Abstract
Free full text

Neuromyelitis optica spectrum disorders
ABSTRACT
Neuromyelitis optica spectrum disorder (NMOSD) is an uncommon antibody-mediated disease of the central nervous system. Long segments of spinal cord inflammation (myelitis), severe optic neuritis, and/or bouts of intractable vomiting and hiccoughs (area postrema syndrome) are classic presentations of the disease and may alert the clinician to the diagnosis. Untreated, approximately 50% of NMOSD patients will be wheelchair users and blind, and a third will have died within 5 years of their first attack. Unlike multiple sclerosis, a progressive clinical course is very unusual and the accrual of disability is related to relapses. Approximately 75% of patients have antibodies against aquaporin-4, a water channel expressed on astrocytes. Relapses are treated aggressively to prevent residual disability with high-dose steroids and often plasma exchange. Relapse prevention is crucial and achieved with long-term immunosuppression. In this article we review the pathogenesis, clinical features, diagnosis and management of NMOSD.
Case vignette
A 24-year-old male developed nausea, vomiting, and pain between his shoulder blades with leg weakness. Over the next 72 hours he developed rapidly progressive leg weakness, numbness, and urinary retention, and attended his local emergency department. He was admitted but quickly progressed to flaccid tetraplegia. There were no preceding infections or inciting events. His medical history was unremarkable except for ‘cyclical vomiting syndrome’. Numerous investigations and treatments including a cholecystectomy had been unsuccessful.
He had urgent magnetic resonance imaging (MRI) of the spine, which demonstrated a long segment of high T2 signal and oedema from C1-T4 with contrast enhancement. His cerebrospinal fluid demonstrated a lymphocytic pleocytosis (white cell count 40 cells/mm3) with normal protein, glucose ratio, and negative oligoclonal bands. Acute inflammatory transverse myelitis was diagnosed and he was given 1 gram of IV methylprednisolone for 5 days. His serum was sent for aquaporin-4 (AQP4) and myelin oligodendrocyte glycoprotein (MOG) antibodies (Abs). The steroid treatment did not result in significant improvement. There was concern of ascending myelitis and respiratory compromise. He was given 5 cycles of plasma exchange and continued on a tapering course of prednisolone. After 4 weeks his arm strength had improved but he remained paraplegic and was transferred to a rehabilitation facility. During his admission he complained of severe itching (to the point of excoriation) over his chest as well as painful lower limb spasms, which were successfully treated with gabapentin and carbamazepine respectively. His AQP4-Abs were positive and he was commenced on mycophenolate mofetil with prednisolone to prevent further relapses. At follow-up 3 months later he remained relapse-free but wheelchair-dependent with a spastic paraplegia. He used intermittent self-catheterisation four times per day and required regular bowel irrigation for constipation. He was commenced on baclofen for spasticity and sildenafil for erectile dysfunction. Interestingly, following plasma exchange his nausea and vomiting resolved completely suggesting that the vomiting may have been AQP4-Ab mediated area postrema syndrome.
Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is an uncommon antibody-mediated disease of the central nervous system. In the UK patients often present to and are first assessed in emergency departments or acute medical units (AMU). Early diagnosis and treatment are important to reduce the risk of long-term disability and death.
The term neuromyelitis optica (derived from neuro-myélite optique aiguë) was first described by Eugène Devic and his doctoral student Fernand Gault in 1894.1 The disease was therefore previously referred to as Devic’s disease. Until recently it was unclear whether neuromyelitis optica was a separate disease or merely a more severe form of ‘optico-spinal’ multiple sclerosis (MS). It was not until 2004 when the putative antigenic target, the aquaporin-4 water channel was identified, and the two diseases could be reliably distinguished through the detection of AQP4-Abs.2 The latest iteration of diagnostic guidelines unify antibody-negative and positive forms under the umbrella of NMOSDs.3
Since the discovery of AQP4-Abs there have been over 3,000 publications on NMOSD in the PubMed database alone. More recently another antigenic target, myelin oligodendrocyte glycoprotein (MOG) has been identified in patients with NMOSD.4 This review aims to provide a basic overview of the pathogenesis, diagnosis, and management of NMOSD.
