Abstract
Free full text

Upregulated expression of MNX1-AS1 long noncoding RNA predicts poor prognosis in gastric cancer
Abstract
As important regulators of gene expression long noncoding RNAs (lncRNAs) are implicated in various physiological and pathological processes, including cancer. An oncogenic role of MNX1 antisense RNA 1 (MNX1-AS1) lncRNA has been suggested in cervical cancer and glioblastoma. In this study, we investigated the clinicopathological significance and biological function of MNX1-AS1 in gastric cancer (GC). The expression of MNX1-AS1 was analyzed by qRT-PCR in 96 GC and adjacent non-tumor tissues in relation to clinicopathological features and overall survival (OS) of patients, and in five human GC cell lines compared to a normal gastric epithelial cell line. Loss-of-function experiments using small interfering RNA (siRNA) targeting MNX1-AS1 (si-MNX1-AS1) were carried out in AGS and MGC-803 GC cell lines. Cell proliferation (CCK-8 assay), migration (Transwell) and invasion (Transwell Matrigel), and protein expression of proliferating cell nuclear antigen (PCNA), E-cadherin, N-cadherin, vimentin and matrix metallopeptidase 9 (MMP-9) were analyzed in transfected GC cells. Expression of MNX1-AS1 was significantly higher in GC vs. adjacent non-tumor tissues. Higher MNX1-AS1 expression was significantly associated with tumor size, TNM stage and lymph node metastasis. Kaplan–Meier analysis showed that GC patients with higher MNX1-AS1 expression had worse OS compared to patients with lower MNX1-AS1 expression. Multivariate analysis showed that MNX1-AS1 is an independent poor prognostic factor in GC. Knockdown of MNX1-AS1 significantly inhibited proliferation, migration and invasion of AGS and MGC-803 cells, and resulted in increased E-cadherin and decreased PCNA, N-cadherin, vimentin and MMP-9 expression. Taken together, these results suggest that MNX1-AS1 has an oncogenic function in GC and potential as a molecular target in GC therapy.
INTRODUCTION
In 2017, the American Cancer Society estimated 28,000 new gastric cancer (GC) cases and 10,960 GC deaths to occur in the United States [1]. Due to high tumor aggressiveness, limited therapeutic options and late diagnosis, GC remains the third leading cause of cancer-related death globally [2]. Risk factors associated with GC include infection with Helicobacter pylori and Epstein-Barr virus, and family history of GC [3]. Dysregulation of oncogenes and tumor suppressor genes plays a crucial role in the progression of GC [4], however, the underlying mechanisms are largely unknown.
Noncoding RNAs represent a significant portion of the human genome and include, among others, microRNAs (miRNAs), long noncoding RNAs (lncRNAs), small nuclear RNAs (snRNAs), and small nucleolar RNAs (snoRNAs) [5]. LncRNAs are transcripts longer than 200 nucleotides that have an important role in transcriptional and posttranscriptional regulation of gene expression. These ncRNAs are implicated in various physiological and pathological processes, including inflammation, development, neurodegenerative and cardiovascular diseases [6]. In cancer, aberrantly expressed lncRNAs lead to epigenetic and transcriptional changes of cancer-related genes, which suggests they could be used as diagnostic and prognostic markers, as well as therapeutic targets [7,8]. For example, Liang et al. showed that H19 lncRNA accelerates tumor growth in colorectal cancer (CRC) and promotes epithelial to mesenchymal transition (EMT), by competitively binding to miR-200a and miR-138 and upregulating the expression of vimentin, zinc finger E-box binding homeobox 1 (ZEB1) and ZEB2 [9]. Increased expression of lncRNA plasmacytoma variant translocation 1 (PVT1) in pancreatic ductal adenocarcinoma (PDAC) is associated with clinical stage, N-classification, and poor overall survival (OS) [10]. Enhanced expression of lncRNA-activated by transforming growth factor beta [TGF-β] (lncRNA-ATB) predicts poor prognosis and promotes metastasis in colon cancer by repressing E-cadherin expression [11].
