Abstract
Free full text

Complete amino acid sequence of human thyroxine-binding globulin deduced from cloned DNA: close homology to the serine antiproteases.
Abstract
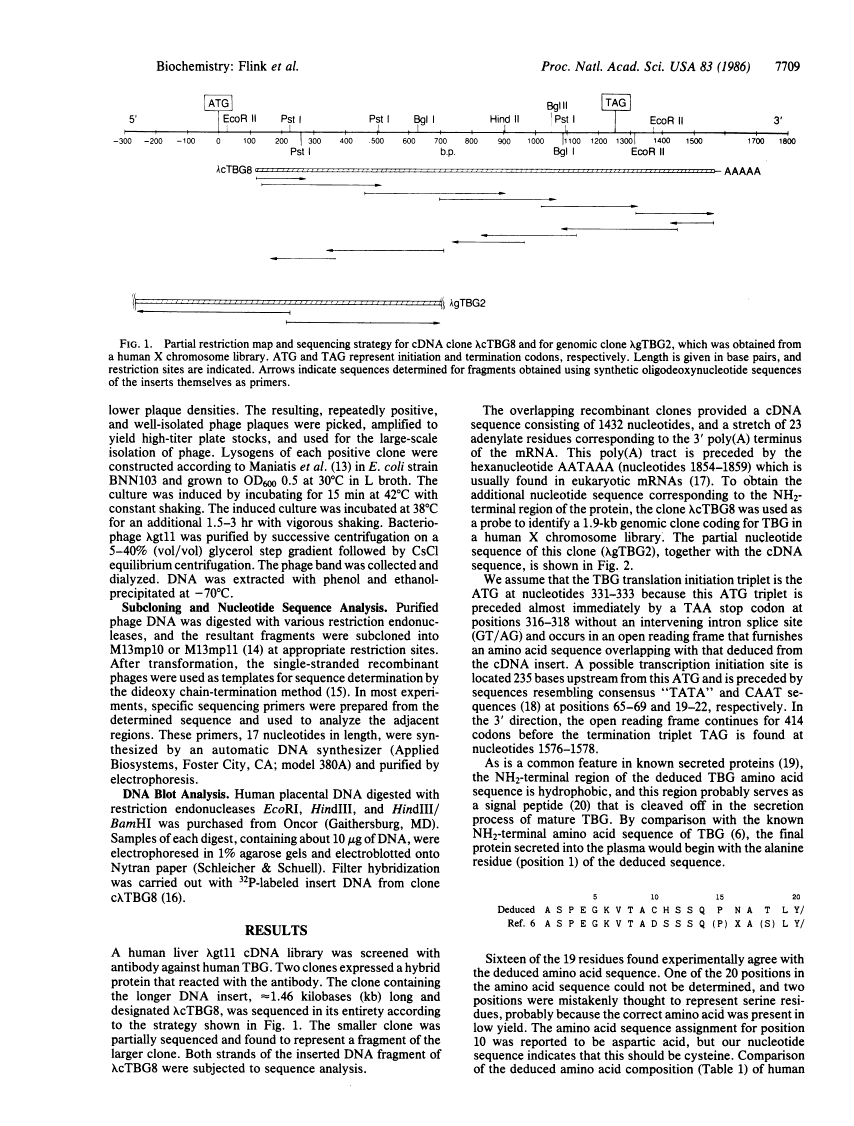
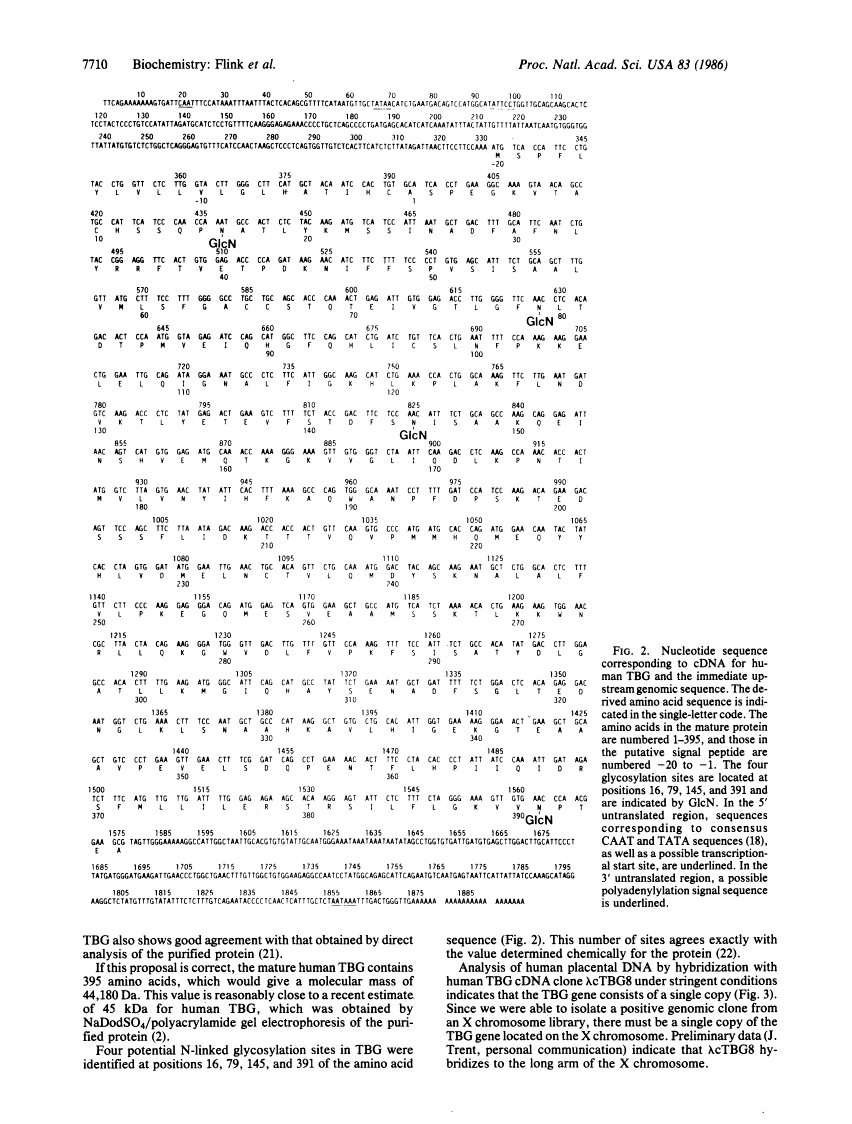
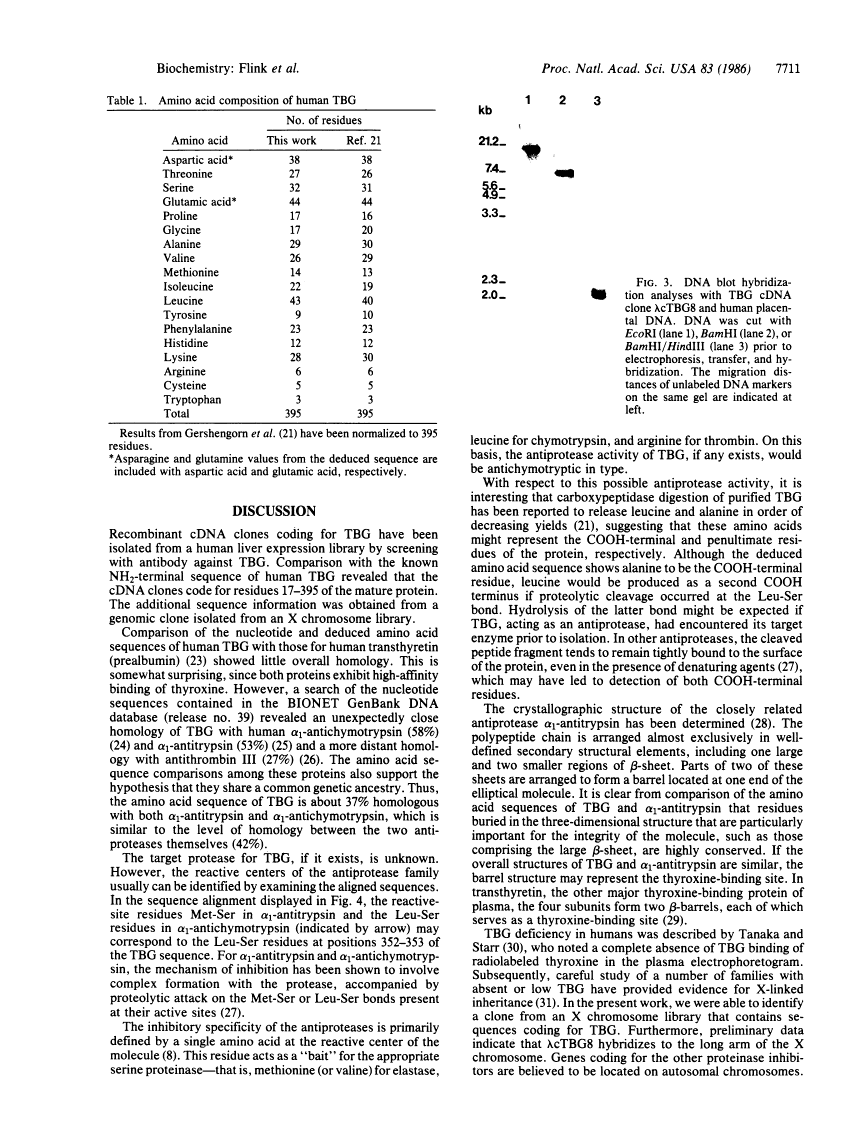
Antibodies directed against thyroxine-binding globulin (TBG) have been used to screen a human liver lambda gt11 expression library. A 1.46-kilobase clone was identified which encodes nearly the complete amino acid sequence, beginning at amino acid 17 of the mature protein. To complete the protein sequence, the cDNA clone was used to identify a genomic clone coding for TBG in a human X chromosome library. The overlapping recombinant clones contained an open reading frame coding for 415 amino acids followed by a polyadenylylation signal (AATAAA) located 275 nucleotides from a TAG termination codon. Beginning at residue 21, the deduced amino acid sequence agrees closely with the known NH2-terminal sequence of the mature peptide. The preceding 20 amino acid residues are hydrophobic in character and presumably represent a leader sequence. Four glycosylation sites were identified, corresponding to the number determined for the purified protein. DNA blot hybridization revealed a single-copy gene, which by chromosomal analysis was found to be located on the long arm of the X chromosome. Unexpectedly, the nucleotide sequence of TBG is closely homologous to those encoding the plasma serine antiproteases alpha 1-antichymotrypsin and alpha 1-antitrypsin. However, there is little overall homology between TBG and transthyretin (prealbumin), the other major thyroxine-binding protein of human plasma.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.0M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Refetoff S, Robin NI, Fang VS. Parameters of thyroid function in serum of 16 selected vertebrate species: a study of PBI, serum T4, free T4, and the pattern of T4 and T3 binding to serum proteins. Endocrinology. 1970 Apr;86(4):793–805. [Abstract] [Google Scholar]