Pathogenesis
AQP4 is the most widely expressed water channel in the brain, spinal cord, and optic nerves. Within the brain, AQP4 is located in regions in contact with cerebrospinal fluid, and is specifically localised to the foot processes of astrocytes at the blood brain barrier.2,5 AQP4 is also present in the collecting ducts of the kidney, parietal cells of the stomach, airways, secretory glands, and skeletal muscle.6 However, these organs are relatively protected from antibody-mediated damage due to local complement inhibitors which are absent in the brain.7
AQP4-Abs are predominantly of the IgG1-isotype. Experimental data suggest that AQP4-Abs induce interleukin-6 (IL-6) production in astrocytes expressing AQP4, and that IL-6 signalling to endothelial cells reduces blood-brain barrier function.8 Once bound to the extracellular domain of the AQP4 receptor, AQP4-Abs result in complement- and cell-mediated astrocytic damage, in addition to internalisation of the glutamate transporter EAAT-2.9 The astrocyte is subsequently rendered powerless, ultimately culminating in withdrawal of support for surrounding cells such as oligodendrocytes and neurons. Granulocyte infiltration ensues, matched by oligodendrocyte damage and demyelination (Fig (Fig11).10 In contrast to MS, the demyelination that is seen in NMOSD is a secondary event and occurs as a consequence of primary damage to astrocytes.
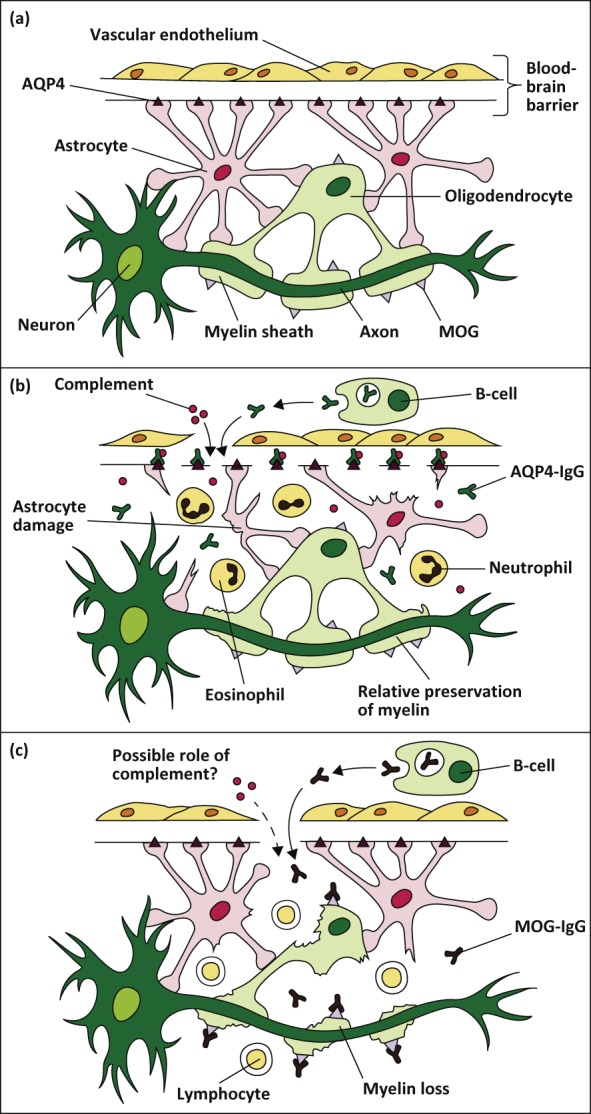
(a) This figure illustrates the sites of expression of aquaporin-4 (AQP4) and myelin oligodendrocyte glycoprotein (MOG) in the central nervous system (CNS). AQP4 is expressed on astrocyte 'foot-like' processes at the blood-brain barrier. MOG is expressed on oligodendrocytes on the outermost lamellae of myelin sheaths. (b) AQP4-Abs (IgG) are produced systemically by mature B-cells, and upon crossing the blood-brain barrier, activate complement-mediated astrocyte damage. There is relative preservation of myelin initially. The inflammatory milieu consists of neutrophils and eosiniphils. (c) MOG-Abs (IgG) are also produced outside the CNS, and causes demyelination by mechanisms that are poorly understood. Reprinted with permission from Whittam D, Wilson M, Hamid S et al. What’s new in neuromyelitis optica? A short review for the clinical neurologist. J Neurol 2017;264:2330–44.
