Abstract
Free full text

Evolution of the immunoglobulin kappa light chain locus in the rabbit: evidence for differential gene conversion events.
Abstract
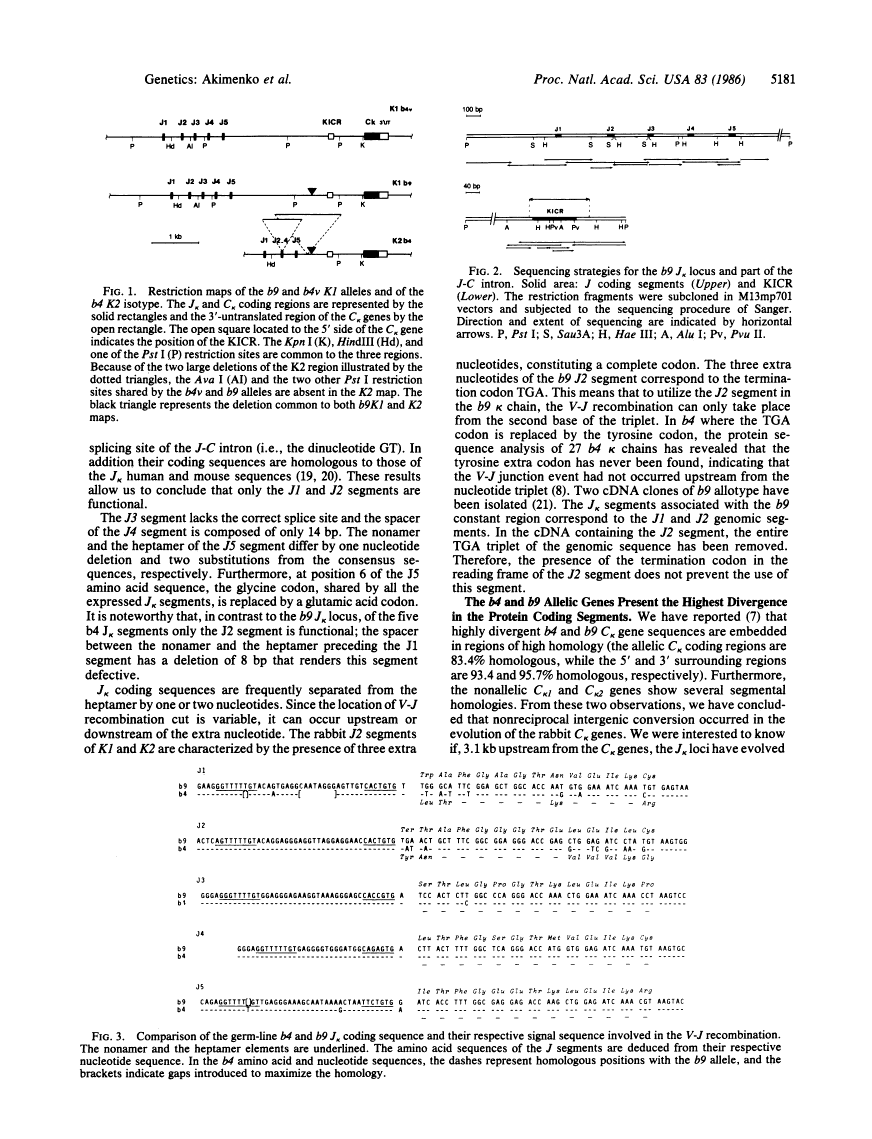
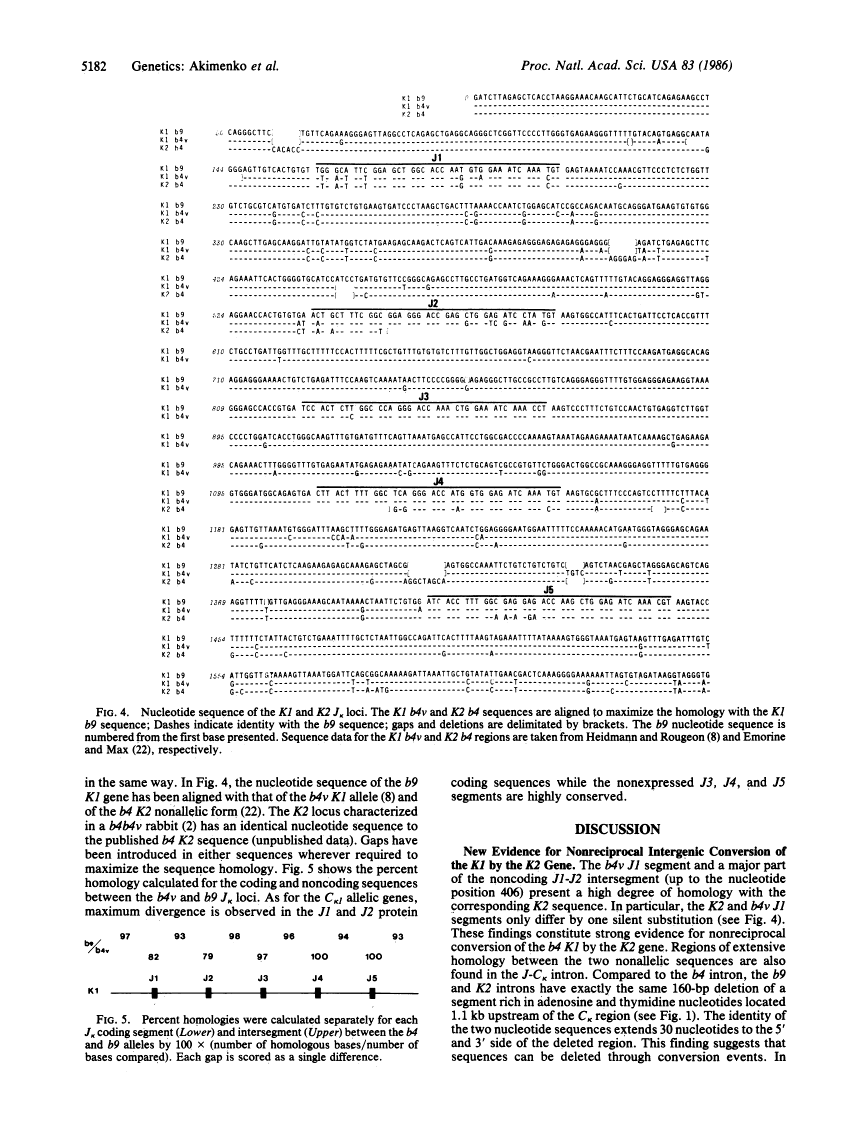
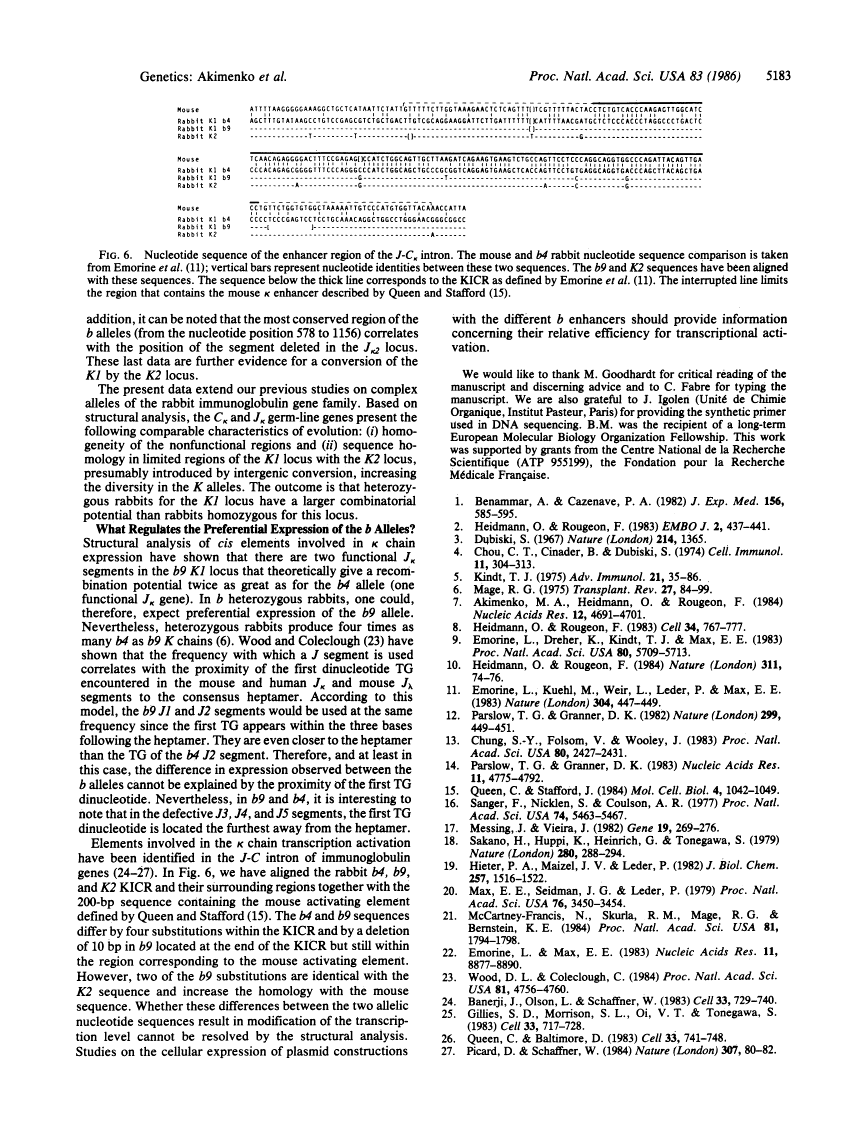
The rabbit kappa light chain gene family is characterized by the presence of two constant region (C kappa) genes; the C kappa 1 gene encodes the constant region of the principal rabbit immunoglobulin light chain, the C kappa 2 gene being not or very poorly expressed in domestic rabbits. There exist four major K1 alleles (b4, b5, b6, and b9), which are unequally expressed in heterozygous rabbits at the K1 locus. Here, we compare the nucleotide sequences of the joining (J) clusters of the kappa light chain gene (J kappa) linked to the b4K2 locus and to the b4 and b9 alleles at the K1 locus. As for C kappa genes, there is evidence for intergenic conversion between the J kappa 1 and J kappa 2 clusters as well as maximum divergence in the expressed J segments. The b9 J kappa 1 cluster differs from its b4 counterpart in that two out of the five J kappa segments (J1 and J2) are expressed instead of only one. This implies that preferential expression of the b4 allele as compared to the b9 allele is not only correlated to the number of available J kappa pieces. The b9 J2 segment is functional in spite of the presence of a termination codon immediately upstream of its coding region. Two major structural differences were observed between the J-C intron sequences of the b9 and b4 alleles; namely a 160-base-pair deletion of an A + T-rich sequence in b9 (which also occurs in the K2 locus) and a 10-base-pair deletion plus some substitutions in the region corresponding to the mouse kappa intron activating element. These differences could underlie the lower transcriptional rate of the b9 allele.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (827K), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benammar A, Cazenave PA. A second rabbit kappa isotype. J Exp Med. 1982 Aug 1;156(2):585–595. [Europe PMC free article] [Abstract] [Google Scholar]
- Heidmann O, Rougeon F. Multiplicity of constant kappa light chain genes in the rabbit genome: a b4b4 homozygous rabbit contains a kappa-bas gene. EMBO J. 1983;2(3):437–441. [Europe PMC free article] [Abstract] [Google Scholar]
