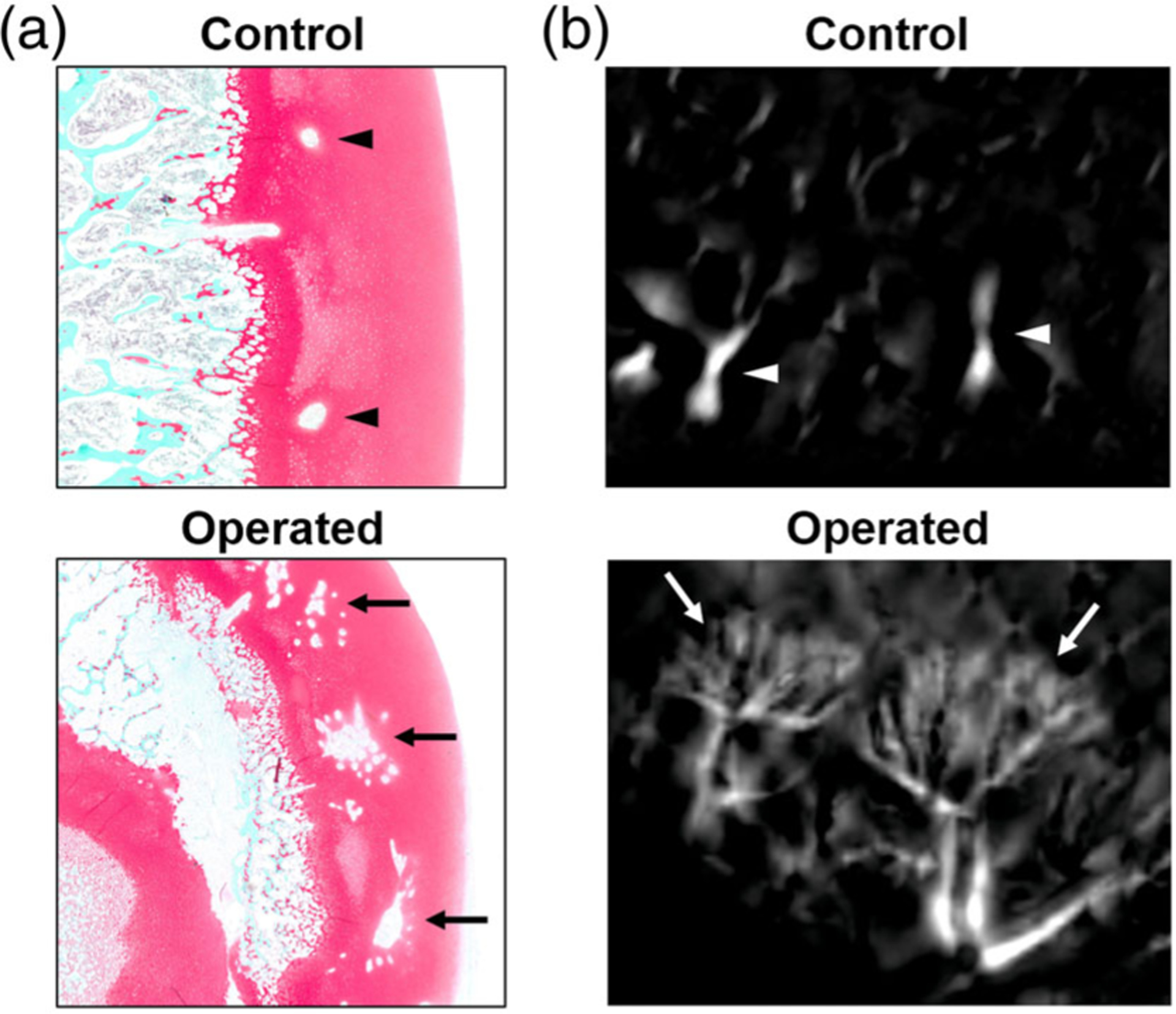
Histological appearance of cartilage canals in control and operated femoral heads 4 weeks following surgical induction of complete femoral head ischemia. (a) Histological sections stained with safranin-O show distinct differences in the appearance of cartilage canals in a control vs. operated femoral head. In the images, the cartilage is stained red (articular cartilage is on the right side), the trabecular bone of the secondary ossification center is stained blue/green, and the clear spaces in the cartilage represent vessels. While the cartilage canals are singular and have a circular appearance in the control femoral head (arrowheads), they are multiplied (with a large central vessel surrounded by many smaller vessels) in the operated femoral head (arrows). (b) The distinct cartilage canal morphology seen histologically is consistent with the 3D appearance of the cartilage canals using QSM, from which targeted MIPs of cartilage canals are shown on a similar scale as the histology images. Although cartilage canals in control femoral heads have a normal linear appearance (arrowheads), cartilage canals in operated femoral heads have dense brush-like architecture (arrows).