Epidemiological and genetic factors
The reported incidence and prevalence of NMOSD are dependent on geographical location and ethnicity. Asians and those of African ancestry are at increased risk, with high mortality rates reported in the latter.11,12 The incidence and prevalence of NMOSD ranges from 0.05–0.40 and 0.52–4.4 per 100,000 people respectively.13 In contrast to MS, a latitude gradient for the prevalence of NMOSD has not been substantiated.14 As with many autoimmune diseases, females are more susceptible than males (3:1–9:1).15 A female hormonal basis for this association may be a factor but requires further study.16 The median age at presentation is 39 years, but 15–20% of patients may present to paediatricians (under 16 years) or elderly care physicians (greater than 65 years).15 Clustering of NMOSD in families is rare but recognised, suggesting complex genetic susceptibility.17 A recent whole-genome sequence study identified genetic variants in the major histocompatibility region that contribute to NMOSD aetiology.18 Approximately one in four patients with AQP4-Ab positive NMOSD have another coexisting autoimmune disease, eg myasthenia gravis, systemic lupus erythematosus (SLE), Sjogren’s and coeliac disease.19–22 Accordingly, specialties such as rheumatology should have a low threshold for testing AQP4-Abs; eg SLE patients with intractable vomiting/hiccoughs or optic neuritis without obvious cause. In one series 46% of myelitis cases thought secondary to anti-phospholipid/SLE syndrome were subsequently found to harbour AQP4-Abs.23
Clinical presentation
Table Table11 summarises the 2015 diagnostic criteria for NMOSD, which incorporates classic clinical presentations of the disease.3 These involve regions of the central nervous system (CNS) where AQP4 is most abundantly expressed; spinal cord (longitudinally extensive transverse myelitis), optic nerve (optic neuritis), dorsal medulla (area postrema syndrome), brainstem (acute brainstem syndromes), and thalamus/hypothalamus (acute diencephalic syndromes eg symptomatic narcolepsy). Attacks are frequently severe and often reach nadir in less than a week. Longitudinally extensive transverse myelitis (LETM) is the most specific presentation of NMOSD and is uncommon in MS (Fig (Fig2a).2a). LETM typically consists of inflammation affecting the central gray matter, extending over three or more contiguous vertebral bodies.24 LETM often results in para- or tetraplegia depending on the spinal cord level involved. A sensory level and bladder involvement are useful distinguishing features from other causes of rapid evolving weakness such as Guillain–Barré syndrome. Table Table22 summarises the differential diagnosis of transverse myelitis. Other useful clinical clues include intense itching (due to inflammation of itch specific fibres in the spinothalamic tract) and tonic spasms (brief recurrent, usually painful episodes of increased muscle tone with abnormal posturing of the affecting limb).25,26 Importantly, up to 14% of NMOSD patients may also present with short spinal cord lesions, which may mimic MS.27 In such cases the absence of MS-typical brain lesions eg ‘Dawson’s fingers’ should increase the suspicion of NMOSD.
Table 1.
Summary of 2015 international diagnostic criteria for neuromyelitis optica spectrum disorder3
Diagnosis with AQP4-Abs
|
Diagnosis without AQP4-Abs/unknown status
|
Core clinical characteristics
|
Additional MRI requirements for NMOSD without AQP4-Abs/unknown status
|
APS = area postrema syndrome; AQP4-Abs = aquaporin-4 antibodies; LETM = longitudinally extensive transverse myelitis; NMOSD = neuromyelitis optica spectrum disorder; MRI = magnetic resonance imaging.
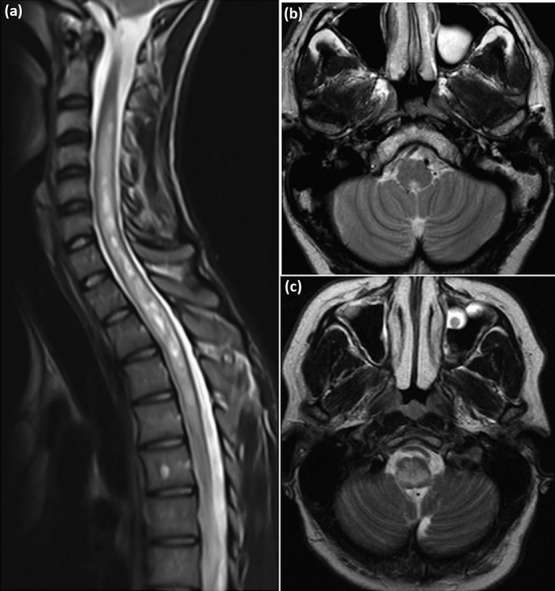
Magnetic resonance imaging (MRI) in neuromyelitis optica. (a) Longitudinally extensive transverse myelitis – sagittal T2 weighted image of cervicothoracic spine demonstrating T2 hyperintensity extending longitudinally from C2–T4. (b) Area postrema syndrome – axial T2 weighted image demonstrating dorsal T2 signal hyperintensity in the medulla. (c) Brainstem syndrome – axial T2 weighted image demonstrating diffuse symmetrical T2 signal hyperintensity in the medulla.