- Bartalena L, Tata JR, Robbins J. Characterization of nascent and secreted thyroxine-binding globulin in cultured human hepatoma (Hep G2) cells. J Biol Chem. 1984 Nov 10;259(21):13605–13609. [Abstract] [Google Scholar]
- Marshall JS, Pensky J, Williams S. Studies on human thyroxine-binding globulin. 8. Isoelectric focusing evidence for microheterogeneity of thyroxine-binding globulin. Arch Biochem Biophys. 1973 Jun;156(2):456–462. [Abstract] [Google Scholar]
- Gärtner R, Henze R, Horn K, Pickardt CR, Scriba PC. Thyroxine-binding globulin: investigation of microheterogeneity. J Clin Endocrinol Metab. 1981 Apr;52(4):657–664. [Abstract] [Google Scholar]
- Young RA, Davis RW. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. [Abstract] [Google Scholar]
- Cheng SY. Partial amino acid sequence of human thyroxine-binding globulin. Further evidence for a single polypeptide chain. Biochem Biophys Res Commun. 1977 Dec 21;79(4):1212–1218. [Abstract] [Google Scholar]
- Hunt LT, Dayhoff MO. A surprising new protein superfamily containing ovalbumin, antithrombin-III, and alpha 1-proteinase inhibitor. Biochem Biophys Res Commun. 1980 Jul 31;95(2):864–871. [Abstract] [Google Scholar]
- Travis J, Salvesen GS. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. [Abstract] [Google Scholar]
- Chandra T, Stackhouse R, Kidd VJ, Woo SL. Isolation and sequence characterization of a cDNA clone of human antithrombin III. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1845–1848. [Europe PMC free article] [Abstract] [Google Scholar]
- Davies KE, Young BD, Elles RG, Hill ME, Williamson R. Cloning of a representative genomic library of the human X chromosome after sorting by flow cytometry. Nature. 1981 Oct 1;293(5831):374–376. [Abstract] [Google Scholar]
- Benton WD, Davis RW. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. [Abstract] [Google Scholar]
- Rigby PW, Dieckmann M, Rhodes C, Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. [Abstract] [Google Scholar]
