Abstract
Free full text

Helper T cell differentiation
Abstract
CD4+ T helper cells are key regulators of host health and disease. In the original model, specialized subsets of T helper cells are generated following activation through lineage-specifying cytokines and transcriptional programs, but recent studies have revealed increasing complexities for CD4+ T-cell differentiation. Here, we first discuss CD4+ T-cell differentiation from a historical perspective by highlighting the major studies that defined the distinct subsets of T helper cells. We next describe the mechanisms underlying CD4+ T-cell differentiation, including cytokine-induced signaling and transcriptional networks. We then review current and emerging topics of differentiation, including the plasticity and heterogeneity of T cells, the tissue-specific effects, and the influence of cellular metabolism on cell fate decisions. Importantly, recent advances in cutting-edge approaches, especially systems biology tools, have contributed to new concepts and mechanisms underlying T-cell differentiation and will likely continue to advance this important research area of adaptive immunity.
Introduction
CD4+ T helper cells are an essential and complex component of the immune system. Upon recognition of antigen-major histocompatibility complex molecules and proper costimulation, naive CD4+ T cells exit from quiescence to undergo clonal expansion. Cytokines within the immediate milieu program the differentiating cells into a specific effector cell type. The mechanisms that govern these processes have been actively investigated for over 30 years, yielding many groundbreaking discoveries that have laid the foundation for the therapeutic approaches for cancer, autoimmunity, and infectious disease.
Following the initial discovery of T helper 1 (TH1) and TH2 cells, the identification of additional functionally distinct T cells, mainly via traditional immunological approaches, has significantly expanded our view from the dichotomous existence of these cells. More recently, technological advances have uncovered complexities underlying the mechanisms for CD4+ T-cell effector programming. Indeed, the heterogeneity and plasticity of T helper cells are becoming increasingly appreciated as major players in the adaptive immune system as they adapt and react to different stimuli.
The goal of this review is to provide both a historical perspective and an updated view of T helper cell differentiation. First, we summarize the landmark discoveries in T helper cell subsets and how cytokines shape their programming. Next, we discuss the signaling and transcriptional events underlying T helper cell differentiation. We then describe current research areas, including the plasticity and heterogeneity of T cells in inflammatory states and within tissues and the roles of metabolic reprogramming in cell fate decisions. We also highlight emerging concepts as revealed, in part, by cutting-edge systems biology approaches.
A brief history of T helper cell subsets
In 1986, the groundbreaking study describing TH1 and TH2 cells, classified according to differential cytokine production and surface marker expression, was published1 (Fig. 1). TH1 cells produce the cytokines interferon-γ (IFN-γ), interleukin (IL)-2, and tumor necrosis factor-α and are important for antiviral and antibacterial immunities. TH2 cells are defined by the expression of their signature cytokines IL-4, IL-5, and IL-13 and are immunologically important against extracellular pathogens, such as worm infections. In 1995, nearly a decade after the first description of TH1 and TH2 cells, another landmark discovery identified an immunosuppressive T-cell type that constitutively expresses CD25, later termed regulatory T cells (Tregs).2 The dichotomous paradigm of proinflammatory T helper cells reigned for nearly two decades until a third subset, TH17 cells, were designated in 2005.3,4 The discovery of TH17 cells was based, in part, on earlier studies that clarified the roles of IL-12 and IL-23 that share the p40 subunit.5,6 TH17 cells are critical for anti-fungal responses and for host defense against bacterial infection (intracellular and extracellular)7; in addition, TH17 cells are abundant within the gut to help regulate the gut microbiota. The signature cytokines of TH17 cells include IL-17A, IL-17F, and IL-22. Another distinguished T helper subset of cells, T follicular helper (TFH) cells, promotes humoral immunity within germinal centers (GCs).8–10 These cells produce IL-21, which is critical for B-cell stimulation,11 and can be defined by CXCR5 (C-X-C chemokine receptor type 5) expression12,13 and coexpression with programmed cell death-1 (PD-1) and/or ICOS (Inducible T-cell COStimulator). TFH cells may also produce IL-4, which is important for immunoglobulin class switching in B cells.14
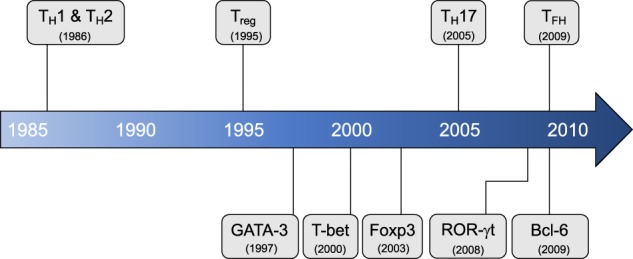
Timeline of major T helper cell discoveries. T helper populations (top) and major transcriptional regulators (bottom) listed in chronological order of the first published observation and/or the commonly agreed upon period establishing a separate lineage
Additional “unconventional” T helper subsets have also been defined but will not be the focus of this review. TH3 cells, first described in 1994, are an immunosuppressive subset marked by high expression of transforming growth factor-β (TGF-β).15 Discovered in 1997, another immunosuppressive subset named TR1 cells secrete IL-10 and are distinct from Tregs.16 TH9 cells were designated as IL-9 producers that are distinct from IL-9-producing TH2 cells in 2008.17,18 Finally, TH22 cells were described in 2009 as a subset with exclusive IL-22 expression, distinguishing them from IL-22-producing TH17 cells.19,20 Further information on these cells can be found in other reviews.21,22
Overall, the production of signature cytokines defines T helper cell subsets and functional capacities. Moreover, cytokine signals, typically produced by antigen presenting cells, orchestrate lineage decisions of activated CD4+ T cells. Differentiation of TH1 cells is promoted by the cytokine IL-12,23 while IL-4 drives TH2 cell differentiation.24 TFH cells are induced by IL-21 and IL-6.25 Unlike other effector CD4+ T-cell subsets, Tregs can be generated directly within the thymus (tTregs) during thymocyte development.2 Tregs may also be induced peripherally (pTregs) and in vitro by the cytokines TGF-β and IL-2.26 TH17 cell differentiation is promoted by IL-1β, IL-6, IL-21, IL-23, IL-1β, and TGF-β.27–34 Aberrant TH17 responses are strongly associated with autoimmune disorders.5 Interestingly, different TH17-promoting cytokines can induce unique types of TH17 cells as follows: TGF-β promotes an IL-10-producing and less pathogenic subtype, while IL-1β promotes a more proinflammatory subtype of TH17 cells.31,35,36 Thus, cytokines are intricately linked to CD4+ T-cell differentiation due to polarizing capability and being lineage-defining products of differentiated cells.