- Dubiski S. Suppression of synthesis of allotypically defined immunoglobulins and compensation by another sub-class of immunoglobulin. Nature. 1967 Jun 24;214(5095):1365–1366. [Abstract] [Google Scholar]
- Chou CT, Cinader B, Dubiski S. Unequal expression of allelic allotypic specificities in circulating immunoglobulins, experimentally-elicited antibodies, and receptor-carrying cells. Cell Immunol. 1974 Mar 30;11(1-3):304–313. [Abstract] [Google Scholar]
- Kindt TJ. Rabbit immunoglobulin allotypes: structure, immunology, and genetics. Adv Immunol. 1975;21:35–86. [Europe PMC free article] [Abstract] [Google Scholar]
- Mage RG. Allotype suppression in rabbits: effects of anti-allotype antisera upon expression of immunoglobulin genes. Transplant Rev. 1975;27:84–99. [Abstract] [Google Scholar]
- Akimenko MA, Heidmann O, Rougeon F. Complex allotypes of the rabbit immunoglobulin kappa light chains are encoded by structural alleles. Nucleic Acids Res. 1984 Jun 11;12(11):4691–4701. [Europe PMC free article] [Abstract] [Google Scholar]
- Heidmann O, Rougeon F. Diversity in the rabbit immunoglobulin kappa chain variable regions is amplified by nucleotide deletions and insertions at the V-J junction. Cell. 1983 Oct;34(3):767–777. [Abstract] [Google Scholar]
- Emorine L, Dreher K, Kindt TJ, Max EE. Rabbit immunoglobulin kappa genes: structure of a germline b4 allotype J-C locus and evidence for several b4-related sequences in the rabbit genome. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5709–5713. [Europe PMC free article] [Abstract] [Google Scholar]
- Heidmann O, Rougeon F. Immunoglobulin kappa light-chain diversity in rabbit is based on the 3' length heterogeneity of germ-line variable genes. Nature. 1984 Sep 6;311(5981):74–76. [Abstract] [Google Scholar]
- Emorine L, Kuehl M, Weir L, Leder P, Max EE. A conserved sequence in the immunoglobulin J kappa-C kappa intron: possible enhancer element. Nature. 1983 Aug 4;304(5925):447–449. [Abstract] [Google Scholar]
- Parslow TG, Granner DK. Chromatin changes accompany immunoglobulin kappa gene activation: a potential control region within the gene. Nature. 1982 Sep 30;299(5882):449–451. [Abstract] [Google Scholar]