Table 2.
Differential diagnosis of transverse myelitis
| Structural | Degenerative disc disease, tumours (primary or secondary), syrinx, abscess |
| Autoimmune/inflammatory | MS, GBS, AQP4-Ab, MOG-Ab, GFAP-Ab, sarcoid, stiff-person syndrome, SLE, Behcet’s, Sjogren’s, MCTD |
| Vascular | Anterior spinal artery occlusion, dural arteriovenous fistula, CNS vasculitis |
| Nutritional | B12/folate/copper (zinc intoxication), vitamin E deficiency |
| Viral/post-viral | DNA viruses eg CMV, varicella-zoster, HSV-2, EBV |
| RNA viruses eg HIV, HTLV-1/2, polio, influenza, measles, mumps, dengue, West Nile, enterovirus-D68, 70/71, hepatitis A/C | |
| Other infections | Mycoplasma, Borrelia burgdorferi, Treponema pallidum, aspergillus, neurocysticercosis, schistosoma, angiostrongylosis |
| Genetic/inborn metabolic errors | eg Leukodystrophies, peroxisomal disorders, biotinidase deficiency, hereditary spastic paraplegias, dopa-responsive dystonia, Friedreich’s ataxia, Hexosaminidase deficiency |
| Paraneoplastic syndromes | Amphiphysin-, Ri-, CV2/CRMP5-, Ma1/2-, Hu-antibodies |
| Toxic | Post-radiation |
| Nitrous oxide | |
| Heroin | |
| Electrical injury | |
| Decompression sickness | |
| Anterior horn cell disease | Amyotrophic lateral sclerosis |
| Primary lateral sclerosis | |
| Poliomyelitis |
Ab = antibody; AQP4 = aquaporin-4; CMV = cytomegalovirus; CNS = central nervous system; CV2/CRMP5 = collapsin response mediator protein 5; DNA = deoxyribonucleic acid; EBV = Epstein-Barr virus; GBS = Guillain–Barre syndrome; GFAP = glial fibrillary acid protein; HSV = herpes simplex virus; HTLV = human T-lymphocytic virus; MCTD = mixed connective tissue disease; MOG = myelin oligodendrocyte glycoprotein; MS = multiple sclerosis; SLE = systemic lupus erythematosus; RNA = ribonucleic acid.
Optic neuritis (ON) in NMOSD is also typically longitudinally extensive and has a predilection for posterior optic nerve segments, particularly the optic chiasm. Bilateral simultaneous involvement and painful severe visual loss with poor recovery (<6/60) are often clues to the diagnosis.
Area postrema syndrome (APS) results in intractable nausea, vomiting and/or hiccoughs secondary to inflammation in the emetic reflex centre located in the rhomboid fossa of the 4th ventricle (Fig (Fig22b).28 Patients may initially present with suspected gastroenteritis or cyclical vomiting syndrome. APS is the initial presenting feature of NMOSD in approximately 12% of cases.11
Acute brainstem syndromes overlap with APS but also include patients who present with oculomotor dysfunction (eg diplopia and nystagmus), or other cranial nerve palsies, depending on location (Fig (Fig22c).29
AQP4 is highly expressed in the hypothalamic periventricular regions and bilateral lesions may affect hypothalamic hypocretin neuronal function. Several cases of narcolepsy, with diencephalic lesions and low cerebrospinal fluid (CSF) hypocretin levels have been reported.30
Although the term neuromyelitis optica might suggest exclusive spinal cord and optic nerve inflammation, brain involvement can be seen in 60% of patients, though the majority of observed changes are non-specific.31 Cerebral involvement may be asymptomatic, but can cause encephalopathy, seizures, and hemiparesis. Lesions often enhance with contrast on MRI and maybe mistaken for a primary CNS tumour. AQP4 is expressed at high density in the periependymal regions outlining the ventricular system, and characteristic lesions involving this region may be seen.