Transcriptional networks regulating T helper cell differentiation
Following cytokine stimulation, distinct signaling transducer and activator of transcription (STAT) family proteins are activated and drive T helper cell differentiation. STAT4 is activated downstream of IL-12 and is critical for TH1 cell differentiation,37,38 while STAT6 is induced downstream of IL-4 for TH2 cell differentiation.39–41 It was later shown that TH1 cell differentiation is also influenced by IFN-γ signaling through STAT1,42 thus revealing a possible autocrine regulation, similar to IL-4 in TH2 cells. In agreement with the elevated expression of CD25, strong IL-2-STAT5 signaling is important for Treg cell differentiation,43,44 though it is also important in TH2 cell differentiation.45 The differentiation of both TH17 and TFH cells is dependent on STAT3 signaling9,25,32 through IL-6/IL-23 and IL-21, respectively.
The ability of naive CD4+ T cells to undergo lineage polarization into distinct effector subsets is mediated by master transcription factors (Fig. 2). These multifunctional transcription factors are able to dictate cell fate by either (I) inducing the expression of lineage-specific genes and coactivators or (II) repressing the expression of genes that are associated with alternate lineages. These regulatory effects can be carried out by directly binding to the promoter and enhancer regions or indirectly by influencing epigenetic changes that are conducive to subset-specific gene programs. GATA-3 was the first master transcription factor identified to induce T helper cell differentiation, which promotes TH2 cell differentiation downstream of IL-4-STAT6.46,47 This finding was followed by the discovery of the master regulator of TH1 cell differentiation, T-bet.48 These master transcription factors play opposing roles in TH1/TH2 cell fate decisions; GATA-3 is induced during TH2 cell differentiation and strongly suppresses TH1-associated gene expression,49 and vice versa for T-bet with TH2-associated gene expression during TH1 cell differentiation.50 T-bet also directly binds to GATA-3 to mediate its suppression of TH2 programs.51 Furthermore, forced expression of GATA-3 in TH1 cells can induce IL-4 expression,49,52 while forced expression of T-bet in TH2 cells can induce IFN-γ expression.53,54 Interestingly, the absence of GATA-3 permits TH1 cell differentiation independently of IL-12 and IFN-γ53, suggesting a primary role for GATA-3 in regulating TH1 vs. TH2 cell differentiation. In fact, GATA-3 functions primarily as an epigenetic modifier of the TH2 cytokine loci, and GATA-3 is not required for Il4 transcription in fully differentiated TH2 cells.55 T-bet also functions to suppress the production of IL-17A.56
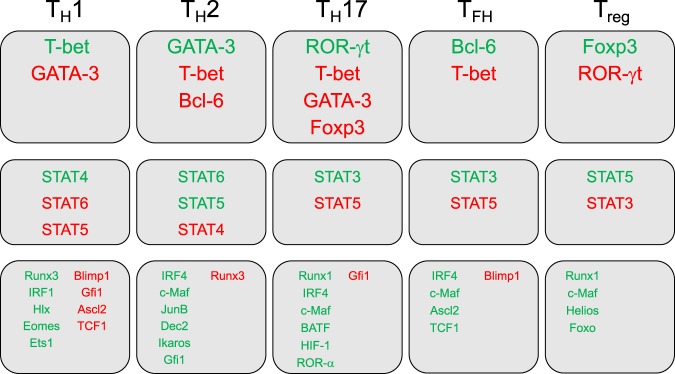
Transcriptional regulators of T helper cells. T helper cell subsets and associated positive (green) and negative (red) transcriptional regulators are separated by master regulators (top), signaling transducer and activator of transcription (STAT) molecules (middle), and additional important transcription factors (bottom)
Downstream of STAT3 signaling is the TH17 master regulator ROR-γt (retinoic acid receptor-related orphan receptor-γt).57 This transcription factor directly regulates the expression of IL-17A and IL-17F, along with other TH17-specific genes,58 and TH17 cytokine production is drastically reduced in ROR-γt-deficient cells.57 The transcriptional regulator of TFH cells is B-cell lymphoma-6 (Bcl-6), which is a characteristic that is shared with GC B cells.8,10 Mice with germline deficiency in Bcl-6 do not generate TFH cells and develop TH2-dominant immune disease.59–61 Interestingly, while Bcl-6 induces TFH-associated surface molecules (e.g., CXCR5 and PD-1) and represses alternate T helper subset cytokines, such as IFN-γ and IL-17A,61 it does not directly promote IL-21 expression.59