LncRNAs associated with GC include homeobox A11 antisense (HOX11-AS) [12], maternally expressed 3 (MEG3) [13], HOX transcript antisense RNA (HOTAIR) [14], and growth arrest-specific 5 (GAS5) [15].
MNX1 antisense RNA 1 (MNX1-AS1) is a lncRNA located on chromosome 7. In patients with ovarian cancer, MNX1-AS1 expression is associated with the International Federation of Gynecology and Obstetrics (FIGO) stage, tumor grade, distant metastasis and poor OS, which suggests it could be used as a prognostic marker [16]. Moreover, downregulation of MNX1-AS1 by RNA interference suppresses cell proliferation, colony formation, cell migration ability and promotes apoptosis in ovarian cancer cell lines, indicating that MNX1-AS1 has an oncogenic role in ovarian cancer [17]. Similarly, Gao et al. [18] showed that MNX-AS1 promotes glioblastoma cell proliferation, invasion and migration, by suppressing miR-4443. However, the biological function and clinicopathological significance of MNX1-AS1 in GC remain unclear.
In this study, we first analyzed the expression of MNX1-AS1 in GC and adjacent non-tumor tissues in relation to clinicopathological features and 5-year OS of GC patients. Next, we assessed MNX1-AS1 expression in five human GC cell lines compared to a normal gastric epithelial cell line. Finally, we performed loss-of-function experiments in two GC cell lines using RNA interference, to investigate the effect of MNX1-AS1 on GC cell proliferation, migration and invasion. This study provides novel findings on the diagnosis and treatment of GC.
MATERIALS AND METHODS
Patients and tissue samples
A total of 96 primary GC and adjacent non-tumor tissues (>3 cm away from the tumor margin) were collected from patients diagnosed with GC by pathology at the Affiliated Hospital of Jining Medical University. All tissue samples were immediately frozen in liquid nitrogen and stored at -80 °C until analysis. None of the patients had received preoperative radiotherapy or chemotherapy. Follow-up data and written informed consent were obtained for all patients. Clinicopathological data, including gender, age, tumor-node-metastasis (TNM) staging and tumor size, were recorded and are summarized in Table 1. OS was defined as the time from the date of primary surgery to the date of death or to the end of follow-up. This study was approved by the ethical committees of Affiliated Hospital of Jining Medical University.
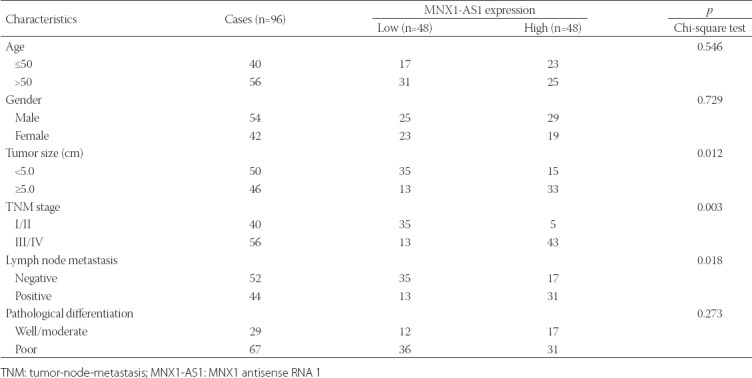
TABLE 1
Association between MNX1-AS1 expression and clinicopathological characteristics of patients with gastric cancer
Cell culture and transfection
Five human GC cell lines (MKN-45, SGC-7901, AGS, MGC-803, and BGC-823) and one normal gastric epithelial cell line (GES-1) were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). MKN-45, MGC-803, BGC-823, and GES-1 cells were cultured in RPMI 1640 medium (Gibco) supplemented with 10% fetal bovine serum (FBS). SGC-7901 and AGS cells were cultured in DMEM (Gibco) with 10% FBS. All cells were maintained at 37 °C, under an atmosphere containing 5% CO2.