Diagnostic considerations
The presence of one of the six core clinical characteristics (LETM, ON, APS, symptomatic brainstem, diencephalic, or cerebral syndromes) with AQP4-Abs is sufficient to make a diagnosis of NMOSD. In seronegative cases the criteria are more stringent and MRI requirements must also be fulfilled. For instance, at least three vertebral segments of spinal cord inflammation/atrophy must be demonstrated in LETM and in APS, a dorsal medulla lesion must be present.3 Lesions considered typical for MS can be seen in approximately 10% of cases but there are specific MRI features (lesions adjacent to body of lateral ventricle and in inferior temporal lobe, S-shaped U-fibre lesions, and presence of Dawson’s finger-type lesions) that help distinguish MS from NMOSD.31,32 Table Table333 summarises the differences between NMOSD and MS.
Table 3.
Differences between neuromyelitis optica spectrum disorder and multiple sclerosis
| MS | NMOSD | |
|---|---|---|
| Frequency of disease | Common | Rare |
| Latitude gradient | Present | Not proven |
| Female sex, % | 70 | 90 |
| Ethnic variation | Common in Caucasians | Common in Africans and Asians |
| Age at onset | 20–40 | 40–60 |
| Progressive course | Common | Rare |
| Coexistent autoimmune disease | Rare | Common: MG, SLE, Sjogren’s, thyroid, APL |
| Tissue involvement | White matter | White and gray matter |
| Necrosis/cavitation | Rare | Common |
| Leukocyte infiltrate | T and B lymphocytes | Neutrophils and eosinophils |
| Perivascular IgG and complement | Common | Common |
| Attack severity | Often mild | Often severe |
| Spinal cord | Short-segment peripheral cord lesions | Usually LETM, central cord involvement |
| May be asymptomatic | Extension into medulla | |
| Usually symptomatic | ||
| Acute T1 hypointensity | ||
| Optic nerve | Short segment inflammation, anterior | Long segment inflammation posterior |
| Unilateral | Unilateral/bilateral | |
| Good recovery | Poor recovery | |
| Brainstem | Any location | Area postrema/dorsal medulla MRI lesion |
| Ventral or dorsal pontine lesion | May be contiguous with spinal lesion | |
| Clearly defined borders | ||
| Diencephalon | Uncommon | Hypothalamic, thalamic, periependymal 3rd ventricle region |
| Corpus callosum | Very common | Uncommon |
| Small lesions | Long lesions | |
| Anterior/posterior CC | Callosal-septal interface in middle and posterior thirds of CC | |
| Cerebral hemispheres | Ovoid lesions perpendicular to lateral ventricle (Dawson’s fingers) | Large, confluent subcortical or deep white matter lesions |
| Lesion adjacent to body of lateral ventricle and in inferior temporal lobe | Long corticospinal tract lesions | |
| Juxtacortical U-fibre lesions | ||
| CSF | Mild pleocytosis; mononuclearOCBs 85% | Occasional prominent pleocytosis, lymphocytes, PMN and mononuclear cells |
| OCBs uncommon | ||
| Permanent disability | Usually in later progressive disease phase | Usually attack-related |
| Treatment | ||
| Relapse | Consider steroids (5 days) for severe acute relapses | High dose steroids |
| Earlier treatment is essential. Escalation to PLEX if needed | ||
| Long-term | In the UK disease modifying therapies are considered in active MS (≥2 relapses in 2 years) | AZA/MMF/RTX for all AQP4-Ab positive NMOSD or relapsing seronegative NMOSD |
APL = antiphospholipid syndrome; AQP4-Ab = aquaporin-4 antibodies; AZA = azathioprine; CC = corpus callosum; CSF = cerebrospinal fluid; Ig = immunoglobulin; LETM = longitudinally extensive transverse myelitis; MAC = membrane attack complex; MG = myasthenia gravis; MMF = mycophenolate mofetil; MRI = magnetic resonance imaging; OCBs = oligoclonal bands; PLEX = plasma exchange; PMN = polymorphonuclear cells; RRMS = relapsing remitting multiple sclerosis; RTX = rituximab; SLE = systemic lupus erythematosus.