Research to identify a master transcriptional regulator and/or definitive markers for Tregs was guided by genetic studies. These studies demonstrated that the lymphoproliferative disorder, known as immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX), is caused by mutations in the gene encoding FOXP3 (forkhead box P3),62,63 while the mutation of the mouse homolog Foxp3 gene is responsible for the ”Scurfy” phenotype.64,65 Indeed, the function and identity of Tregs are dependent upon Foxp3 expression.66,67 Furthermore, a regulatory phenotype is imparted upon conventional T helper cells with enforced Foxp3 expression.66–68 TGF-β can promote Treg and TH17 cell differentiation, yet TH17-associated factors suppress Foxp3 expression through ROR-γt binding or STAT3 signaling.69 Though both tTregs and pTregs are categorically Foxp3-expressing Tregs, it is now understood that there are distinct functional properties and cis control elements between the two populations.70,71 tTregs mediate self-tolerance and prevention of autoimmunity, while pTregs enforce peripheral immune tolerance and general suppression of inflammation.
Aside from their respective master regulators, additional transcription factors are also critical regulators of T helper cell differentiation. The runt-related transcription factor (Runx) family is important for T-cell development and function. Runx3 promotes IFN-γ expression and represses Il4 gene expression in TH1 cells.72,73 Runx1 is critical for Treg cell function and Foxp3 stability74–76 and for the identity and function of TH17 cells by promoting the expression of ROR-γt and IL-17A.77 The interferon regulatory factor (IRF) family also regulates T helper cell differentiation. IFN-γ signaling induces IRF1, which assists TH1 identity through the upregulation of IL-12Rα.78 IRF4 upregulates GATA-3 and thus is important for TH2 cell function.79,80 Interestingly, TH17 and TFH cells also utilize IRF4 for differentiation.81,82 Transcription factors can also be part of negative-feedback mechanisms affecting differentiation. Both TH1 and TFH generation are impaired by Blimp-1 expression, which is induced by IL-2 signaling.60,83 In fact, IL-2-STAT5 signaling inhibits Bcl-6 due to similarities in binding sites near TFH genes.84 c-Maf is another important transcription factor for T helper cell differentiation that has context-specific functions based on chromatin availability,85 making it both a positive and negative regulator of cytokine genes within the same cell. Downstream of TCR signaling, c-Maf is a known positive regulator of Il10 expression,58,86,87 yet it promotes Il4 expression in TH2 cells88,89 and is also involved in TH1758,87 and TFH90 cell differentiation. Furthermore, c-Maf is critical for the cell ular function of Tregs in the gut.91 More comprehensive descriptions of additional transcription factors involved in T helper cell differentiation, including roles for ROR-α for TH17 cell generation92 and Ascl2 and T-cell factor 1 (TCF-1) for regulating TFH vs. TH1 or TH17 cell differentiation,93–95 are reviewed elsewhere.96,97 Future studies will continue to determine the transcriptional networks imparting context-specific functions of T helper cell subsets.
While master transcriptional regulators play critical roles in T helper cell differentiation, transcriptional mediators working in a coordinated network are required to drive cell fate decisions. The first reported descriptions of large-scale, transcriptional network-dependent control of CD4+ T-cell differentiation were focused on TH17 cells.58,98 These studies used chromatin immunoprecipitation-sequencing (ChIP-seq)58 and small interfering RNA98 screening methods, together with computational analyses, to reconstruct the dynamic regulatory network of TH17 cell differentiation. Recently, using a combination of CRISPR-Cas9 (clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9) screening and next-generation sequencing, including RNA-seq, ChIP-seq, and ATAC-seq (Assay for Transposase-Accessible Chromatin using sequencing), researchers generated a quantitative “atlas” of TH2 cell differentiation.99 Complex data integration, via a new biocomputational methodology, grants novel insight into regulatory networks involving different transcription factors, metabolic regulators, and cytokine signaling pathways.99 Future adaptation of these integrative and systems-level analyses to modern topics in immunology is an exciting prospect.