Discussion
Our results demonstrate that QSM can detect vascularity in the epiphyseal cartilage of the developing femoral head without the use of an exogeneous contrast agent in a piglet model of LCPD. Furthermore, QSM can detect increased density of vessels in the epiphyseal cartilage following ischemic injury to the femoral head, which can be attributed to neovascularization during the early repair phase. Histological assessment confirmed the finding.
Our findings at the 4-week timepoint support the hypothesis that QSM can detect neovascularization in the epiphyseal cartilage. Comparison of the control and operated femoral heads revealed significantly greater vessel density in the operated femoral heads despite having significantly greater cartilage volume due to interruption of endochondral ossification.6 The greater vessel density was primarily localized to the epiphyseal cartilage near the metaphyseal physis, which is where vessels supplying the SOC are known to originate.6,10 Importantly, the vessel density of the operated femoral heads was also greater at 4 weeks vs. 48 hours postsurgery. This supports the previously reported finding in the piglet model that the epiphyseal cartilage expresses increased levels of angiogenic factors that promote new vessel ingrowth into the cartilage after ischemic injury.6 The dense, brush-like appearance of the cartilage canals in the operated femoral heads at the 4-week timepoint were fundamentally different than the linear appearance of cartilage canals for all other examined femoral heads. This brush-like orientation of vessels was confirmed histologically to be indicative of neovascularization. A similar appearance of neovascularization was recently observed in a goat model of osteochondritis dissecans, another developmental disease that affects epiphyseal vascularity.17
Our results at the 4-week timepoint are consistent with the μCTA findings of Kim et al6 using the same piglet model and a similar experimental design. Kim et al also found that both vessel volume and cartilage volume were significantly greater in the operated vs. control femoral heads. Kim et al also concluded, based on differences between the measurements in operated femoral heads at 48 hours and 4 weeks postsurgery, that the increase in vessel volume (despite a concomitant increase in cartilage volume) is a result of neovascularization. However, there were some discrepancies between our results and those of Kim et al at the 4-week timepoint. While cartilage volume measurements were similar using μCTA and QSM, vessel volume measurements using μCTA were about a factor of 10 lower than those using QSM. This discrepancy may be due to several factors: 1) μCTA images the vessel lumen of the cartilage canals, whereas QSM images the cartilage canal structure enclosing the lumen and may overestimate the size of the cartilage canals due to magnetic field disturbances near the cartilage canals; 2) QSM images both patent and occluded vessels, whereas μCTA images only patent vessels (ie, those that are accessible via injection of iodinated contrast material); and 3) our QSM images appear to show greater vessel detail than the μCTA images, which suggests that the QSM images had greater spatial resolution and/or sensitivity. Another discrepancy is that Kim et al found that the operated and control femoral heads had similar vessel density, while we found the operated femoral heads had significantly greater vessel density. This is primarily due to detection of a greater difference in vessel volume between the operated and control femoral heads using QSM. As noted above, this may due to QSM detecting finer vessel detail and/or the presence of both patent and occluded vessels. In either case, our finding of increased vessel density is consistent with the growth or invasion of new vessels.
The lack of a difference between the operated and control femoral heads at the 48-hour timepoint is a result of the sensitivity of QSM to the presence of cartilage canal vessels independent of blood flow. In contrast, the μCTA study by Kim et al did not detect vessels in the epiphyseal cartilage 48 hours postsurgery,6 since iodine contrast material injected into the extraosseous vessels feeding the femoral head was unable to reach the epiphyseal cartilage vessels due to the surgically induced vascular occlusion. Thus, while QSM has the advantage of being a noninvasive technique that may be suitable for in vivo imaging of children, its potential to detect vascular occlusion (eg, by measuring vessel oxygenation levels28,29) has not yet been explored in the epiphyseal cartilage. However, it has been previously observed that cartilage canal profiles can become less distinct and even vanish over an extended period in response to ischemic injury to epiphyseal cartilage,17 thus degenerative as well as proliferative changes in the cartilage canals may eventually be detectable.