- Chung SY, Folsom V, Wooley J. DNase I-hypersensitive sites in the chromatin of immunoglobulin kappa light chain genes. Proc Natl Acad Sci U S A. 1983 May;80(9):2427–2431. [Europe PMC free article] [Abstract] [Google Scholar]
- Parslow TG, Granner DK. Structure of a nuclease-sensitive region inside the immunoglobin kappa gene: evidence for a role in gene regulation. Nucleic Acids Res. 1983 Jul 25;11(14):4775–4792. [Europe PMC free article] [Abstract] [Google Scholar]
- Queen C, Stafford J. Fine mapping of an immunoglobulin gene activator. Mol Cell Biol. 1984 Jun;4(6):1042–1049. [Europe PMC free article] [Abstract] [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. [Europe PMC free article] [Abstract] [Google Scholar]
- Messing J, Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. [Abstract] [Google Scholar]
- Sakano H, Hüppi K, Heinrich G, Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. [Abstract] [Google Scholar]
- Hieter PA, Maizel JV, Jr, Leder P. Evolution of human immunoglobulin kappa J region genes. J Biol Chem. 1982 Feb 10;257(3):1516–1522. [Abstract] [Google Scholar]
- Max EE, Seidman JG, Leder P. Sequences of five potential recombination sites encoded close to an immunoglobulin kappa constant region gene. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3450–3454. [Europe PMC free article] [Abstract] [Google Scholar]
- McCartney-Francis N, Skurla RM, Jr, Mage RG, Bernstein KE. Kappa-chain allotypes and isotypes in the rabbit: cDNA sequences of clones encoding b9 suggest an evolutionary pathway and possible role of the interdomain disulfide bond in quantitative allotype expression. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1794–1798. [Europe PMC free article] [Abstract] [Google Scholar]
- Emorine L, Max EE. Structural analysis of a rabbit immunoglobulin kappa 2 J-C locus reveals multiple deletions. Nucleic Acids Res. 1983 Dec 20;11(24):8877–8890. [Europe PMC free article] [Abstract] [Google Scholar]
- Wood DL, Coleclough C. Different joining region J elements of the murine kappa immunoglobulin light chain locus are used at markedly different frequencies. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4756–4760. [Europe PMC free article] [Abstract] [Google Scholar]
- Banerji J, Olson L, Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983 Jul;33(3):729–740. [Abstract] [Google Scholar]