T-cell plasticity and heterogeneity
Differentiated T helper cells adapt to ever-changing microenvironments and surrounding molecular cues. Thus, these cells are able to continuously adapt to unique circumstances to provide proper immunity.100 Earlier work hinted at this phenomenon by forced expression of master regulators in differentiated T cells.49,52–54 Indeed, differentiated CD4+ T cells can adopt alternate expression profiles and produce cytokines that are associated with alternate T-cell lineages,101–105 including TFH cells106 (Fig. 3a). This duality is due, in part, to bivalent histone modifications (e.g., H3K4me3 and H3K27me3) that are maintained in T helper cells near all master regulator genes and are independent of the differentiated status of the cell.107 In other words, T helper cells remain “poised” to adopt alternative subset transcriptional programs upon receiving the appropriate signals. Extracellular cues that drive subset differentiation from naive T cells are also involved in this process. TH1 cells can be repolarized to produce IL-4 under TH2 conditions,102 and TH2 cells will express IFN-γ when cultured with IL-12, IFN-γ, and type I IFNs.108 IL-12 can also cause TH17 cells to express IFN-γ.103,109,110 Interestingly, TFH cells can be made to coexpress IL-21 and any other subset-specific cytokines when cultured under respective conditions.106 Both TH17 and TFH cells demonstrate plasticity within peripheral tissues111 and are discussed in more detail below.
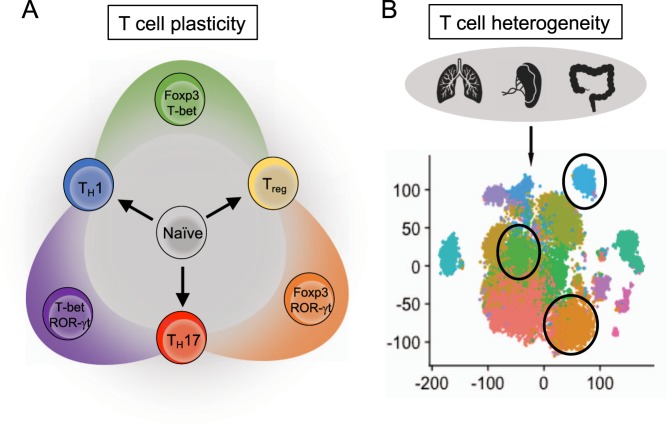
T helper cell plasticity and heterogeneity. a Representative diagram of plasticity in selected T helper cells. Differentiated T helper cells can coexpress master transcriptional regulators from other lineages. b Diagram with example tSNE (T-distributed stochastic neighbor embedding) plot generated from single-cell RNA-sequencing (scRNA-seq) data. Single-cell analysis allows for the discovery of subpopulations with unique expression profiles
Diversity among individual T helper subsets was established with the observations that master transcription factors could be coexpressed within certain subpopulations. One common example is a T-bet+ROR-γt+ T-cell population found in the gut and within the inflamed central nervous system (CNS) that produces both IFN-γ and IL-17.109 Arguably the most well-established example of functional plasticity are Tregs, which are reprogrammed to suppress specific types of inflammation (e.g., TH1 vs. TH2 inflammation).112,113 For instance, in the context of TH1 inflammation, Tregs receiving IFN-γ and/or IL-12 signals will express T-bet and the TH1-chemokine receptor CXCR3, which enables migration and accumulation within areas of TH1 inflammation.114,115 This “TH1-like” capability is a critical part of the cell-mediated immune homeostasis of Tregs, as loss of T-bet+ Tregs (but not T-bet expression itself) results in TH1-dominant immune disease.116 Importantly, Tregs must maintain Foxp3 expression for suppressive function, despite the extrinsic TH1 influence. This regulation occurs by ongoing IL-2-STAT5 signaling, which reinforces Foxp3 expression117 and limits the expression of IL-12Rβ2.118 Similar functional effects in Tregs are observed for TH2 inflammation, where IL-4 induces IRF4 and GATA-3119–121 or in TH17 inflammation with IL-6-STAT3 signaling for ROR-γt upregulation.122–124 TFH responses are controlled by a unique class of Tregs, termed T follicular regulatory (TFR) cells, which express Bcl-6 and TFH surface markers CXCR5 and PD-1.125–127 Notably, while the overall importance of effector-like transcriptional signatures in Tregs is clear, Tregs expressing effector-like transcriptional programs are also associated with aberrant immune diseases and show defective suppressive capacity ex vivo. Increased frequencies of TH1-like Tregs are observed in patients with type I diabetes128 and multiple sclerosis.115 Likewise, TH2-like Tregs are increased in patients with food allergies,121 and TH17-like Tregs are enriched in the synovium of rheumatoid arthritis patients.129 The mechanisms for this notable disparity between effector-like Tregs in healthy function and disease states remain unclear.
Heterogeneity has also become a key area of research due to the availability of powerful experimental methods, such as single-cell RNA-seq (scRNA-seq).130 Single-cell resolution allows for the discovery of unique subpopulations of T cells that would otherwise be “lost” in bulk RNA-seq analyses (Fig. 3b). With the inference of multiple developmental or transitional “states” of the same cell type, this method can garner more information when combined with “pseudotime” analysis, which generates a visualized continuum of cells as they progress, regress, or transgress along a developmental trajectory. This analysis is especially applicable to T helper cell differentiation to provide a temporal dimension of gene regulation as opposed to a snap-shot of the end result.