Small interfering RNA (siRNA) targeting MNX1-AS1 (si-MNX1-AS1) and non-targeting negative control siRNA (si-NC) were purchased from RiboBio Co., Ltd. (Guangzhou, China). For transfection experiments, AGS and MGC-803 cells were seeded in 6-well plates and cultured to reach 80% confluence. The cells were transfected with si-MNX1-AS1 or si-NC using RNAiMAX (Invitrogen, Shanghai, China) according to the manufacturer’s instructions. Forty-eight hours after transfection, the cells were harvested for subsequent experiments.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA was extracted from tissue samples or cells using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) and complementary DNA (cDNA) was synthesized from isolated RNA using PrimeScript RT Kit (TaKaRa, Dalian, China), according to the manufacturer’s instructions. qRT-PCR was performed on an ABI 7500 system (Applied Biosystems, Foster City, CA, USA) using SYBR Premix Ex Taq (TaKaRa, Dalian, China). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was the internal control. The following primers were used: MNX1-AS1 forward 5′-CCCGCATT TTCAGATTCAC-3′, reverse 5′-GCTCTCAG CCTCGCCATA-3′; GAPDH forward 5′-GCACCGTCAA GGCTGAGAA-3′, reverse 5′-GTGAAGACGCCA GTGGACTC-3′. Each sample was analyzed in triplicate. The relative expression of MNX1-AS1 was normalized to GAPDH mRNA by the 2-ΔΔCt method.
Cell proliferation assay
Cell proliferation was analyzed by Cell Counting Kit-8 (CCK-8) assay (Beyotime Institute of Biotechnology, Shanghai, China), according to the manufacturer’s instructions. Briefly, around 3,000 transfected cells were seeded in a 96-well plate and cultured overnight. CCK-8 solution was added to the wells at 24, 48, 72 or 96 hours of incubation and the cells were incubated for another 2 hours at 37 °C. The absorbance value (OD) of each well was measured at 450 nm on a microplate reader. The experiments were independently performed three times.
Cell migration and invasion assays
Cell migration and invasion assays were performed using 24-well Transwell chambers with 8 µm pore size (BD Biosciences). For migration assay, approximately 1×105> of transfected cells were seeded into the upper chamber (with a permeable membrane) in serum-free medium and incubated for 24 hours. A medium containing 10% FBS, which serves as a chemoattractant, was added to the lower chamber. After 24 hours of incubation, cells that migrated to the bottom of the membrane were fixed with 4% formaldehyde for 10 minutes and stained with 0.5% crystal violet solution. The stained cells were observed and counted under a microscope (Olympus Corp., Tokyo, Japan) at 200× magnification. The protocol was identical for cell invasion assay, except that the upper chambers were precoated with 40 µl of diluted Matrigel (BD Biosciences).
Western blot analysis
Total protein was extracted from cells using RIPA lysis buffer (Beyotime, Beijing, China) and protein concentration was determined using a BCA protein assay kit (Beyotime, Beijing, China). Subsequently, 30 µg of protein was separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, Bedford, MA, USA). The membrane was blocked with 5% skim milk and incubated with primary antibodies against proliferating cell nuclear antigen (PCNA), E-cadherin, N-cadherin, vimentin, matrix metallopeptidase 9 (MMP-9), and GAPDH as a control (all from Abcam, Cambridge, UK). After washed with phosphate buffered saline (PBS) with Tween 20 for 25 minutes (5 times × 5 minutes), the membrane was incubated with goat anti-rabbit horseradish peroxidase (HRP)-conjugated polyclonal secondary antibody, followed by signal detection with enhanced chemiluminescence detection kit (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
Statistical analysis
Statistical analysis was performed using SPSS Statistics for Windows, Version 17.0. (SPSS Inc., Chicago, IL, USA). Ninety-six GC tissues were divided into groups with high and low expression, according to the median expression levels of MNX1-AS1. The association between MNX1-AS1 expression and clinicopathological characteristics of GC patients was analyzed using the chi-square test. The OS of GC patients was estimated by the Kaplan–Meier method and compared between the groups with low and high MNX1-AS1 expression using the log-rank test. In vitro experiments were performed in triplicate and quantitative data are expressed as mean ± standard deviation (SD). Two-tailed Student’s t-test was used to determine significant differences between si-MNX1-AS1 and si-NC groups. A p-value <0.05 was considered statistically significant.
