Abstract
Aims
Obinutuzumab (G) is a humanized type II, Fc-glycoengineered anti-CD20 monoclonal antibody used in various indications, including patients with previously untreated front-line follicular lymphoma. We investigated sources of variability in G exposure and association of progression-free survival (PFS) with average concentration over induction (CmeanIND ) in front-line follicular lymphoma patients treated with G plus chemotherapy (bendamustine, CHOP, or CVP) in the GALLIUM trial.Methods
Individual exposures (CmeanIND ) were obtained from a previously established population pharmacokinetic model updated with GALLIUM data. Multivariate Cox proportional hazard models and univariate Kaplan-Meier plots investigated relationships of PFS with exposure and other potential prognostic factors.Results
Overall, G exposure was lower in high body-weight patients and in males, and slightly lower in patients with high baseline tumour burden. Analysis of clinical outcomes showed that variability in G exposure did not impact PFS in G-bendamustine-treated patients; PFS was inferior in males and patients with FCGR2a/2b T232 T low-affinity receptor variant, and superior in patients with FCGR2a/2b I232T variant. In G-CHOP/CVP arms, PFS improved with increasing CmeanIND (hazard ratio = 1.74 and 0.394 at 5th and 95th percentile compared to median CmeanIND ) and was inferior in patients with high baseline tumour size and B symptoms.Conclusions
It remains unclear whether for G-CHOP/CVP patients lower G exposure is a consequence of adverse disease biology and/or resistance to chemotherapy backbone (higher clearance in nonresponder patients, as demonstrated for rituximab) rather than being the cause of poorer clinical outcome. A study with >1 dose level of G could help resolve this uncertainty.Free full text

Role of obinutuzumab exposure on clinical outcome of follicular lymphoma treated with first‐line immunochemotherapy
Associated Data
Abstract
Aims
Obinutuzumab (G) is a humanized type II, Fc‐glycoengineered anti‐CD20 monoclonal antibody used in various indications, including patients with previously untreated front‐line follicular lymphoma. We investigated sources of variability in G exposure and association of progression‐free survival (PFS) with average concentration over induction (CmeanIND) in front‐line follicular lymphoma patients treated with G plus chemotherapy (bendamustine, CHOP, or CVP) in the GALLIUM trial.
Methods
Individual exposures (CmeanIND) were obtained from a previously established population pharmacokinetic model updated with GALLIUM data. Multivariate Cox proportional hazard models and univariate Kaplan–Meier plots investigated relationships of PFS with exposure and other potential prognostic factors.
Results
Overall, G exposure was lower in high body‐weight patients and in males, and slightly lower in patients with high baseline tumour burden. Analysis of clinical outcomes showed that variability in G exposure did not impact PFS in G‐bendamustine‐treated patients; PFS was inferior in males and patients with FCGR2a/2b T232 T low‐affinity receptor variant, and superior in patients with FCGR2a/2b I232T variant. In G‐CHOP/CVP arms, PFS improved with increasing CmeanIND (hazard ratio = 1.74 and 0.394 at 5th and 95th percentile compared to median CmeanIND) and was inferior in patients with high baseline tumour size and B symptoms.
Conclusions
It remains unclear whether for G‐CHOP/CVP patients lower G exposure is a consequence of adverse disease biology and/or resistance to chemotherapy backbone (higher clearance in nonresponder patients, as demonstrated for rituximab) rather than being the cause of poorer clinical outcome. A study with >1 dose level of G could help resolve this uncertainty.
1. INTRODUCTION
Targeting the CD20 antigen with monoclonal antibodies (mAbs) leads to depletion of B‐cells in blood, bone marrow and other tissues. This therapeutic strategy has greatly improved the outcome of patients with B‐cell non‐Hodgkin lymphomas (NHL) and chronic lymphocytic leukaemias (CLL) and is now considered standard of care for these diseases.1, 2, 3 Rituximab, the first anti‐CD20 mAb, is a chimeric, unmodified Type I anti‐CD20 mAb of the IgG1 subclass.4 New insights into the biology of CD20 and technical advances in antibody engineering led to the development of obinutuzumab (G, GA101, Gazyva or Gazyvaro; Hoffmann‐La Roche), a humanized Type II anti‐CD20 mAb glycoengineered to increase its affinity to Fc receptors on effector cells. Obinutuzumab was developed to have more potent direct cell killing activity and enhanced ability to activate effector cells and demonstrated its superiority over equal dosed rituximab in xenograft tumour models.5 Based on three pivotal trials (CLL‐11, GADOLIN, GALLIUM) obinutuzumab was approved for the front‐line (1 L) treatment of patients with CLL, relapsed/refractory (R/R) and follicular lymphoma (FL).6, 7, 8
While the approved rituximab dose is based on patient body surface area, trialists chose a fixed dose for obinutuzumab based on the insights that anti‐CD20 mAbs have wide therapeutic windows and that fixed dosing may provide more convenience for physicians.9, 10 The recommended dose and schedule of obinutuzumab was based on safety, efficacy, and pharmacokinetic (PK) data from two Phase 1b/2 studies10 and confirmed in subsequent Phase 3 trials in CLL and NHL.
Obinutuzumab displays target‐mediated drug disposition (TMDD), i.e. the CD20 target antigen mediates the elimination of the drug. Total obinutuzumab clearance is therefore the sum of two pathways: a time‐independent linear (through nonspecific endocytosis via Fcγ receptors [FCGR]) and a time‐dependent (through the target) pathway.11, 12, 13 Early in treatment, the time‐dependent clearance pathway makes a greater contribution to total clearance due to high levels of CD20+ cells (tumour burden), and diminishes thereafter as the CD20+ tumour volume reduces with repeated dosing. In contrast, the time‐independent linear clearance of obinutuzumab remains constant throughout treatment. PK (i.e. mAb blood concentration) is thought to reflect CD20 occupancy and the dose and schedule of obinutuzumab was designed to ensure full target saturation throughout the entire dosing period.10 Obinutuzumab exposure is mainly influenced by body weight, sex and tumour burden,14 i.e. a male patient with high body weight and large tumour burden will have lower exposure than a female patient with low body weight and small tumour burden. However, if the chosen fixed dose is sufficient to ensure target saturation and hence ensure optimized efficacy in all patient subsets, such differences in exposure should not influence outcome. In patients where the disease progresses after obinutuzumab treatment, insufficient exposure to the drug may be considered as a possible contributor to the suboptimal response; however, there are also intrinsic disease and host factors that may affect patient outcome.