Previous work in TH17 cell differentiation demonstrated both pathogenic and regulatory-like TH17 cell generation from different cytokine stimuli.35 The combination of scRNA-seq and computational approaches shows that in vivo-derived TH17 cells, isolated from an autoimmune environment, are also heterogeneous and have a spectrum of functional states.131,132 Applying computational analysis with scRNA-seq also reveals novel targets for dictating the different cellular fates of TH17 cells.131–133 “Pseudotime” analyses have also demonstrated the relative temporal trajectory of IL-17-producing TH17 cells and TH1-like TH17 cells132 as well as differentiating bifurcations in TH1 and TFH cell fates in a mouse model of malaria.134 In both cases, these cells originate from a common precursor, yet the programming of their differential fates is coincident with the expression of unique chemokine receptors, transcription factors, and functional profiles.132,134 Applications of scRNA-seq and computational approaches have also recently been applied to Tregs in lymphoid tissues, identifying an important role for TCR signal strength in shaping the activation and heterogeneity of Tregs.135 Furthermore, this approach has identified similarities and differences among nonlymphoid tissue-resident Tregs, known to be starkly different from lymphoid-resident cells.136,137
Another emerging concept in T helper cell heterogeneity is cells with stem-like qualities. Even in chronic viral infections that were thought to drive T-cell exhaustion and loss of memory potential, a stem-like population of CD8+ T cells with an enhanced capacity for self-renewal and responsiveness to immune checkpoint therapy was recently identified and depends upon TCF-1–LEF-1 (lymphoid enhancer-binding factor 1) signaling.138–140 Long-lived TH17 cells with stem cell-like attributes of self-renewal and multipotency were also found.141 Interest in stem-like T helper cells has recently increased by reports of their involvement in autoimmune diseases, including chronic colitis142 and experimental autoimmune encephalomyelitis.132 The functional identification of these cells has revealed another dimension in T helper cell differentiation that is only decipherable under specific disease contexts but is physiologically relevant. Furthermore, it remains to be seen if other T helper subsets, particularly highly plastic Tregs, also have stem-like properties during homeostasis or autoimmunity.
Tissue T-cell populations
While cellular heterogeneity and plasticity occur during inflammation,100 recent studies suggest that these phenomena also arise under steady-state conditions. Lineage-tracing genetic systems have been an invaluable tool to demonstrate the physiological relevance of T-cell plasticity.109,143,144 For example, tissue-specific plasticity occurs in Peyer’s patches (PPs) and gut-associated lymphoid tissues where GC B cells capable of producing IgA are generated. TFH cells within the PPs have been shown to develop from gut-derived TH17 cells or pTregs that have been activated through MyD88-coupled receptors.145–147 Furthermore, inflammatory TH17 cells can transdifferentiate into Tregs to resolve inflammatory responses.144 Thus, CD4+ T-cell plasticity can play critical roles under homeostasis.
Both tTregs and pTregs reside in mucosal tissues, including gut-associated lymphoid tissues. Foxp3+ Tregs found within these sites are inherently heterogeneous, as tissue Tregs must coexpress effector T-cell transcription factors for proper function. For instance, intestinal pTregs express ROR-γt to suppress TH1, TH2, and TH17 inflammation in the gut.122,123 Similarly, Tregs migrate into PPs at steady-state conditions and differentiate into Foxp3+Bcl-6+ TFR cells to regulate IgA antibody production.148 Tissue Tregs regulate type 2 immunity in the intestines, lung, and skin under steady-state conditions.70,149–151 This function is supported by the inflammation-triggered upregulation of IRF4120 or GATA-3 by TCR or IL-33 signals, which mediate the stability and function of Tregs.152–154 Notably, functional redundancy of GATA-3 and T-bet in Tregs exists for the proper control of immune homeostasis119 and potentially explains why the conditional deletion of GATA-3 in Tregs does not trigger excessive inflammation.152,153,155 However, the hypercolonization of selective commensals induces potent type 2 inflammation within the skin of mice bearing GATA-3-deficient Tregs.151 Thus, the heterogeneity and plasticity of Tregs are important for tissue homeostasis.
Cytokines and transcriptional networks regulate the differentiation of several memory CD4+ and CD8+ T-cell subsets. Among these memory T-cell subsets are tissue-resident memory T cells (TRM), which were first shown to be induced by pathogens. TRM cells share transcriptional signatures with other effector and memory subsets but also have distinct transcriptional programs, as reviewed elsewhere.156 However, host antigens, such as commensal bacteria, can also induce TRM-like T cells that produce IFN-γ or IL-17 in the skin.157,158 IL-17-producing CD8+ and CD4+ T cells within the skin can coexpress ROR-γt and GATA-3 in the presence of the “alarmins” lL-18, IL-33, or IL-1. This regulation is essential for generating type 2 inflammation that promotes wound healing.151 These studies establish tissue T-cell heterogeneity as a critical node for tissue homeostasis and repair.
How immune cells regulate the tissue microenvironment through cell–cell interactions and signaling networks is an emerging concept in immunological research. For example, Tregs support stem cell homeostasis in the intestines, hair follicle, and bone marrow.159–161 Moreover, Tregs regulate microbiota and metabolite diversity, which controls inflammatory processes at both local and distal sites.70,162,163 Thus, the molecular dissection of how resident and infiltrating T cells modulate the overall tissue microenvironment will likely uncover new regulations of disease etiology and progression.