RESULTS
Clinicopathological significance of MNX1-AS1 expression in GC
To investigate the clinicopathological significance of MNX1-AS1 in GC, we analyzed MNX1-AS1 expression in 96 paired GC and adjacent normal tissues by qRT-PCR. We found that MNX1-AS1 expression was higher in GC compared to adjacent normal tissues (Figure 1A, p < 0.001). Next, we analyzed the association between MNX1-AS1 expression and the clinicopathological characteristics of GC patients. According to the median expression levels of MNX1-AS1, GC tissues were divided into low expression group (n = 48) and high expression group (n = 48). As shown in Table 1, higher MNX1-AS1 expression was significantly associated with tumor size (p = 0.012), TNM stage (p = 0.003) and lymph node metastasis (p = 0.018). On the other hand, no significant association was observed between MNX1-AS1 expression and age, gender and pathological differentiation (all p > 0.05).
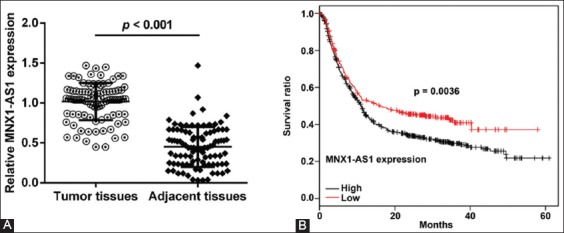
MNX1 antisense RNA 1 (MNX1-AS1) expression is related to poor prognosis of gastric cancer patients. A) Expression of MNX1-AS1 in 96 paired gastric cancer (GC) and adjacent normal tissues was analyzed by quantitative reverse transcription polymerase chain reaction (qRT-PCR). Relative MNX1-AS1 expression was higher in GC compared to adjacent normal tissues (p < 0.001). B) GC tissues were divided into low expression group (n = 48) and high expression group (n = 48), according to the median expression levels of MNX1-AS1. Kaplan–Meier survival analysis with log-rank test was used to evaluate the association between MNX1-AS1 expression and patient overall survival (OS). GC patients with high MNX1-AS1 expression had a poorer 5-year OS compared to those with low MNX1-AS1 expression (p = 0.0036).
High MNX1-AS1 expression is associated with poor prognosis in GC patients
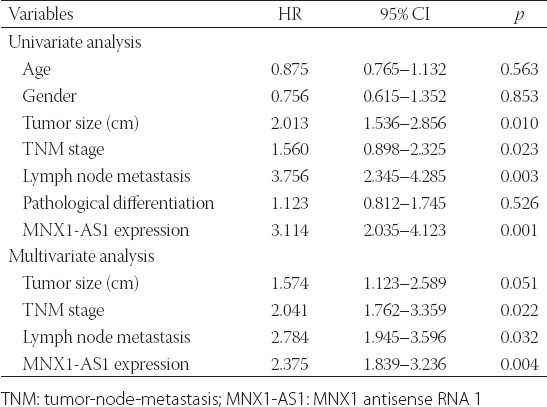
We further explored the prognostic value of MNX1-AS1 in GC. Kaplan–Meier survival analysis with log-rank test was used to analyze the association between MNX1-AS1 expression and patient OS. As shown in Figure 1B, GC patients with high MNX1-AS1 expression had a poorer 5-year OS compared to those with low MNX1-AS1 expression (p = 0.0036). The univariate analysis (Table 2) showed that the tumor size (HR: 2.013, 95% CI: 1.536–2.856, p = 0.010), TNM stage (HR: 1.560, 95% CI: 0.898–2.325, p = 0.023), lymph node metastasis (HR: 3.756, 95% CI: 2.345–4.285, p = 0.003) and MNX1-AS1 expression (HR: 3.114, 95% CI: 2.035–4.123, p = 0.001) were prognostic factors for OS in GC patients. Furthermore, the multivariate analysis (Table 2) confirmed that high MNX1-AS1 expression (HR: 2.375, 95% CI: 1.839–3.236; p = 0.004) is a poor prognostic factor in GC patients independent of TNM stage (HR: 2.041, 95% CI: 1.762–3.359; p = 0.022) and lymph node metastasis (HR: 2.784, 95% CI: 1.945–3.596; p = 0.032).