The GALLIUM study (NCT01332968) showed that obinutuzumab‐based immunochemotherapy followed by obinutuzumab maintenance significantly improved progression‐free survival (PFS) compared to rituximab‐based immunochemotherapy followed by rituximab maintenance.8
In this analysis, we studied the sources of variability in outcome amongst 1 L FL obinutuzumab‐treated patients in the GALLIUM trial with particular emphasis on whether differences in obinutuzumab exposure affected outcome.
2. METHODS
2.1. Study design
The GALLIUM trial is described in detail elsewhere.8 In summary, patients with previously untreated FL were randomized 1:1 to receive intravenous infusions of obinutuzumab (1000 mg per day on Days 1, 8 and 15 of Cycle 1 and on Day 1 of subsequent cycles) or rituximab (375 mg/m2 body surface area on Day 1 of each cycle) for 6 × 28‐day or 8 × 21‐day cycles dependent on the selected chemotherapy regimen. The chemotherapy regimens were site‐specific and included cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP; 6 × 21‐day cycles plus 2 with antibody alone), cyclophosphamide, vincristine and prednisone (CVP; 8 × 21‐day cycles), or bendamustine (6 × 28‐day cycles), at standard doses for each regimen. Patients who attained a partial or complete response at the end of this induction phase then received maintenance therapy every 2 months for up to 2 years with the same antibody and dose they received during induction. Patients who had stable disease at the end of induction were followed on the same schedule but received no maintenance therapy (Figure 1). The primary endpoint was investigator‐assessed PFS in patients with FL. Characterization of PK in 1 L FL patients treated with obinutuzumab was an exploratory objective.
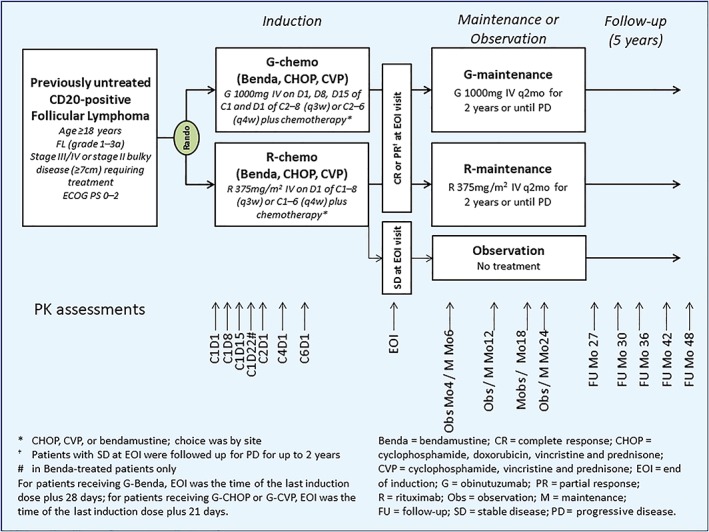
Overview of study design. Samples for assessment of peak concentrations were drawn 0–30 minutes after the end of infusion and trough samples were drawn 0–4 hours before the start of the next infusion. Pharmacokinetic samples were collected from the arm opposite to that receiving the infusion. In patients with indwelling catheters, a pharmacokinetic sample was drawn from the catheter after ample flushing
2.2. PK analysis
PK samples for peak and trough (Ctrough) obinutuzumab concentrations were collected as specified in Figure 1. Serum obinutuzumab concentrations were analysed using a validated sandwich enzyme‐linked immunosorbent assay with a lower limit of quantitation of 4.05 ng/mL14 and were included in a population PK (popPK) analysis using software NONMEM, Version 7.3.0 (ICON Development Solutions15). The popPK model previously developed for obinutuzumab in CLL and NHL,14 was updated and extended, incorporating data from the GALLIUM trial.
Minimum concentration during a dosing interval (Ctrough) reflects the lowest target saturation within this dosing interval. It should be sufficiently high to achieve desired pharmacodynamic effect (maximal B‐cell depletion) and clinical outcomes. The area under the curve over the dosing interval (AUCτ) provides important information regarding overall exposure. Notably, a high correlation exists between Ctrough and AUCτ. As patients treated with obinutuzumab‐CHOP (G‐CHOP) or obinutuzumab‐CVP (G‐CVP), and obinutuzumab‐bendamustine (G‐Benda) were not receiving the same cumulative dose or frequency of obinutuzumab, the average concentration over the induction period (CmeanIND i.e. ratio of cumulative AUC over the induction period to its duration) was derived from the PK model for each patient and was used as a metric of obinutuzumab exposure. In addition to CmeanIND values, categories of CmeanIND (low, medium, and high tertiles) were used as measures of exposure.
In addition, the influence on obinutuzumab exposure (CmeanIND) of several disease‐related covariates (e.g. bone marrow involvement, serum level of β2‐microglobulin, number of malignant cells [i.e. quantification of minimal residual disease {MRD} at baseline]) that were not tested in the popPK model was investigated univariately on GALLIUM data.
2.3. Tumour burden assessment
Tumour burden, defined as the sum of product diameter of target lesions (SPD; as linear and log), was assessed by computed tomography (CT).