- Gillies SD, Morrison SL, Oi VT, Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983 Jul;33(3):717–728. [Abstract] [Google Scholar]
- Queen C, Baltimore D. Immunoglobulin gene transcription is activated by downstream sequence elements. Cell. 1983 Jul;33(3):741–748. [Abstract] [Google Scholar]
- Picard D, Schaffner W. A lymphocyte-specific enhancer in the mouse immunoglobulin kappa gene. Nature. 1984 Jan 5;307(5946):80–82. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.83.14.5180
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc323914?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
Evolutionary genomics of immunoglobulin-encoding Loci in vertebrates.
Curr Genomics, 13(2):95-102, 01 Apr 2012
Cited by: 16 articles | PMID: 23024601 | PMCID: PMC3308330
DNA deamination in immunity.
Immunol Rev, 203:80-97, 01 Feb 2005
Cited by: 35 articles | PMID: 15661023
Review
Allelic variation at the VHa locus in natural populations of rabbit (Oryctolagus cuniculus, L.).
J Immunol, 172(2):1044-1053, 01 Jan 2004
Cited by: 19 articles | PMID: 14707078
High affinity ScFvs from a single rabbit immunized with multiple haptens.
Biochem Biophys Res Commun, 268(2):398-404, 01 Feb 2000
Cited by: 35 articles | PMID: 10679216
Co-existence of somatic hypermutation and gene conversion in hypervariable regions of single Igkappa clones.
Immunology, 95(2):291-301, 01 Oct 1998
Cited by: 4 articles | PMID: 9824489
Go to all (18) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Identification of rabbit immunoglobulin latent Ckappa1 allotype genes alters the concept of allelic inheritance.
Mol Immunol, 35(14-15):965-976, 01 Oct 1998
Cited by: 0 articles | PMID: 9881692
The rabbit kappa 1 b5 immunoglobulin gene: another J region gene cluster with only one functional J gene segment?
J Immunol, 136(3):1107-1111, 01 Feb 1986
Cited by: 9 articles | PMID: 3079796
Interallelic and intergenic conversion events could induce differential evolution of the two rabbit immunoglobulin kappa light chain genes.
Nucleic Acids Res, 15(15):6171-6179, 01 Aug 1987
Cited by: 6 articles | PMID: 3114714 | PMCID: PMC306076
Evolution of variable and constant domains and joining segments of rearranging immunoglobulins.
FASEB J, 3(13):2469-2479, 01 Nov 1989
Cited by: 20 articles | PMID: 2509274
Review