TABLE 2
Univariate and multivariate Cox regression analysis of factors influencing overall survival in gastric cancer patients
MNX1-AS1 knockdown inhibited proliferation, migration and invasion of GC cells
Since MNX1-AS1 expression was higher in GC vs. normal tissues, we speculated that MNX1-AS1 might have an oncogenic role in GC. To validate our hypothesis, we assessed the expression of MNX1-AS1 in five human GC cell lines and the normal gastric cell line GES-1, using qRT-PCR. As shown in Figure 2A, MNX1-AS1 expression was higher in all five GC cell lines (MKN-45, SGC-7901, AGS, MGC-803, and BGC-823) compared to GES-1 cells (p < 0.01 or p < 0.001). Because MNX1-AS1 expression was relatively higher in AGS and MGC-803 compared to the other three GC cell lines, we selected AGS and MGC-803 cell lines for loss-of-function experiments. The qRT-PCR analysis confirmed that the transfection of AGS and MGC-803 cell lines with si-MNX1-AS1 knockdown MNX1-AS1 expression in these cells (Figure 2B, p < 0.001). The CCK-8 assay showed that MNX1-AS1 knockdown significantly impaired proliferation of AGS (Figure 2C, p < 0.001) and MGC-803 cells (Figure 2D, p < 0.001).
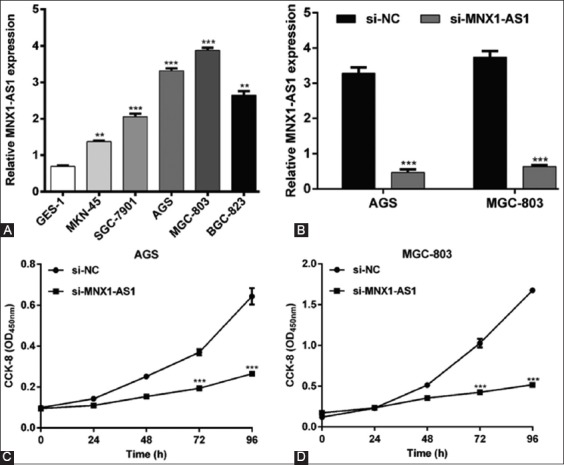
Effects of MNX1-AS1 knockdown on the proliferation of gastric cancer (GC) cells. A) Increased MNX1-AS1 expression in five GC cell lines (MKN-45, SGC-7901, AGS, MGC-803, and BGC-823) compared to the normal gastric epithelial cell line GES-1. **p < 0.01, ***p < 0.001 vs. GES-1. B) Expression of MNX1-AS1 was significantly downregulated after si-MNX1-AS1 transfection in AGS and MGC-803 cells. ***p < 0.001 vs. si-NC. Cell proliferation was measured by CCK-8 assay in C) AGS and D) MGC-803 cells transfected with si-MNX1-AS1 or si-NC. ***p < 0.001 vs. si-NC; Quantitative data are expressed as mean ± standard deviation (SD). MNX1-AS1: MNX1 antisense RNA 1; CCK-8: Cell Counting Kit-8; si-MNX1-AS1: Small interfering RNA (siRNA) targeting MNX1-AS1; si-NC: Non-targeting negative control siRNA.
In addition, we analyzed the effects of MNX1-AS1 knockdown on GC cell migration and invasion ability. The Transwell migration assay showed that the number of migrated AGS and MGC-803 cells was markedly decreased in si-MNX1-AS1 compared to si-NC group (Figure 3A, p < 0.001). Consistently, the Matrigel invasion assay demonstrated that MNX1-AS1 knockdown inhibited the invasion ability of AGS and MGC-803 cells (Figure 3B, p < 0.01 and p < 0.001 compared to si-NC, respectively). These results together indicated that MNX1-AS1 plays a positive role in GC cell proliferation and metastasis.