MRD was assessed by real‐time, quantitative allele‐specific oligonucleotide polymerase chain reaction (PCR), at baseline, mid‐induction, end of induction, and 6‐monthly intervals to 24 months post end of induction/discontinuation.16
2.4. Outcome assessment
PFS was defined as the time from randomization to the first occurrence of disease progression or relapse as assessed by the investigator according to revised response criteria for malignant lymphoma17 without positron emission tomography (PET) and with PET18 (at baseline and at end of induction and where PET was available) or death from any cause, as described previously.8 Response was assessed according to revised response criteria for malignant lymphoma with and without PET (at baseline, mid‐induction and at end of induction and where PET was available). Response was assessed at mid‐induction, end of induction, then assessed every 2 months for 2 years (maintenance phase), and then every 3–6 months, with CT performed every 6–12 months until progression or withdrawal from the study. PFS was defined as the time from randomization to the first occurrence of disease progression or relapse or death from any cause, as described previously.
2.5. Genotyping
Genotyping of FCGR2A H131R, FCGR3A F158 V and FCGR2B I232T single nucleotide polymorphisms (SNPs) was performed in triplicate on genomic DNA samples alongside sequence‐confirmed positive (Coriell Cell Repository, Camden, NJ, USA) and non‐template controls as previously reported.19 FCGR2A rs1801274 and FCGR3A rs396991 SNPs were genotyping using the commercially available TaqMan assays (Life Technologies, Paisley, UK), C_9077561_20 and C_25815666_10, respectively, according to the manufacturer's instructions. FCGR2B rs1050501 was genotyped using custom‐designed primers and TaqMan probes. Each genotyping assay was prepared using the CAS‐1200 PCR setup robot (Corbett Life Science, Qiagen) and amplification and allelic discrimination was performed using a Corbett Rotor‐Gene 6000 (Corbett Life Science) and Rotor‐Gene Q series software 2.0.2 (Build 4), respectively. Genotyping results were confirmed by direct sequencing of PCR products. In cases of discrepancy between sequencing and TaqMan genotype data for FCGR2B, no valid result was recorded.
2.6. Exposure–response analysis
Relationships between exposure, patient characteristics, or disease specific covariates and PFS were first explored graphically using Kaplan–Meier plots. To consider potential confounding factors, an exposure‐response analysis of PFS was then performed in FL patients who received more than half of the planned induction doses of obinutuzumab (i.e. ≥5 CHOP/CVP, ≥4 bendamustine; n = 401) using semiparametric Cox proportional hazards (CPH) models. The relationships between obinutuzumab exposure with PFS was first characterized using the base CPH model. The hazard function in the CPH model is expressed as:
where λ0(t) is the baseline hazard function, Xi is a vector of predictor variables, and β is a parameter vector estimated by maximum partial‐likelihood. Function coxph of the survival package of R language (https://www.r‐project.org/) was used for model fitting.
The base model characterizes the marginal effect of exposure (i.e. average concentration over induction treatment period) on PFS without consideration of covariates. A univariate screening of covariates using the Bayesian information criterion was then used to identify covariates to incorporate in the full covariate model. Covariate effects were specified as proportional on the hazard, as specified in Equation 1. Performance of the exposure–PFS model was evaluated using diagnostic plots and visual predictive check simulations.
As patients receiving G‐CHOP and G‐CVP received the same obinutuzumab dosing regimen (every 3 weeks), they were grouped in the analysis to be of comparable sample size to G‐Benda patients (treated every 4 weeks). A multivariate semi‐parametric CPH model investigated the effects of the following factors: exposure (CmeanIND), age, sex, body mass index, time from diagnosis, chemotherapy regimen and baseline disease characteristics, which included tumour burden, follicular lymphoma international prognostic index score, Eastern Cooperative Oncology Group performance status, bone marrow involvement, presence of bulky disease (defined as ≥7 cm diameter on CT imaging), polymorphism of FCGR 2b/2c/3a, Ann Arbor stage, B symptoms (all symptoms together or separately as fever, night sweats, and weight loss), peripheral blood leucocytes (as cell count and log of cell count), lymphocytes (as cell count and log of cell count), B cell counts (as cell count and log of cell count), serum albumin, lactate dehydrogenase (LDH), β2‐microglobulin, and number of malignant cells at baseline (i.e. MRD at baseline). In this analysis, CmeanIND that accounted for the actual dosing history (including dosing delays and modifications) over the entire induction period was preferred over Ctrough at a specific time point that would mainly account for the latest dose before Ctrough. Additionally, as frequency of obinutuzumab dosing was different for patients receiving different backbone chemotherapies (every three or four weeks), Ctrough values would have been confounded with backbone chemotherapy. This average concentration is similar to using cumulative AUC over induction period, but it better accounted for differences in the duration of induction between patients, and has been previously used for obinutuzumab.14
2.7. Nomenclature of targets and ligands
Key targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY20 and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16.21
3. RESULTS
3.1. PK analysis
The GALLIUM data (n = 408) were added to the original popPK model and the model was updated. The updated model was consistent with the previously developed model in patients with CLL, FL, other indolent NHL (iNHL) subtypes, diffuse large B‐cell lymphoma (DLBCL) and mantle cell lymphoma,14 and confirmed the influence of previously identified covariates i.e. body weight, sex, tumour size (SPD of target lesions; Table 1, Figure 2), serum albumin at baseline, age, disease types and concomitant chemotherapies on obinutuzumab exposure. Final PK parameter estimates and covariate effects are reported in Supporting Information Tables S1 and S2, respectively.