Table 4.
Which patients should be tested for aquaporin-4 antibodies?
| When to test for aquaporin-4 antibodies† |
 1. LETM or ≥ 3 contiguous segments of focal cord atrophy on MRI spine 1. LETM or ≥ 3 contiguous segments of focal cord atrophy on MRI spine |
 2. ‘Idiopathic’ acute transverse myelitis without MS features 2. ‘Idiopathic’ acute transverse myelitis without MS features |
 3. Severe unilateral optic neuritis with poor recovery, and/or involving posterior visual pathway, eg optic chiasm 3. Severe unilateral optic neuritis with poor recovery, and/or involving posterior visual pathway, eg optic chiasm |
 4. Bilateral simultaneous or sequential optic neuritis 4. Bilateral simultaneous or sequential optic neuritis |
 5. Intractable nausea, vomiting, hiccoughs without clear explanation 5. Intractable nausea, vomiting, hiccoughs without clear explanation |
 6. Dorsal medullary lesion on MRI brain 6. Dorsal medullary lesion on MRI brain |
 7. Diencephalic clinical syndrome 7. Diencephalic clinical syndrome |
 8. Cryptogenic leukoencephalopathy 8. Cryptogenic leukoencephalopathy |
 9. MS apparently unresponsive/worsening after starting MS disease modifying therapies 9. MS apparently unresponsive/worsening after starting MS disease modifying therapies |
| 10. CNS inflammation atypical for MS without CSF oligoclonal bands |
| If initial testing is negative, the sensitivity of AQP4-Ab detection is increased with repeat testing 3–6 months later |
†It is reasonable to test for myelin oligodendrocyte glycoprotein antibodies if testing for aquaporin-4 antibodies. AQP4-Ab = aquaporin-4; CNS = central nervous system; CSF = cerebrospinal fluid; LETM = longitudinally extensive transverse myelitis; MRI = magnetic resonance imaging; MS = multiple sclerosis. Adapted from Whittam et al60
Of available AQP4-Ab assays, the cell based assay (CBA) has the highest sensitivity (76.7%) and specificity (99.8%).33 The impact and discriminatory power of detecting AQP4-Abs has likely contributed to the increase in rates of NMOSD diagnosis.34,35 Furthermore, following an episode of LETM the presence of AQP4-Abs confers a 50% risk of further relapse in the next 12 months.36 The presence of non-organ specific antibodies eg anti-nuclear, anti-double-stranded DNA, anti-Ro, anti-La may also be seen.3,22 Cerebrospinal fluid typically contains a mixed pleocytosis (lymphocytes, neutrophils, and monocytes) with a median cell count of 19 cells/μl (range: 6-380). Unlike MS, CSF-restricted oligoclonal bands are uncommon.37 Other useful investigations include visual evoked potentials and optical coherence tomography.38
MOG-antibodies
In 2012 some patients with seronegative NMOSD were found to have MOG-Abs. MOG is expressed on the surface of oligodendrocytes and myelin in the CNS. Though postulated as a candidate autoantigen in CNS autoimmunity for almost 30 years, the advent of the CBA has facilitated the detection of Abs against human MOG expressed in its conformational state, which is essential for diagnostic relevance. MOG-Abs account for approximately 40% of NMOSD patients who are seronegative for AQP4.39 It is therefore reasonable when suspecting NMOSD to test for both AQP4-Abs and MOG-Abs (Table (Table22).