- Messing J, Crea R, Seeburg PH. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. [Europe PMC free article] [Abstract] [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. [Europe PMC free article] [Abstract] [Google Scholar]
- Southern EM. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. [Abstract] [Google Scholar]
- Proudfoot NJ, Brownlee GG. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. [Abstract] [Google Scholar]
- Breathnach R, Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. [Abstract] [Google Scholar]
- Kreil G. Transfer of proteins across membranes. Annu Rev Biochem. 1981;50:317–348. [Abstract] [Google Scholar]
- Silhavy TJ, Benson SA, Emr SD. Mechanisms of protein localization. Microbiol Rev. 1983 Sep;47(3):313–344. [Europe PMC free article] [Abstract] [Google Scholar]
- Gershengorn MC, Cheng SY, Lippoldt RE, Lord RS, Robbins J. Characterization of human thyroxine-binding globulin. Evidence for a single polypeptide chain. J Biol Chem. 1977 Dec 10;252(23):8713–8718. [Abstract] [Google Scholar]
- Zinn AB, Marshall JS, Carlson DM. Preparation of glycopeptides and oligosaccharides from thyroxine-binding globulin. J Biol Chem. 1978 Oct 10;253(19):6761–6767. [Abstract] [Google Scholar]
- Kanda Y, Goodman DS, Canfield RE, Morgan FJ. The amino acid sequence of human plasma prealbumin. J Biol Chem. 1974 Nov 10;249(21):6796–6805. [Abstract] [Google Scholar]
- Chandra T, Stackhouse R, Kidd VJ, Robson KJ, Woo SL. Sequence homology between human alpha 1-antichymotrypsin, alpha 1-antitrypsin, and antithrombin III. Biochemistry. 1983 Oct 25;22(22):5055–5061. [Abstract] [Google Scholar]
- Colau B, Chuchana P, Bollen A. Revised sequence of full-length complementary DNA coding for human alpha 1-antitrypsin. DNA. 1984 Aug;3(4):327–330. [Abstract] [Google Scholar]
- Bock SC, Wion KL, Vehar GA, Lawn RM. Cloning and expression of the cDNA for human antithrombin III. Nucleic Acids Res. 1982 Dec 20;10(24):8113–8125. [Europe PMC free article] [Abstract] [Google Scholar]