Table 1
Obinutuzumab pharmacokinetic parameters during induction treatment in follicular lymphoma patients (n = 401), grouped by chemotherapy backbone and body weight, sex or tumour burden
| Parameter median (range) | G‐Benda* | G‐CHOP/CVP* | ||
|---|---|---|---|---|
| By body weight | ||||
| ≤90 Kg | >90 Kg | ≤90 Kg | >90 Kg | |
| n = 169 | n = 43 | n = 159 | n = 30 | |
| AUCτ μg/mL day | 11 300 (4090–21 200) | 7430 (2010–22 300) | 11 600 (2520–26 100) | 7460 (4360–10 800) |
| CmeanIND μg/mL | 358 (95.7–650) | 253 (68–675) | 454 (88–878) | 292 (145–479) |
| By sex | ||||
|---|---|---|---|---|
|
Female n = 106 |
Male n = 106 |
Female n = 105 |
Male n = 84 | |
| AUCτ μg/mL day | 11 900 (5800–22 300) | 9060 (2010–18 600) | 13 000 (2520–26 100) | 9070 (4020–18 400) |
| CmeanIND μg/mL | 382 (174–675) | 290 (68–524) | 487 (88–878) | 358 (145–716) |
| By tumour burden at baseline (below/above median) | ||||
|---|---|---|---|---|
|
≤5109 mm2
n = 111 |
>5109 mm2
n = 101 |
≤5109 mm2
n = 90 |
>5109 mm2
n = 99 | |
| AUCτ μg/mL day | 10 800 (4840–22 300) | 10 100 (2010–21 200) | 10 800 (5370–21 100) | 10 800 (2520–26 100) |
| CmeanIND μg/mL | 339 (95.7–675) | 327 (68–650) | 423 (249–855) | 429 (88–878) |
AUCτ = area under the curve for one cycle duration (final cycle of induction). Tau = cycle durations, i.e Tau = 28 days for G‐Benda patients, on Cycle 6 (end of induction corresponds to Cycle 6 dose +28 days) and Tau = 21 days for G‐CHOP/CVP patients, on Cycle 8 (end of induction corresponds to Cycle 8 dose +21 days).
CmeanIND = cumulative AUC/induction period.
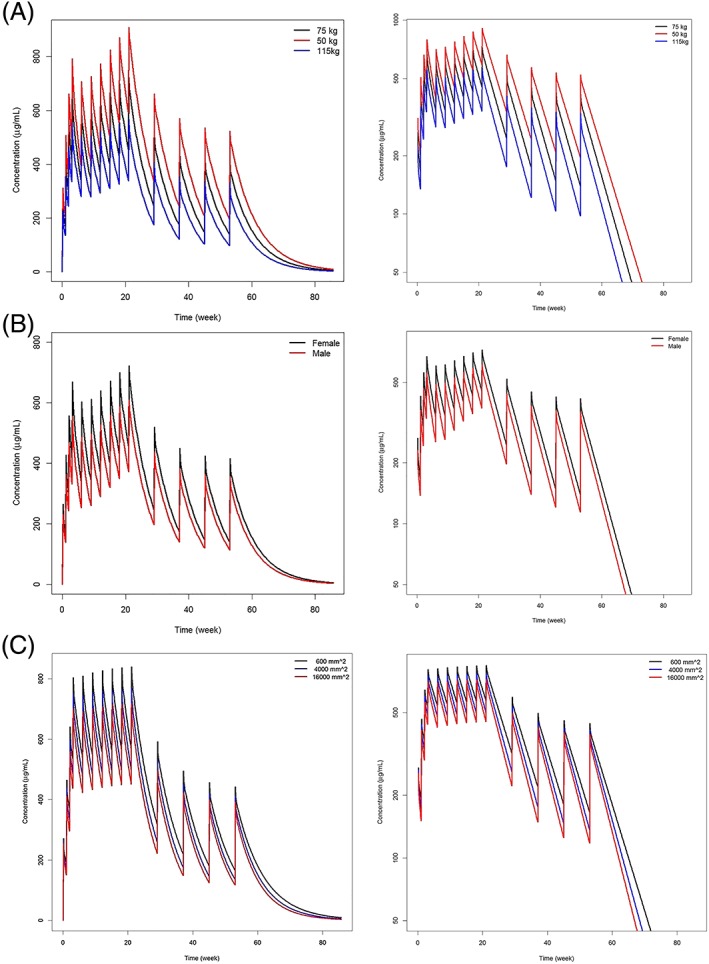
Influence of A, body weight, B, sex, and C, tumour burden on obinutuzumab concentrations in first‐line follicular lymphoma (FL) patients. A, Typical obinutuzumab pharmacokinetic (PK) profiles in FL patients weighing 50, 75 and 115 kg—linear scale (left)— semi‐log scale (right); B, typical obinutuzumab PK profiles in male and female FL patients—linear scale (left)— semi‐log scale (right); C, typical obinutuzumab PK profiles in FL patients with a tumour size at baseline of 600, 4000 and 16 000 mm2—linear scale (left)— semi‐log scale (right)
Nonspecific time‐independent elimination of obinutuzumab is influenced by body weight, sex, tumour size, serum albumin at baseline and age in FL patients. For example, based on model simulations, a patient with a high body weight (115 kg) will have a 36% higher time‐independent clearance, and a patient with low body weight (50 kg) a 25% lower one compared to a patient weighing 75 kg (Figure 2a). Males eliminate obinutuzumab 48% faster than females (Figure 2b). A patient with higher (27 500 mm2) or lower tumour burden (344 mm2) eliminates obinutuzumab 14% faster and 12% slower respectively compared to a patient with a tumour burden at baseline of 3000 mm2 (Figure 2c). Patients with high serum albumin (48.6 g/L) eliminate obinutuzumab 9% slower, and patients with low serum albumin (29.0 g/L) eliminate obinutuzumab 17% faster, than patients with serum albumin of 40 g/L (data not shown).
Tumour burden, sex and backbone chemotherapy influence the exposure to obinutuzumab during the first weeks of treatment by impacting its time‐dependent elimination. As an example, initial time‐dependent clearance of a patient with low (344 mm2) or high (27 500 mm2) tumour burden at baseline is 53% lower or 115% higher, respectively, than for a patient with a tumour burden of 3000 mm2. Initial time‐dependent clearance is 48% higher in males, which further decreases the exposure in males during the first few weeks of treatment.
The initial total clearance that largely represents elimination through the CD20‐target is 4.4‐fold higher than the nonspecific time‐independent clearance, which is similar to other IgG monoclonal antibodies.22
The saturable (time‐dependent) part of clearance decreased with time with a decay rate that is impacted by backbone chemotherapy. In patients treated with G‐Benda, the decay of the time dependent clearance occurred more rapidly with a half‐life of 13.2 days versus 21.6 days in patients treated with G‐CHOP or G‐CVP, suggesting a faster elimination of target CD20+ cells in G‐Benda‐treated patients.