The most frequent presentation of MOG-Ab-associated disease is acute disseminated encephalomyelitis (ADEM) in children under 7 years, and optic neuritis in older children and adults.40,41 MOG-Abs appear to have a predilection for inflammation of the anterior segments of the optic nerve, in contrast to NMOSD with AQP4-Abs, which tends to involve the chiasm and optic tracts.42 Visual outcomes are generally better but visual disability is not uncommon. Although motor outcomes after myelitis are also better, patients can be left with significant sphincter and erectile dysfunction.41 Seizures can also occur as part of an encephalitis-like presentation with cortical involvement, more commonly than with AQP4-Abs.43,44 Importantly a larger proportion of patients with MOG-Abs may have monophasic disease with transient presence of MOG-Abs, meaning that long-term immunosuppression is not always required.41 It is becoming increasingly clear that despite the clinical overlap between CNS inflammation with AQP4- and MOG-Abs there are important biological differences including; disease mechanism, clinical phenotype, relapse rates, and treatment response. It has therefore been suggested that the 2015 NMOSD diagnostic criteria may need revision to account for these important differences.45
Acute treatment
Acute treatment of an NMOSD attack consists of high dose steroids (HDS), typically 1 gram of intravenous methylprednisolone daily for 5 days; oral prednisolone 1 mg/kg is then continued for weeks, followed by gradual taper over months. Earlier treatment is ideal and with severe neurological deficits, if improvement is not seen within days of HDS, plasma exchange (PLEX; 5 cycles) should be commenced. Escalation therapy has been shown to increase response/remission rates and should be offered in appropriate cases.46 There is some data to support PLEX as first-line treatment for relapses, particularly myelitis, but prospective randomised trials are required to substantiate this observation.47 Thromboprophylaxis is advised, particularly in non-ambulant patients with myelitis.
Long-term treatment
Untreated, approximately 50% of NMOSD patients will be wheelchair users and blind, and a third will have died within 5 years of their first attack.48 We therefore treat all patients with AQP4-Abs at their first attack with long-term immunosuppression. The most commonly used first line immunosuppressants (IS) in NMOSD are mycophenolate mofetil (MMF; 2–3 grams/day) and azathioprine (AZA; 2.5–3 mg/kg). Retrospective data suggest that MMF may be superior to AZA (reduction in relapse rate 87.4% versus 72.1% respectively) but prospective data are lacking.49 AZA is often selected in younger female patients as MMF is contraindicated in pregnancy. It should also be noted that MMF can have spermatotoxic effects. Oral prednisolone (5–10 mg) is often given long-term as the combination may be more protective than MMF/AZA alone.50 Importantly, steroids should be given to cover the period of time it takes for AZA/MMF to take full effect (with AZA a 5 fL rise in mean cell volume or mild absolute lymphocyte count suppression are useful indicators of this). Currently in the UK, the B-cell depleting monoclonal antibody, rituximab (RTX) is used as a second-line medication. RTX reduces relapse rates up to 88.2% and is either given 6-monthly or according to monitored B-cell counts (CD19+ lymphocytes).51 Our practice is to re-treat if CD19 counts rise above 1% of the total B-cell population, but other treatment paradigms exist.50 We also periodically check serum immunoglobulin levels as some patients may develop symptomatic secondary hypogammaglobulinaemia.52
Other IS that are occasionally used include tocilizumab, methotrexate, cyclophosphamide, mitoxantrone, intravenous immunoglobulins, tacrolimus, and ciclosporin. Importantly, many of the disease modifying drugs used in the treatment of MS have been associated with futility or exacerbation in NMOSD, and should be avoided eg beta-interferon, fingolimod, and natalizumab.53–55
A detailed review of general symptom management in NMOSD is beyond the scope of this article but three symptoms deserve special mention. Tonic spasms following transverse myelitis can often effectively be treated with a low dose of carbamazepine.50 Neuropathic pain is more severe and disabling as compared with MS and early involvement of a local pain team is helpful.56 Moderate to severe depression and fatigue are common, associated with neuropathic pain and challenging to treat.57 Ideally a multidisciplinary approach with regular review of the effectiveness of pharmacotherapies is recommended.
Preliminary results of the first multi-centre, randomised, double-blind, placebo-controlled trial in NMOSD were recently reported (NCT01892345).58 Publication of the full trial results are awaited but the initial results support the use of eculizumab in the armamentarium of drugs for NMOSD, though its expense is a concern. At the time of review, clinical trials assessing the efficacy of SA237 (satralizumab – anti-interleukin-6 receptor; NCT02073279 and NCT0202884) and MEDI-551 (inebilizumab – humanised monoclonal antibody against CD19; NCT002200770) also reported positive outcomes with reduction in relapse rates between 70–90%.