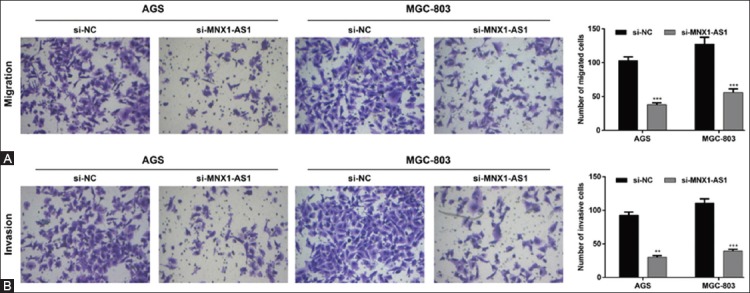
Effects of MNX1-AS1 knockdown on the migration and invasion of gastric cancer (GC) cells. AGS and MGC-803 cells were transfected with si-MNX1-AS1 or si-NC and analyzed by Transwell migration and Matrigel invasion assays. A) Representative images of Transwell migration assay are shown in the left panel and quantification of the number of migrated cells is presented in the right panel. The number of migrated AGS and MGC-803 cells was markedly decreased in si-MNX1-AS1 compared to si-NC group (p < 0.001). B) Representative images of Matrigel invasion assay are shown in the left panel and quantification of the number of invasive cells is presented in the right panel. MNX1-AS1 knockdown inhibited the invasion ability of AGS and MGC-803 cells (p < 0.01 and p < 0.001, respectively). Quantitative data are expressed as mean ± standard deviation (SD). **p < 0.01, ***p < 0.001 vs. si-NC. MNX1-AS1: MNX1 antisense RNA 1; si-MNX1-AS1: Small interfering RNA (siRNA) targeting MNX1-AS1; si-NC: Non-targeting negative control siRNA.
Mechanism of MNX1-AS1 in GC cell proliferation and migration
To further elucidate the molecular mechanisms underlying MNX1-AS1 control of GC cell proliferation and migration, we assessed the effects of MNX1-AS1 knockdown on the expression of related proteins. The Western blot analysis showed that MNX1-AS1 knockdown decreased the expression of proteins involved in cell proliferation (PCNA), EMT (N-cadherin and vimentin) and metastasis (MMP-9), and upregulated E-cadherin expression in both AGS (Figure 4A) and MGC-803 (Figure 4B) cell lines, compared to the corresponding si-NC controls. These results indicated that MNX1-AS1 regulates proliferation, migration and invasion of GC cells at least partially through PCNA and the EMT pathway.
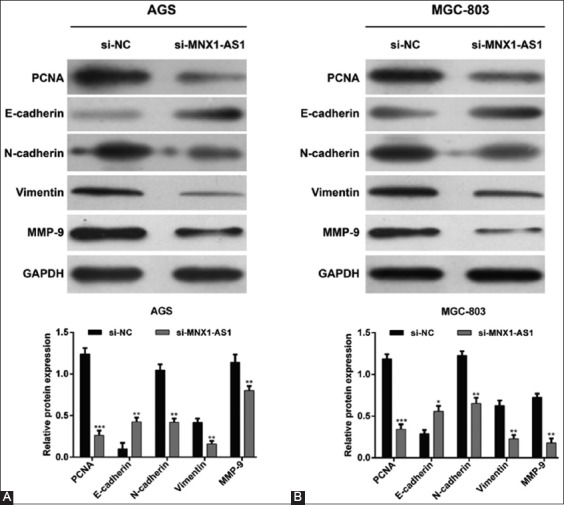
Mechanism of MNX1-AS1 in proliferation and migration of gastric cancer (GC) cells. AGS and MGC-803 cells were transfected with si-MNX1-AS1 or si-NC. Western blot was performed to analyze the expression of PCNA, E-cadherin, N-cadherin, vimentin and MMP-9 in A) AGS and B) MGC-803 cells. GAPDH was used as the internal control. MNX1-AS1 knockdown decreased the expression of proteins involved in cell proliferation (PCNA), EMT (N-cadherin and vimentin), and metastasis (MMP-9), and upregulated E-cadherin expression in both AGS and MGC-803 cells. Quantitative data are expressed as mean ± standard deviation (SD). *p < 0.05, **p < 0.01, ***p < 0.001 vs. si-NC. MNX1-AS1: MNX1 antisense RNA 1; si-MNX1-AS1: Small interfering RNA (siRNA) targeting MNX1-AS1; si-NC: Non-targeting negative control siRNA; PCNA: Proliferating cell nuclear antigen; MMP-9: Matrix metallopeptidase 9; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase; EMT: Epithelial to mesenchymal transition.