- Morii M, Travis J. Amino acid sequence at the reactive site of human alpha 1-antichymotrypsin. J Biol Chem. 1983 Nov 10;258(21):12749–12752. [Abstract] [Google Scholar]
- Loebermann H, Tokuoka R, Deisenhofer J, Huber R. Human alpha 1-proteinase inhibitor. Crystal structure analysis of two crystal modifications, molecular model and preliminary analysis of the implications for function. J Mol Biol. 1984 Aug 15;177(3):531–557. [Abstract] [Google Scholar]
- Blake CC, Geisow MJ, Swan ID, Rerat C, Rerat B. Strjcture of human plasma prealbumin at 2-5 A resolution. A preliminary report on the polypeptide chain conformation, quaternary structure and thyroxine binding. J Mol Biol. 1974 Sep 5;88(1):1–12. [Abstract] [Google Scholar]
- TANAKA S, STARR P. Clinical observations on serum globulin thyroxine-binding capacity, using a simplified technique. J Clin Endocrinol Metab. 1959 Jan;19(1):84–91. [Abstract] [Google Scholar]
- Refetoff S, Robin NI, Alper CA. Study of four new kindreds with inherited thyroxine-binding globulin abnormalities. Possible mutations of a single gene locus. J Clin Invest. 1972 Apr;51(4):848–867. [Europe PMC free article] [Abstract] [Google Scholar]
- Cox DW, Markovic VD, Teshima IE. Genes for immunoglobulin heavy chains and for alpha 1-antitrypsin are localized to specific regions of chromosome 14q. Nature. 1982 Jun 3;297(5865):428–430. [Abstract] [Google Scholar]
- Burr WA, Ramsden DB, Hoffenberg R. Hereditary abnormalities of thyroxine-binding globulin concentration. A study of 19 kindreds with inherited increase or decrease of thyroxine-binding globulin. Q J Med. 1980;49(195):295–313. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.83.20.7708
Read article for free, from open access legal sources, via Unpaywall:
https://www.pnas.org/content/pnas/83/20/7708.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1073/pnas.83.20.7708
Article citations
Partial Thyroid Hormone-Binding Globulin Deficiency: A Case Report and Literature Review.