UK NMOSD service
The UK NMOSD service (www.nmouk.nhs.uk) is funded by NHS England Highly Specialised Services, and aims to provide high-quality, up-to-date, evidence-based multidisciplinary care. The team consists of adult and paediatric neurologists, clinical fellows, nurse specialists, occupational therapists, physiotherapists, ophthalmologists/orthoptists, dieticians, and clinical psychologists. The service operates out of the Walton Centre Foundation Trust in Liverpool and John Radcliffe Hospital in Oxford but telephone/email advice, home visits, and satellite clinics help to ensure countrywide accessibility of care. Referrals are accepted from GPs and all medical specialities. The NMOSD service has also produced guidelines to unify treatment approaches across the UK, runs educations days, webinars, and provides direct patient access. Relapse rates and morbidity from NMOSD have both improved since the introduction of the service in 2010.59 The service has an active research portfolio and has facilitated the development of the NMO Spectrum-UK charity (www.nmouk.org). It has also received a Royal College of Physicians Excellence in Patient Care Award in 2016.
Take-home messages
Neuromyelitis optica spectrum disorder (NMOSD) is a relapsing central nervous system disease associated with aquaporin-4 antibodies.
Common presentations include longitudinally extensive myelitis, severe optic neuritis, and area postrema syndrome.
Prompt and aggressive treatment of relapses with high dose steroids +/- plasma exchange improves outcomes.
All patients with aquaporin-4 antibodies should be immunosuppressed indefinitely to prevent further attacks.
The NMOSD service based in Liverpool (Walton Centre NHS Foundation Trust) and Oxford (John Radcliffe Hospital) provides a quaternary service for all NMOSD patients in the UK. Referrals are accepted from GPs and all medical specialties.
Conflicts of interest
Dr Jacob has received research grants from Biogen Idec, Alexion Pharmaceuticals and speaker fees from Biogen, Chugai, Sanofi-Genzyme, and Terumo-BCT.
References
Articles from Clinical Medicine are provided here courtesy of Royal College of Physicians
Full text links
Read article at publisher's site: https://doi.org/10.7861/clinmedicine.19-2-169
Read article for free, from open access legal sources, via Unpaywall:
https://www.rcpjournals.org/content/clinmedicine/19/2/169.full.pdf
Citations & impact
Impact metrics
Article citations
Assessment of disability and disease burden in neuromyelitis optica spectrum disorders in the CIRCLES Cohort.
Sci Rep, 14(1):26150, 30 Oct 2024
Cited by: 0 articles | PMID: 39477975 | PMCID: PMC11525583
A Case of Myelin Oligodendrocyte Glycoprotein Antibody Disease in a Pediatric Patient: Clinical Presentation, Treatment Response, and Follow-Up Considerations.
Cureus, 16(9):e70518, 30 Sep 2024
Cited by: 0 articles | PMID: 39479073 | PMCID: PMC11524172
CXCR2 mediated trafficking of neutrophils and neutrophil extracellular traps are required for myelin clearance after a peripheral nerve injury.
Exp Neurol, 382:114985, 03 Oct 2024
Cited by: 0 articles | PMID: 39368532
Neuromyelitis Optica: A Peek Into the Brain Through the Eyes.
Cureus, 16(8):e67408, 21 Aug 2024
Cited by: 0 articles | PMID: 39310466 | PMCID: PMC11414768
HLA-transgenic mouse models to study autoimmune central nervous system diseases.
Autoimmunity, 57(1):2387414, 21 Aug 2024
Cited by: 0 articles | PMID: 39167553
Review
Go to all (92) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials (2)
- (1 citation) ClinicalTrials.gov - NCT02073279
- (1 citation) ClinicalTrials.gov - NCT01892345
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Do modern therapies change natural history of Neuromyelitis optica?
Rev Neurol (Paris), 177(5):567-570, 18 Aug 2020
Cited by: 2 articles | PMID: 32826068
Review
MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: Epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome.
J Neuroinflammation, 13(1):280, 27 Sep 2016
Cited by: 370 articles | PMID: 27793206 | PMCID: PMC5086042
Clinicoradiological comparative study of Aquaporin-4-IgG seropositive neuromyelitis optica spectrum disorder (NMOSD) and MOG antibody associated disease (MOGAD): A prospective observational study and review of literature.
J Neuroimmunol, 361:577742, 08 Oct 2021
Cited by: 8 articles | PMID: 34655992
Seropositive Neuromyelitis Optica in a Case of Undiagnosed Ankylosing Spondylitis: A Neuro-Rheumatological Conundrum.
Qatar Med J, 2022(3):29, 07 Jul 2022
Cited by: 1 article | PMID: 35864917 | PMCID: PMC9272764