Bone marrow involvement, serum level of β2‐microglobulin, number of malignant cells (MRD) at baseline had no impact on obinutuzumab exposure (CmeanIND).
3.2. Correlation between obinutuzumab exposure, outcome and chemotherapy backbone in GALLIUM
In total, 401 were included in the PFS analysis. This subpopulation was representative of the full intent‐to‐treat population with respect to baseline patient/disease characteristics despite a lower proportion of patients with follicular lymphoma international prognostic index‐1 high risk (Supporting Information Table S3).
Exploratory graphical Kaplan–Meier analyses of PFS with patients grouped by category of exposure (CmeanIND as low, intermediate and high) and chemotherapy (Figure 3a), show that exposure did not affect PFS in bendamustine‐treated patients (n = 212, Figure 3b), but suggested that exposure might influence outcome in G‐CHOP/CVP patients (n = 189, Figure 3c). The effect seen in G‐CHOP/CVP patients seems to be driven by patients with high tumour burden at baseline (>observed median; Figure 4). In those with low tumour burden at baseline (<observed median), obinutuzumab exposure seems not to impact PFS (Supporting Information Figure S1).
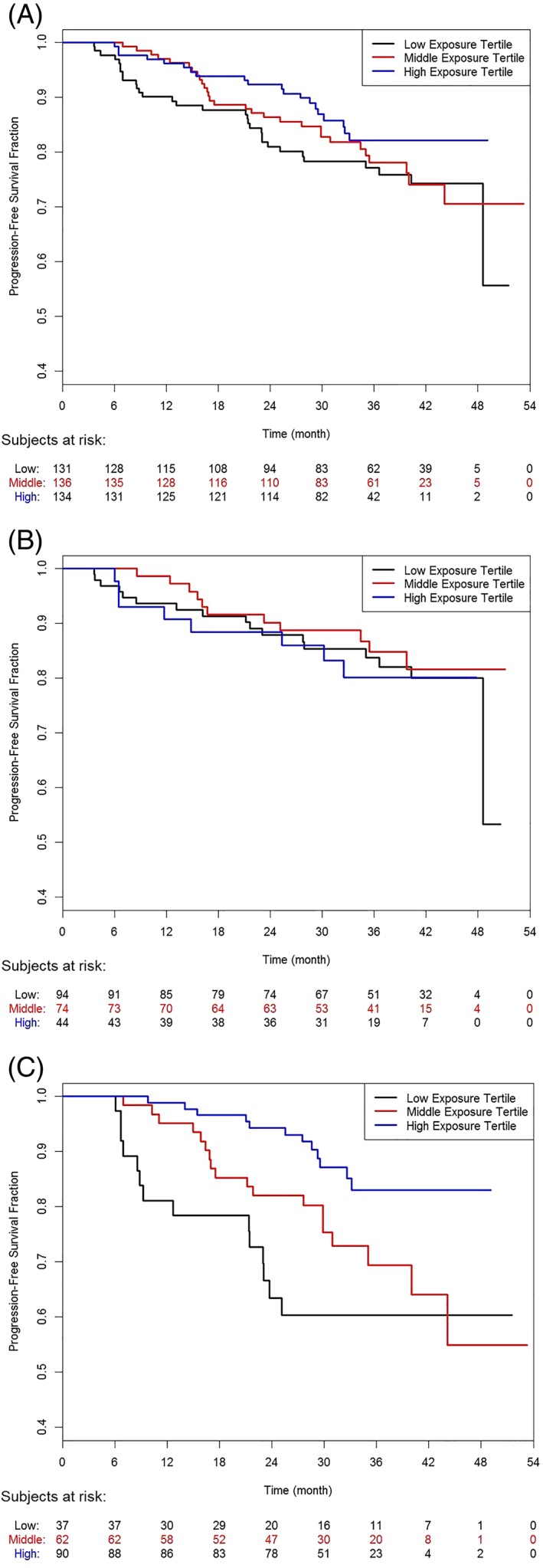
Kaplan–Meier plots of progression‐free survival by exposure category. Lower tertile: CmeanIND = 68–313 μg/mL; middle tertile: CmeanIND = 315–433 μg/mL; high tertile: CmeanIND = 433–878 μg/mL
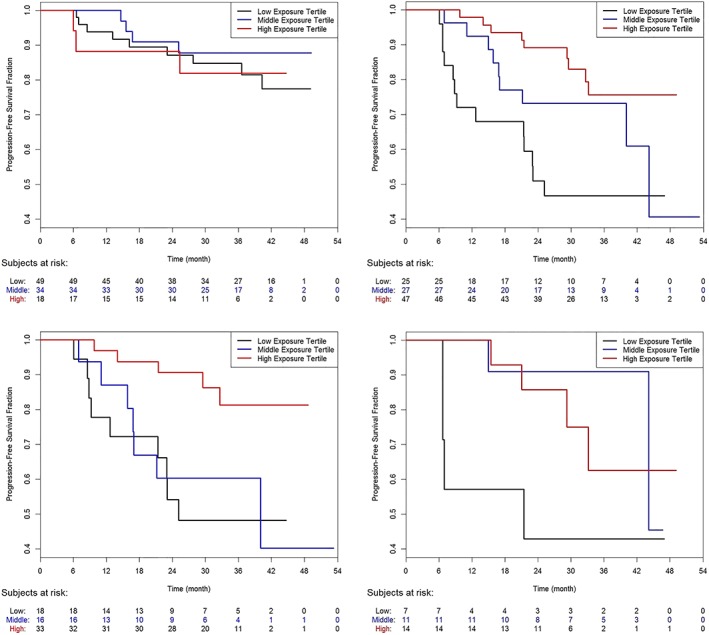
Kaplan–Meier plots of progression‐free survival in patients with high tumour burden (>5110 mm2) at baseline by exposure category and chemotherapy backbone. Obinutuzumab–bendamustine patients (top left), obinutuzumab–CHOP (bottom left), obinutuzumab–CHOP/CVP (top right) and obinutuzumab–CVP patients (bottom right). Lower tertile: CmeanIND = 68–313 μg/mL; middle tertile: CmeanIND = 315–433 μg/mL; high tertile: CmeanIND = 433–878 μg/mL
The multivariate exposure–response analysis of PFS using Cox proportional hazard model showed that G‐Benda treatment was associated with a more favourable outcome than G‐CHOP or G‐CVP (Figure 5). High tumour burden at baseline negatively influenced PFS while presence of the low/high affinity FCGR2b I232T genotype positively influenced PFS. Presence of other polymorphisms such as FCGR3a‐158F and FCGR2a‐H131R did not impact PFS. In patients treated with G‐Benda (n = 212), the PFS benefit was similar across all obinutuzumab exposure categories while it increased with increasing obinutuzumab exposure in G‐CHOP/CVP patients (Figure 5).