DISCUSSION
LncRNAs are implicated in different pathophysiological processes and are considered to be promising diagnostic/prognostic markers and therapeutic targets. In recent years, considerable effort has been made to identify mechanisms of lncRNAs in cancer initiation and progression [19]. Previous studies suggested that MNX1-AS1 lncRNA has an oncogenic role in ovarian cancer [17] and glioblastoma [18], however, the biological function and clinicopathological significance of MNX1-AS1 in GC have not been systematically investigated so far. In this study, we analyzed the expression of MNX1-AS1 in GC and adjacent non-tumor tissues in relation to clinicopathological features and 5-year OS of GC patients. Furthermore, we conducted loss-of-function experiments to investigate the function and mechanism of MNX1-AS1 in GC. We showed that MNX1-AS1 expression was higher in GC compared to adjacent normal tissues. High MNX1-AS1 expression in GC patients was significantly associated with tumor size, TNM stage and lymph node metastasis and, according to multivariate analysis, it was an independent predictor of survival in GC. The in vitro analysis showed that MNX1-AS1 expression was higher in GC cell lines MKN-45, SGC-7901, AGS, MGC-803, and BGC-823 compared to normal gastric cell line GES-1. The knockdown of MNX1-AS1 expression in AGS and MGC-803 cell lines significantly inhibited cell proliferation, migration and invasion, indicating that MNX1-AS1 has an oncogenic function in GC.
Carcinogenesis is a complex and multistage process characterized by uncontrolled cell proliferation [20]. Changes in the expression of proteins involved in DNA replication, DNA repair and cell cycle progression, such as PCNA, are associated with tumor growth and progression [21]. As a non-histone nuclear protein, PCNA (36 kDa) is synthesized before the S-phase of the cell cycle and functions as a cofactor of DNA polymerase δ and ε during DNA replication [22]. Expression of PCNA and two other proliferative markers, Ki-67 and minichromosome maintenance complex component 2 (MCM-2), in pT3, G2 CRC is associated with development of lymph node metastasis [23]. Conversely, reduced expression of PCNA and Ki-67 is at least partially related to decreased proliferation of CRC cells [24]. In lung adenocarcinoma, miR-363-3p impairs cell proliferation by targeting PCNA [21]. A clinical study showed higher survival rates in patients with anorectal malignant melanoma who have lower PCNA scores compared to those with higher PCNA scores [25]. The association between high PCNA expression and poor survival was also observed in GC [26], oral squamous cell carcinomas [27], and cervical cancer [28] among others. In our study, PCNA expression in MNX1-AS1-silenced AGS and MGC-803 GC cells was decreased compared to si-NC control cells, suggesting that the reduction in PCNA expression contributed to the decreased proliferation of GC cells. We hypothesize that the positive regulation of PCNA by MNX1-AS1 is one of the mechanisms underlying the significant association of high MNX1-AS1 expression with tumor size, TNM stage, lymph node metastasis, and poor survival in GC patients.
The EMT, a biological process in which epithelial cells transform into mesenchymal cells, is considered to be a major mechanism of carcinogenesis, leading to increased cell migration, acquisition of invasiveness and metastasis [29,30]. The EMT markers E-cadherin, N-cadherin, and vimentin were found to be prognostic in GC [31-33]. Transcriptional downregulation of the cell adhesion protein E-cadherin leads to the loss of cell-cell contact thus enabling cancer cells to acquire migratory and invasive abilities [34]. On the other hand, N-cadherin, a calcium-dependent cell adhesion molecule that mediates epithelial-mesenchymal cell interactions, and the intermediate filament protein vimentin are upregulated during the EMT of cancer cells [35]. Furthermore, to escape the primary tumor and migrate to new locations cancer cells utilize proteolytic enzymes such as MMPs to degrade the extracellular matrix (ECM) [36]. In the present study, si-MNX1-AS1-transfected GC cells exhibited increased E-cadherin, and decreased N-cadherin, vimentin and MMP-9 protein expression compared to si-NC-transfected cells. These results indicate that the knockdown of MNX1-AS1 inhibited the EMT and degradation of the ECM in GC cells and therefore suppressed their migration and invasion. This may be another mechanism explaining the observed association between high MNX1-AS1 expression and clinicopathological characteristics and prognosis of GC patients.