Diabetes Metab Syndr Obes, 16:2225-2232, 26 Jul 2023
Cited by: 0 articles | PMID: 37525823 | PMCID: PMC10387242
The Serpin Superfamily and Their Role in the Regulation and Dysfunction of Serine Protease Activity in COPD and Other Chronic Lung Diseases.
Int J Mol Sci, 22(12):6351, 14 Jun 2021
Cited by: 32 articles | PMID: 34198546 | PMCID: PMC8231800
Review Free full text in Europe PMC
Compound hemizygous variants in SERPINA7 gene cause thyroxine-binding globulin deficiency.
Mol Genet Genomic Med, 9(2):e1571, 07 Feb 2021
Cited by: 4 articles | PMID: 33554479 | PMCID: PMC8077092
A review of species differences in the control of, and response to, chemical-induced thyroid hormone perturbations leading to thyroid cancer.
Arch Toxicol, 95(3):807-836, 05 Jan 2021
Cited by: 9 articles | PMID: 33398420
Review
Evolution of thyroid hormone distributor proteins.
Mol Cell Endocrinol, 459:43-52, 27 Feb 2017
Cited by: 14 articles | PMID: 28249735
Review
Go to all (65) article citations
Other citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Molecular cloning and primary structure of rat thyroxine-binding globulin.
Biochemistry, 30(22):5406-5411, 01 Jun 1991
Cited by: 10 articles | PMID: 1903654
Replacement of Leu227 by Pro in thyroxine-binding globulin (TBG) is associated with complete TBG deficiency in three of eight families with this inherited defect.
J Clin Endocrinol Metab, 70(3):804-809, 01 Mar 1990
Cited by: 27 articles | PMID: 2155256
Nucleotide sequence of cloned cDNA for human sphingolipid activator protein 1 precursor.
Proc Natl Acad Sci U S A, 84(23):8652-8656, 01 Dec 1987
Cited by: 17 articles | PMID: 2825202 | PMCID: PMC299604
[Cloning of human thyroxine-binding globulin cDNA, isolation of the gene, and its transcriptional regulation].
Nihon Rinsho, 52(4):875-879, 01 Apr 1994
Cited by: 1 article | PMID: 8196173
Review
Funding
Funders who supported this work.
NCRR NIH HHS (1)
Grant ID: IU42 RR-01685-02
NHLBI NIH HHS (2)
Grant ID: 5 P01 HL20984-09
Grant ID: R01 HL35751-01