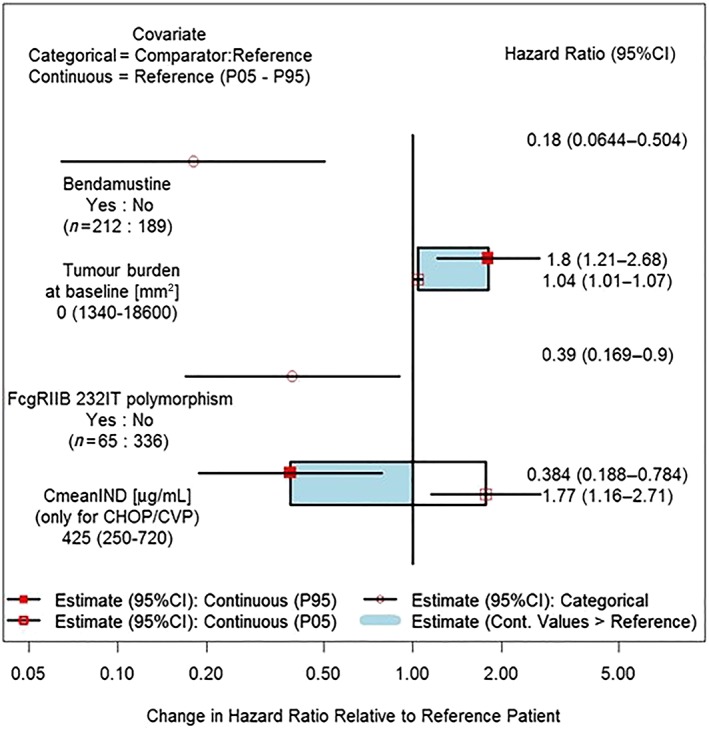
Forrest plot of multivariate analysis on progression‐free survival: Covariate effects on the progression‐free survival hazard ratio for final Cox proportional hazard model in patients with follicular lymphoma. CmeanIND: average concentration up to the last dose of induction period assuming actual dosing history in GALLIUM. Results for continuous variables are presented as box plots, and categorical variables are presented as ratios (95% confidence intervals, CI). Note: for FCGR2B polymorphism, 292 patients were FCGR2b I232I, 65 patients were FCGR2b I232T, 7 patients were FCGR2b T232T and 37 patients had missing data
Due to differences in response for different backbone chemotherapies, separate analyses for only G‐Benda or G‐CHOP/CVP patients were also performed. The results were consistent with the combined analysis regarding influence of exposure on PFS, and differed slightly between G‐Benda and G‐CHOP/CVP regarding other covariates. In G‐Benda‐treated patients, PFS was impaired in males and in patients with the FCGR2a/2b T232T receptor variant, and was improved in patients with the FCGR2a/2b I232T receptor variant (data not shown). In patients treated with G‐CHOP/CVP, low obinutuzumab exposure (5th percentile of CmeanIND) increased the risk of disease progression or death by 74% (HR = 1.74), while high exposure (95th percentile of CmeanIND) decreased the risk of disease progression or death by 61% (HR = 0.394) compared to patients with the median value of obinutuzumab CmeanIND (Figure 5). In those patients, PFS was also longer in patients with low baseline tumour size and absence of B symptoms (data not shown).
4. DISCUSSION
Strategies to determine the optimal dosing of therapeutic antibodies have been refined over the past decades. For obinutuzumab, a novel type‐II glycoengineered anti‐CD20 mAb, two Phase 1b/2 studies were conducted to determine the optimal dose and schedule that would rapidly saturate the target and maintain this saturation.10
PK characteristics have been thoroughly described for mAbs undergoing TMDD and are indicative of two clearance processes: (i) a concentration‐ and/or time‐varying clearance which is associated with TMDD and which is saturable as the target is either fully occupied by the mAb or eliminated through the mechanism of action of the mAb; and (ii) a nonsaturable clearance process common to IgG‐like mAbs where the mAb is cleared by nonspecific proteolytic action.11, 12, 13 For obinutuzumab, the time‐varying clearance has been described previously in iNHL and CLL patients.14 This initial obinutuzumab model was used to support the choice of an appropriate dosing regimen in CLL and iNHL patients. In order to achieve optimal clinical response, a high CD20 target saturation was desired throughout the dosing interval of obinutuzumab treatment.
In accordance with earlier results in patients with CLL or R/R FL, the exposure to obinutuzumab in 1 L treatment of patients with advanced FL in the GALLIUM trial apart from backbone chemotherapy was most strongly influenced by body weight, sex and tumour burden at baseline, but also serum albumin level and age. In prior studies, there was no evidence of any PK drug–drug interaction between obinutuzumab and backbone chemotherapies (Zelenetz et al., manuscript in preparation).23
The analysis of the relationships between clinical outcome and obinutuzumab exposure is complicated by the underlying relationships between exposure, tumour burden, CD20‐target expression and the biology of disease/resistance to treatment.