The limitations of our study are as follows: 1) the function of MNX1-AS1 was not investigated in in vivo animal models; 2) the activity of MMP-9 in GC cells should be further confirmed using Gelatin zymography; 3) a larger number of GC tissue samples should be analyzed to confirm the expression of PCNA, E-cadherin, N-cadherin, vimentin and MMP-9 in relation to MNX1-AS1.
CONCLUSION
In summary, we showed that high MNX-AS1 expression is associated with tumor size, TNM stage, and lymph node metastasis in GC. It appears that MNX1-AS1 is implicated in GC cell proliferation, invasion and migration through the regulation of PCNA, EMT-related markers and MMP-9. Characterization of MNX1-AS1 function in GC should enable a better understanding of its role in human cancer.
REFERENCES
Articles from Bosnian Journal of Basic Medical Sciences are provided here courtesy of Association of Basic Medical Sciences of Federation of Bosnia and Herzegovina
Full text links
Read article at publisher's site: https://doi.org/10.17305/bjbms.2019.3713
Read article for free, from open access legal sources, via Unpaywall:
http://www.bjbms.org/ojs/index.php/bjbms/article/download/3713/1208
Citations & impact
Impact metrics
Citations of article over time
Article citations
Pan-Cancer Analysis Identifies MNX1 and Associated Antisense Transcripts as Biomarkers for Cancer.
Cells, 11(22):3577, 11 Nov 2022
Cited by: 0 articles | PMID: 36429006 | PMCID: PMC9688723
lncRNA MNX1‑AS1 promotes prostate cancer progression through regulating miR‑2113/MDM2 axis.
Mol Med Rep, 26(1):231, 26 May 2022
Cited by: 6 articles | PMID: 35616155 | PMCID: PMC9178709
Constructing a prognostic immune-related lncRNA model for colon cancer.
Medicine (Baltimore), 101(38):e30447, 01 Sep 2022
Cited by: 3 articles | PMID: 36197160 | PMCID: PMC9509170
LncRNA MNX1-AS1 contributes to lung adenocarcinoma progression by targeting the miR-34a/SIRT1 axis.
Am J Transl Res, 14(7):4977-4989, 15 Jul 2022
Cited by: 1 article | PMID: 35958481 | PMCID: PMC9360842
Clinical significance of long noncoding RNA MNX1-AS1 in human cancers: a meta-analysis of cohort studies and bioinformatics analysis based on TCGA datasets.
Bioengineered, 12(1):875-885, 01 Dec 2021
Cited by: 4 articles | PMID: 33685348 | PMCID: PMC8291812
Review Free full text in Europe PMC
Go to all (16) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
TEAD4 modulated LncRNA MNX1-AS1 contributes to gastric cancer progression partly through suppressing BTG2 and activating BCL2.
Mol Cancer, 19(1):6, 10 Jan 2020
Cited by: 70 articles | PMID: 31924214 | PMCID: PMC6953272
Knockdown of TPT1-AS1 inhibits cell proliferation, cell cycle G1/S transition, and epithelial-mesenchymal transition in gastric cancer.
Bosn J Basic Med Sci, 21(1):39-46, 01 Feb 2021
Cited by: 10 articles | PMID: 32156253 | PMCID: PMC7861632
Long noncoding RNA MNX1-AS1 overexpression promotes the invasion and metastasis of gastric cancer through repressing CDKN1A.
Eur Rev Med Pharmacol Sci, 23(11):4756-4762, 01 Jun 2019
Cited by: 13 articles | PMID: 31210302
LncRNA MNX1-AS1: A novel oncogenic propellant in cancers.
Biomed Pharmacother, 149:112801, 12 Mar 2022
Cited by: 5 articles | PMID: 35290890
Review