Metabolic control of T helper cell function
Metabolic control of differentiation
The discovery that different T helper cells have shared, yet distinct, metabolic programs has established that metabolism can influence CD4+ T-cell fates.164–166 Specifically, TH1, TH2 and especially TH17 cells maintain high levels of glycolysis compared with in vitro-derived Tregs.167–169 These observations have since been extended to TFH cells, which have increased glycolytic metabolism relative to non-TFH cells; however, cell-autonomous IL-2 dampens the differentiation and metabolic programs of TFH cells at the expense of TH1 programs through the induction of Blimp-1.170–172 Additionally, glutaminolysis programs increase in TH17 cells compared with TH1 or in vitro-derived Tregs,173 while fatty acid synthesis is a critical regulator of TH17 and, to a lesser extent, TH1 cell generation and function.174,175 In contrast, fatty acid synthesis opposes the differentiation of Tregs,174 demonstrating that this pathway also balances TH17 vs. Treg cell programming. Tregs require high levels of mitochondrial activity to support their differentiation, homeostasis, and function in vitro and in vivo.149,150,167,169,176 Using in vitro systems with the drug etomoxir,167,169 Tregs were found to rely on fatty acid oxidation to induce mitochondrial oxidative metabolism, but this model has recently been challenged in vivo.177 Moreover, genetic studies show that metabolic programs are altered in accordance with the requirements for the activation, functional reprogramming, and migration of tTregs and likely pTregs,149,150,176,178–182 thus establishing layers of complexity remaining to be fully addressed.
The kinase mammalian target of rapamycin (mTOR), which functions via one of the two distinct complexes mTORC1 or mTORC2, and the transcription factors c-Myc, hypoxia-inducible factor-1α (HIF-1α), and nuclear factor of activated T-cells (NFAT) cooperatively induce the expression of metabolic enzymes and nutrient transporters (e.g., glucose, amino acids, fatty acids, and minerals) that promote anabolic metabolism.168,183–187 Activation programs also lead to increased expression of the high-affinity IL-2 receptor and induction of its downstream signaling, which can further amplify Akt- and mTOR-dependent signaling to tune cellular metabolism.188 Also downstream of mTORC1, HIF-1α signaling is a critical determinant of the reciprocal differentiation of TH17 and Tregs.168,189 HIF-1α activity induces glycolysis under hypoxic conditions. This oxygen-sensing function is enforced by the von Hippel-Lindau (VHL) complex and opposed by prolyl-4-hydroxylase domain (PHD) proteins. Upon activation, VHL-deficient CD8+ T cells have augmented glycolytic function and reduced mitochondrial oxidative metabolism, which are associated with increased effector programming, terminal effector-memory T-cell differentiation, and increased cell death.190,191 In contrast, PHD-deficient CD4+ T cells have increased and decreased differentiation of TH1 and Tregs, respectively, which can boost anti-tumor immunity in hyperoxic environments, such as the lung.192 These results implicate oxygen sensing by upstream regulators of HIF-1α as determinants of TH1 and Treg cell fate decisions.
Metabolic signaling can also impart functional reprogramming of effector T cells by epigenetic mechanisms (Fig. 4). mTOR signaling promotes TH1 and TH2 cell differentiation, perhaps in part by inducing epigenetic-related metabolites.183,193–195 The lactate dehydrogenase A (LDHA)-dependent aerobic glycolytic flux maintains high levels of cytosolic acetyl-coenzyme A,196 which can posttranslationally modify proteins via the enzymatic activities of histone acetyltransferases.164 Indeed, LDHA-deficient T cells have reduced TH1 responses owing to reduced H3K9Ac on the Ifng locus.196 A recent study has shown that glutaminolysis can regulate the differentiation and function of TH1 and TH17 cells through discrete mechanisms. TH17 cell differentiation is reduced in the absence of glutaminolytic metabolism due to an accumulation of reactive oxygen species in part alter H3K27me3 levels and chromatin accessibility in TH17-related genes.167,173 In contrast, TH1 cell proliferation and function are only temporarily impaired when glutaminolysis is suppressed and are associated with a loss of the α-ketoglutarate (α-KG)-dependent suppression of H3K27me3 that is reversed and elevated by increased IL-2-mTORC1 signaling.173 Additional studies will continue to dissect how mTOR and metabolic networks orchestrate the differentiation and function of CD4+ T cells at different stages of effector programming.