The GALLIUM trial showed that obinutuzumab‐based immunochemotherapy significantly improved PFS versus rituximab‐based immunochemotherapy.8 In this analysis, our objective was to explore among patients from the obinutuzumab–chemotherapy arms, if individual and disease factors contribute to clinical response; specifically, if lower obinutuzumab exposure affects clinical outcome. Despite a higher cumulative dose during induction (3‐weekly regimen for 6–8 cycles) and generally higher obinutuzumab exposure in G‐CHOP/CVP compared to G‐Benda patients (4‐weekly regimen for 6 cycles), we found that lower exposure was associated with shorter PFS in patients treated with G‐CHOP or G‐CVP but that PFS was similar across the range of obinutuzumab exposure in patients treated with G‐Benda.
The faster decrease of time‐dependent obinutuzumab clearance in G‐Benda compared to G‐CHOP/CVP patients (half‐life of decrease of 13.2 vs 21.6 days), elucidated by the PK analysis suggests a faster reduction of CD20+ target by G‐Benda treatment. While the relative potency of the chemotherapy backbone in FL is debated, our findings supported the fact that bendamustine is often considered to be more effective than CHOP24 and CHOP is considered to be more effective than CVP (without anthracycline).25, 26, 27 Although it might have been desirable to conduct the analysis of exposure and PFS outcome for each chemotherapy backbone, we needed to pool the CHOP and CVP cohorts in order not to have too disparate sample size in the analysis. Of note, chemotherapy backbones were not randomized, and their comparison may therefore be confounded by differences in cohorts (e.g. younger patients and more aggressive disease in CHOP [median tumour burden of 5466 mm2 compared to 5116 mm2 for G‐Benda‐treated patients]; older patients with comorbidities in G‐Benda‐treated patients).26
In cancer patients, exposure–response analyses often indicate poorer response in patients with lower exposure suggesting that increasing the dose (and theoretically concentration) may improve the outcome of such patients.28 Several studies with rituximab also indicated an association between lower exposure and inferior outcome.29, 30 Several other groups have described a correlation between low exposure to the therapeutic antibody and outcome parameters.31, 32, 33, 34, 35 Typically, these findings have been based on small sample size and not all parameters that influence outcome were included in the analyses. Nevertheless, the concept of increasing the dose of rituximab was tested in clinical trials, but the results were mixed. While smaller studies seemed to corroborate this concept,29, 36, 37 others, including a large phase 3 in 1 L DLBCL (HOVON 84) did not show any benefit of rituximab dose intensification.38, 39 These results are not entirely surprising since lower exposure to the therapeutic mAb may not only be due to a higher baseline tumour burden, but also related to innate or acquired mechanisms of target cell resistance. For example, it is theoretically possible that other biological features such as loss of CD20 antigen or high rate of CD20 antigen cellular internalization upon drug binding by the tumour cell, a high intrinsic resistance of the tumour cell to direct cell killing or a high rate of CD20 production by the tumour cells, may lead to a loss of efficacy of the mAb as well as to a lower exposure.40, 41 It is conceivable that other factors can also influence both exposure and outcome, without lower exposure being the cause of the poorer outcome.12, 42, 43 For example, Tout et al.44 found that lower rituximab exposure in DLBCL patients was a consequence of high tumour burden leading to poorer prognosis (low rate of complete molecular response, PFS and overall survival) rather than a cause of inferior response.44, 45 Likewise, exposure‐response analyses of trastuzumab treatment in patients with HER2+ cancers have suggested that patients with low drug exposures had shorter overall survival times; however, increasing the dose did not lead to an improvement in efficacy.28 Similar results have been found in CLL and DLBCL patients treated with dose dense treatment regimens of rituximab.46, 47, 48, 49 Recently, a Food and Drug Administration pharmacometric reviewer highlighted the complexity of such analyses: “When both the response and the drug target can affect the exposure, the causal relationship between the two becomes confounded. Assessments of exposure–response relationships can then be biased even if baseline confounding factors are technically correctly adjusted.”50
In clinical practice, the parameters that could influence outcome are not yet routinely measured making definitive conclusions difficult. Total tumour burden is usually not directly assessable and only a small fraction of lesions (the target lesions) are measured. We assess the tumour burden using SPD, presence/absence of bulky disease and/or bone marrow involvement. Rates of target internalization or target re‐production are not yet measurable in vivo. In the GALLIUM trial, novel parameters such as MRD in peripheral blood and bone marrow, biological factors such as numbers of natural killer (NK) cells at baseline,51 and the single nucleotide polymorphisms in FCG receptor genes (2a H131R, 2b/2c I232T [CD32] and 3 V158F [CD16]) were collected as exploratory parameters.
Defining exposure as the primary determinant of inferior clinical outcome should therefore be done with caution as other factors, such as intrinsic patient and disease characteristics could be relevant in PFS. In this analysis, we found that high tumour burden and presence of the FCG polymorphism 2b T232 T SNP increased the risk of disease progression or death. The FCGR2b 232 T‐allele has been linked to reduced ability of the bound‐mAb‐CD20 complex to move to lipid rafts.52 The FCGR2b acts as an inhibitory receptor for rituximab since its presence leads to internalization of the mAb/antigen complexes. This process compromises mAb efficacy as it both consumes the mAb and downregulates the target so that cells become invisible to effector mechanisms.41, 53, 54
In conclusion, exposure to obinutuzumab is influenced by several factors such as patient demographics, tumour burden and chemotherapy backbone (during the first few weeks of treatment) and is therefore variable among patients.
Nevertheless, this variability in exposure does not correlate with clinical outcome in patients treated with G‐Benda. In those patients, intrinsic patient characteristics (male sex and tumour burden) are relevant contributors to the differences in PFS.
In patients treated with G‐CHOP/CVP, the analysis suggests a correlation between lower exposure to obinutuzumab and poorer outcome. In those patients, higher obinutuzumab clearance (and therefore lower exposure) could be a consequence of poorer prognosis or resistance to chemotherapy backbone rather than the cause of poorer clinical outcome, as demonstrated for rituximab.55 To resolve this uncertainty a randomized study with >1 dose level of obinutuzumab would be needed.