The QSM technique may play an important role in the clinical management of patients with LCPD, as it potentially can help determine initiation of neovascularization and restoration of the epiphyseal cartilage function to sustain femoral head growth. The technique may also be useful for determining the efficacy of proangiogenic treatments to accelerate epiphyseal cartilage neovascularization and subsequent epiphyseal healing.6 However, further work is needed to translate this method for in vivo imaging at clinical magnetic field strengths. The use of long scan times and a preclinical 9.4T MRI system enabled detailed depiction of the vascularity of the epiphyseal cartilage, but image spatial resolution and quality will need to be reduced to accommodate in vivo imaging. This is potentially feasible, as QSM has recently been applied to examine the vascularity of the distal femoral condyle in children.18,19 Additionally, the distinct appearance of neovascularization compared with normal vasculature may aid in its detection at lower spatial resolution.
Scientific limitations of our study include its cross-sectional design, a somewhat limited sample size, and the use of an ex vivo animal model. Future work will focus on translation of these methods for in vivo studies to longitudinally assess changes in the vascularity of the epiphyseal cartilage during normal femoral head development and in response to ischemic injury.
In conclusion, QSM can detect neovascularization in the epiphyseal cartilage following ischemic injury to the femoral head. This noncontrast-enhanced method may prove useful for understanding the pathophysiology of LCPD and related childhood hip disorders, clinical staging of these diseases, and evaluating the efficacy of proangiogenic treatments.
Supplementary Material
supp fig 1
movie 1
movie 2
supp fig 2
Acknowledgments
Funding for this study was provided by the National Institute for Arthritis and Musculoskeletal and Skin Diseases (K01AR070894), the National Institute for Biomedical Imaging and Bioengineering (P41EB015894), the W. M. Keck Foundation, and Texas Scottish Rite Hospital for Children.
We thank Paula Overn for assistance with the preparation of the histological sections.
Footnotes
Additional supporting information may be found in the online version of this article.
References
Full text links
Read article at publisher's site: https://doi.org/10.1002/jmri.26552
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc7249674?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
Biochanin A inhibits endothelial dysfunction induced by IL‑6‑stimulated endothelial microparticles in Perthes disease via the NFκB pathway.
Exp Ther Med, 27(4):137, 13 Feb 2024
Cited by: 0 articles | PMID: 38476892 | PMCID: PMC10928846
MR-based techniques for intracortical vessel visualization and characterization: understanding the impact of microvascular disease on skeletal health.
Curr Opin Endocrinol Diabetes Obes, 30(4):192-199, 19 Jun 2023
Cited by: 1 article | PMID: 37335282 | PMCID: PMC10461604
Review Free full text in Europe PMC
Effects of acute femoral head ischemia on the growth plate and metaphysis in a piglet model of Legg-Calvé-Perthes disease.
Osteoarthritis Cartilage, 31(6):766-774, 22 Jan 2023
Cited by: 1 article | PMID: 36696941
Naturally occurring osteochondrosis latens lesions identified by quantitative and morphological 10.5 T MRI in pigs.
J Orthop Res, 41(3):663-673, 25 Jun 2022
Cited by: 0 articles | PMID: 35716161 | PMCID: PMC9759621
Quantitative T2 and T1ρ mapping are sensitive to ischemic injury to the epiphyseal cartilage in an in vivo piglet model of Legg-Calvé-Perthes disease.
Osteoarthritis Cartilage, 30(9):1244-1253, 26 May 2022
Cited by: 2 articles | PMID: 35644462 | PMCID: PMC9378508
Go to all (8) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Quantitative T2 and T1ρ mapping are sensitive to ischemic injury to the epiphyseal cartilage in an in vivo piglet model of Legg-Calvé-Perthes disease.
Osteoarthritis Cartilage, 30(9):1244-1253, 26 May 2022
Cited by: 2 articles | PMID: 35644462 | PMCID: PMC9378508
T1ρ and T2 mapping detect acute ischemic injury in a piglet model of Legg-Calvé-Perthes disease.
J Orthop Res, 40(2):484-494, 16 Apr 2021
Cited by: 5 articles | PMID: 33788301 | PMCID: PMC8481332
Detection of early metaphyseal changes in a piglet model of Legg-Calvé-Perthes disease using quantitative mapping of MRI relaxation times.
J Orthop Res, 42(10):2277-2286, 26 May 2024
Cited by: 0 articles | PMID: 38796746 | PMCID: PMC11486590
Can large doses of glucocorticoids lead to Perthes? a case report and review of the literature.
BMC Pediatr, 21(1):339, 12 Aug 2021
Cited by: 0 articles | PMID: 34384372 | PMCID: PMC8359607
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NIAMS NIH HHS (1)
Grant ID: K01 AR070894
NIBIB NIH HHS (1)
Grant ID: P41 EB015894
NIH HHS (1)
Grant ID: K01 OD021293