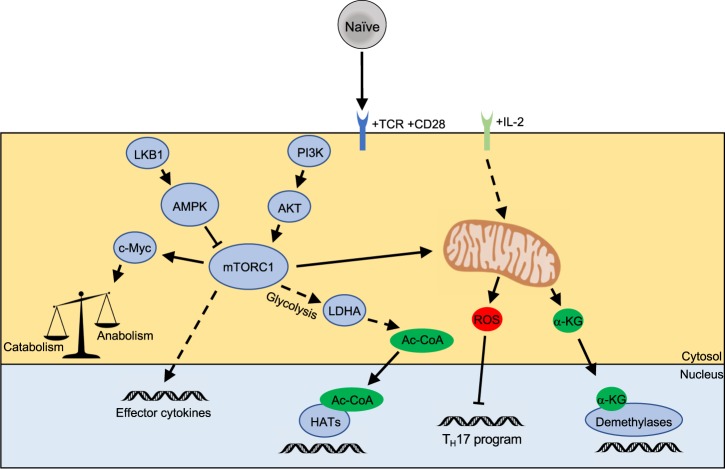
Metabolic control of T helper cell differentiation. Activated T cells undergo metabolic reprogramming, which leads to permissive epigenetic alterations of T effector genes. Much of this pathway is coordinated by mammalian target of rapamycin (mTOR) signaling and mitochondrial function to generate metabolic co-factors of epigenetic-modifying enzymes
Metabolic programs are also key regulators of the activation of naive CD4+ T cells. During antigen-driven quiescence exit, naive T cells rapidly rewire their metabolic programs, switching from a catabolic to anabolic state by upregulating glycolysis, glutaminolysis, and mitochondrial metabolism.197 Defects in metabolic reprogramming during quiescence exit manifest in reduced T-cell expansion by suppressing cell proliferation and/or survival, as well as impaired TH1, TH2, TH17, and TFH responses as reviewed elsewhere.164–166 It remains unclear whether alterations in effector CD4+ T-cell programming are distinct from, or a consequence of, proliferation defects. However, a recent study suggested that the demethylase-inducing effects of α-KG mediate the CTCF-dependent induction of TH1 signature genes separate from effects on proliferation.198 These results thus provide evidence that metabolic networks can prime CD4+ T-cell differentiation independently of effects on proliferation.
Metabolic control of plasticity and heterogeneity
The metabolic landscape also shapes T-cell plasticity and heterogeneity. Metabolic disruptions due to excessive phosphoinositide 3-kinase (PI3K) and mTOR activities can lead to increased methylation of the Foxp3 locus and reduce stability of Tregs, leading to uncontrolled TH1 and TFH cell responses.180,181 Defects in metabolic reprogramming are also associated with an exhausted-like state in Tregs, characterized by the hyperexpression of coinhibitory molecules that impairs their stability and suppression of TH2 immunity.150 High levels of glycolytic metabolism limit Foxp3 induction to promote TH17 cell conversion at the expense of Tregs.167,168 Strong glycolytic signals also reduce the stability of tTregs, leading to the generation of IFN-γ- or IL-17-producing ex-Tregs,179,199 and these observations further establish the link between metabolic programming and plasticity or instability of Tregs.
Using a lineage-tracing system and scRNA-seq, we recently found that IL-17-producing TH17 cells can adopt different metabolic states in accordance with their pathogenicity; a stem-like signature of IL-17-producing cells has low mTORC1 activity and downstream anabolic programs and an IFN-γ-producing state has high mTORC1 activity and anabolic metabolism (glycolytic and mevalonate-related pathways).132 This metabolic heterogeneity is mediated by a balance of TCF-1 and T-bet-dependent programs, and it is also associated with altered histone acetylation and chromatin accessibility at the Ifng locus. Thus, metabolic and functional flexibility, together with the dynamic regulation of transcriptional and epigenetic networks, mediate heterogeneity within specific effector T-cell subsets. Exploring the mechanisms for effector T-cell heterogeneity will be crucial as more precision-based therapies are used to treat various diseases.
Metabolic control of tissue T-cell responses
One emerging downstream consequence of metabolic reprogramming is the capacity to modulate T-cell accumulation within tissues at steady-state conditions,149,170,182 where extracellular nutrients further tune their function. This phenomenon is perhaps best exemplified within the tumor microenvironment, where T cells compete for limited nutrient sources, including glutamine and glucose.200 Many in vitro studies have been informative for understanding the role of nutrients in T helper cell differentiation and function. For instance, defects in TH1 and TH17 cell differentiation occur when glutamine or leucine are limited, owing in part to the modulation of mTORC1 or AMPK (5' adenosine monophosphate-activated protein kinase) activity,173,201–203 though the precise mechanisms remain elusive. The manipulation of extracellular glucose concentrations can also alter effector T-cell function through multiple mechanisms in vitro. First, AMPK, which is activated when glucose levels are low, regulates the function of TH1 and TH17 cells under contexts of nutrient limitations in vitro and following bacterial and viral challenges.203 Second, glycolytic flux promotes the translation of Ifng mRNA by disengaging the glycolytic enzyme GAPDH (glyceraldehyde 3-phosphate dehydrogenase) from its 3’-untranslated region,204 in addition to mediating the acetylation of H3K9 at the Ifng promoter.196 Third, glucose deprivation diminishes the production of phosphoenolpyruvate, which helps to sustain Ca2+/NFAT signaling that can itself promote glycolytic reprogramming in T cells.187,205 Fourth, glucose and glutamine metabolism regulate Myc expression by inducing O-linked N-acetylglucosaminylation.206 Thus, one functional consequence of glucose and glutamine metabolism is the feedforward reinforcement of transcriptional networks that promote metabolic reprogramming.
While changes in nutrient concentrations are likely to have impacts beyond the tumor microenvironment, we lack a complete understanding of the role of nutrient sensing under physiologically relevant conditions, especially under steady-state conditions. Indeed, the deletion of transporters or anabolic-related enzymes or the in vitro manipulation of these programs (where other nutrients are still likely to be in excess), as described above, cannot fully mimic the dynamics of nutrient sensing and metabolic programming of the tissue microenvironment. Moreover, in vitro systems cannot fully recapitulate the cell–cell interactions or other factors (e.g., cytokines and microbiota) of specific tissues, or if complex microenvironments may shape how T cells respond to nutrients. Therefore, much remains to be discovered about the functional roles of nutrients and metabolic pathways in T helper cell responses, especially in vivo.