COMPETING INTERESTS
C.J., V.B., D.S., G.M.L., N.F. and G.F.R. are employees of F. Hoffmann‐LaRoche, and may own stock. G.C. has received funding for consultancy from Roche, Celgene and honoraria from Roche, Celgene, Gilead, Sanofi and Janssen. J.C.S. has received research funding from Roche. J.F.S. and W.H. have received honoraria, consultancy and research funding from Roche, Gilead and Janssen. R.M. has received honoraria, lecture fees and travel support from Roche. J.F.S. has received honoraria from Roche. E.G. and L.G. have received funding for consultancy from Roche. C.E.H. has no conflicts of interest to declare.
CONTRIBUTORS
All authors reviewed and contributed to the manuscript and approved the final version. C.J. was involved in the study, analyses, interpretation of the results and co‐wrote the paper. E.G. and L.G. were involved in the PK and exposure‐response analyses. V.B. was involved in data preparation and study analysis. D.S. was involved in the design of the study, and analysis of the data. G.C., R.M., W.H., J.F.S. and J.C.S. were involved in the design and conduct of the study. J.C.S. and C.E.H. conducted the genotyping analysis. G.M.‐L., and N.F. were involved in the study, analyses, and interpretation of the results. G.F.‐R. was involved in the design of the study, analysis of the data and co‐wrote the paper.
Supporting information
Table S1
Parameters estimates for the final population pharmacokinetics model
Table S2 Covariates effects in the final population pharmacokinetics model
Table S3 Comparison of baseline patient demographics and disease characteristics between pharmacokinetics/progression‐free survival evaluable follicular lymphoma population and follicular lymphoma intent‐to‐treat population treated by obinutuzumab‐chemotherapy regimen.
Figure S1 Kaplan–Meier plots of progression‐free survival in patients with low tumour burden (≤5110 mm2) at baseline by exposure category and chemotherapy backbone. Obinutuzumab–bendamustine patients (top left), obinutuzumab–CHOP (bottom left), obinutuzumab–CHOP/CVP (top right) and obinutuzumab–CVP patients (bottom right). Lower tertile: CmeanIND = 68–313 μg/mL; middle tertile: CmeanIND = 315–433 μg/mL; high tertile: CmeanIND = 433–878 μg/mL.
ACKNOWLEDGEMENTS
We like to thank all patients/caregivers and all physicians who participated in the study and contributed to PK assessment, and Samantha Abel (Valley Writing Solutions Ltd) for medical writing assistance.
Notes
Jamois C, Gibiansky E, Gibiansky L, et al. Role of obinutuzumab exposure on clinical outcome of follicular lymphoma treated with first‐line immunochemotherapy. Br J Clin Pharmacol. 2019;85:1495–1506. 10.1111/bcp.13920 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
PI Statement: The authors confirm that R. Marcus and W. Hiddermann were joint PIs for the GALLIUM study and that they had direct clinical responsibility for patients
ClinicalTrials.gov number: NCT01332968
REFERENCES
Articles from British Journal of Clinical Pharmacology are provided here courtesy of British Pharmacological Society
Full text links
Read article at publisher's site: https://doi.org/10.1111/bcp.13920
Read article for free, from open access legal sources, via Unpaywall:
https://bpspubs.onlinelibrary.wiley.com/doi/pdfdirect/10.1111/bcp.13920
Citations & impact
Impact metrics
Article citations
Population pharmacokinetic and exposure-response analyses of intravenous and subcutaneous rituximab in patients with chronic lymphocytic leukemia.
CPT Pharmacometrics Syst Pharmacol, 10(8):914-927, 16 Jul 2021
Cited by: 6 articles | PMID: 34110098 | PMCID: PMC8376135
Quantitative Clinical Pharmacology Supports the Bridging From i.v. Dosing and Approval of s.c. Rituximab in B-Cell Hematological Malignancies.
Clin Pharmacol Ther, 110(5):1261-1272, 26 Jun 2021
Cited by: 0 articles | PMID: 34041738 | PMCID: PMC8597022
Sex as decisive variable in lymphoid neoplasms-an update.
ESMO Open, 6(1):100001, 31 Dec 2020
Cited by: 2 articles | PMID: 33399069 | PMCID: PMC7808098
Rituximab maintenance significantly reduces early follicular lymphoma progressions in patients treated with frontline R-CHOP.
EJHaem, 1(1):170-180, 31 Jul 2020
Cited by: 1 article | PMID: 35847728 | PMCID: PMC9175682
Role of obinutuzumab exposure on clinical outcome of follicular lymphoma treated with first-line immunochemotherapy.
Br J Clin Pharmacol, 85(7):1495-1506, 17 May 2019
Cited by: 5 articles | PMID: 30866056 | PMCID: PMC6595360
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT01332968
SNPs (3)
- (1 citation) dbSNP - rs1050501
- (1 citation) dbSNP - rs396991
- (1 citation) dbSNP - rs1801274
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Immunochemotherapy With Obinutuzumab or Rituximab for Previously Untreated Follicular Lymphoma in the GALLIUM Study: Influence of Chemotherapy on Efficacy and Safety.
J Clin Oncol, 36(23):2395-2404, 01 Jun 2018
Cited by: 83 articles | PMID: 29856692
Pharmacokinetics, exposure, efficacy and safety of obinutuzumab in rituximab-refractory follicular lymphoma patients in the GADOLIN phase III study.
Br J Clin Pharmacol, 85(9):1935-1945, 12 Jul 2019
Cited by: 5 articles | PMID: 31050355 | PMCID: PMC6710522
Prognostic Impact of Natural Killer Cell Count in Follicular Lymphoma and Diffuse Large B-cell Lymphoma Patients Treated with Immunochemotherapy.
Clin Cancer Res, 25(15):4634-4643, 03 May 2019
Cited by: 42 articles | PMID: 31053601
Rituximab for the first-line treatment of stage III-IV follicular lymphoma (review of Technology Appraisal No. 110): a systematic review and economic evaluation.
Health Technol Assess, 16(37):1-253, iii-iv, 01 Jan 2012
Cited by: 10 articles | PMID: 23021127
ReviewBooks & documents Free full text in Europe PMC
Funding
Funders who supported this work.

 1
1