Human T helper cells
Though most of the seminal work in T helper cell differentiation was performed in murine cells, the basic tenants of human CD4+ T-cell differentiation are well conserved across species. On the whole, mouse and human T helper cell subsets are similar with respect to definitive cytokines, master transcriptional regulators, and signaling pathways, as reviewed elsewhere.207 In contrast to laboratory mice, the human immune system is subjected to continuous and diverse pathogen exposure, which is reflected in the relatively more substantial degree of heterogeneity and plasticity in T helper cells. Additional factors, such as age, genetics, microbiota, and location (among many other factors), further add to this complexity and are an active area of investigation.
The elucidation of human T-cell heterogeneity and plasticity is emerging as a powerful tool to discover new mechanistic targets and to optimize current therapies for patient-specific and/or disease-specific purposes. Techniques, such as scRNA-seq, performed on primary tissues from melanoma patients have demonstrated differential cellular mechanisms of action caused by checkpoint therapies208 and have enabled the characterization of T-cell subsets that best respond to these therapies.209 Thus, from a meta-analysis or clinical perspective, the heterogeneity within human T helper cells can be viewed as an opportunity, instead of a caveat, to discover novel mechanisms of T-cell differentiation and function as reviewed in more detail elsewhere.210,211
Concluding remarks
Over the past three decades, the field of T helper cell differentiation has seen exponential growth. Starting from a relatively simplistic view of TH1 and TH2 cells, T helper cell differentiation has become one of the most actively studied areas in immunology. The seminal studies described in this review have laid the foundations for our current understanding of the mechanisms that both prevent and potentially cause immune-mediated diseases. Despite the significant advancements, many important questions remain to be answered.
T helper cells are receptive to many extrinsic and intrinsic signals that influence transcriptional programs and cell identity throughout their lifespan. Master transcriptional networks are cross-regulatory but can also be coexpressed in unique subpopulations. We are beginning to unravel the complex mechanisms of T helper cell plasticity and heterogeneity, which are highly integrated with metabolic regulation and tissue homeostasis. As scRNA-seq and other next-generation techniques become more accessible, these techniques can be used to address large-scale and systems-level questions about the crosstalk between different tissues and the regulation of T helper cell differentiation. Thus, the generation and integration of “big data” will be an integral component in future investigations. Overall, the outlook for this field remains bright, and we look forward in anticipation to what the next 30 years will bring.
Acknowledgements
The authors apologize to all colleagues whose key contributions could not be individually cited due to space limitations. We thank Yanyan Wang for manuscript editing. This work was supported by the National Institutes of Health and the National Multiple Sclerosis Society.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Articles from Cellular and Molecular Immunology are provided here courtesy of Nature Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1038/s41423-019-0220-6
Read article for free, from open access legal sources, via Unpaywall:
https://www.nature.com/articles/s41423-019-0220-6.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1038/s41423-019-0220-6
Article citations
Th17 cell function in cancers: immunosuppressive agents or anti-tumor allies?
Cancer Cell Int, 24(1):355, 27 Oct 2024
Cited by: 0 articles | PMID: 39465401 | PMCID: PMC11514949
Review Free full text in Europe PMC
Dynamic chromatin architecture identifies new autoimmune-associated enhancers for <i>IL2</i> and novel genes regulating CD4+ T cell activation.
Elife, 13:RP96852, 20 Sep 2024
Cited by: 1 article | PMID: 39302339 | PMCID: PMC11418197
Posttransplant complications: molecular mechanisms and therapeutic interventions.
MedComm (2020), 5(9):e669, 02 Sep 2024
Cited by: 0 articles | PMID: 39224537 | PMCID: PMC11366828
Review Free full text in Europe PMC
Diet-Induced Obesity in Mice Affects the Maternal Gut Microbiota and Immune Response in Mid-Pregnancy.
Int J Mol Sci, 25(16):9076, 21 Aug 2024
Cited by: 0 articles | PMID: 39201761 | PMCID: PMC11354285
Synergistic Photothermal Tumor Immunotherapy by 1-MT Based on Zeolitic Imidazolate Framework-8 with pH-High Sensitivity.
Int J Nanomedicine, 19:8501-8517, 20 Aug 2024
Cited by: 0 articles | PMID: 39185344 | PMCID: PMC11344551
Go to all (208) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Roles for helper T cell lineage-specifying transcription factors in cellular specialization.
Adv Immunol, 124:171-206, 01 Jan 2014
Cited by: 9 articles | PMID: 25175776
Review
In Vitro Differentiation of Effector CD4+ T Helper Cell Subsets.
Methods Mol Biol, 1960:75-84, 01 Jan 2019
Cited by: 19 articles | PMID: 30798522
Multilayer regulation of CD4 T cell subset differentiation in the era of single cell genomics.
Adv Immunol, 141:1-31, 03 Jan 2019
Cited by: 13 articles | PMID: 30904130
Review
Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells.
Science, 327(5969):1098-1102, 01 Feb 2010
Cited by: 852 articles | PMID: 20185720 | PMCID: PMC2997673
Review Free full text in Europe PMC
Funding
Funders who supported this work.






