Abstract
Free full text
Liraglutide 3.0 mg for the management of insufficient weight loss or excessive weight regain post‐bariatric surgery
Summary
To assess the effectiveness of liraglutide 3.0 mg in post‐bariatric surgery patients, and to determine whether this would differ based on the type of bariatric surgery. One hundred seventeen post‐bariatric surgery patients from the Wharton Medical Clinic were analysed. Changes in weight while taking liraglutide 3.0 mg were examined for all patients, and by three types of bariatric surgery—Roux‐en‐Y gastric bypass, gastric banding and gastric sleeve. Patients primarily underwent Roux‐en‐Y gastric bypass (n = 53, 45.3%) or gastric banding (n = 50, 42.7%). Over 7.6 ±
± 7.1 months taking liraglutide 3.0 mg, patients lost a statistically significant amount of weight (−6.3
7.1 months taking liraglutide 3.0 mg, patients lost a statistically significant amount of weight (−6.3 ±
± 7.7 kg, P
7.7 kg, P <
< .05) regardless of the type of surgery they had (P
.05) regardless of the type of surgery they had (P >
> .05). This decrease in weight remained significant after 1‐year of taking liraglutide 3.0 mg (P
.05). This decrease in weight remained significant after 1‐year of taking liraglutide 3.0 mg (P <
< .05). Nausea was the most prevalent side effect, reported by 29.1% patients. While options for excess weight management in post‐bariatric surgery patients are limited, results of this study suggest that post‐bariatric surgery patients can lose a significant amount of weight while taking liraglutide 3.0 mg regardless of the type of surgery they had. Further, similar to non‐surgical populations, post‐bariatric surgery patients taking liraglutide 3.0 mg may experience gastrointestinal side effects such as nausea and can continue to lose weight up to 1 year.
.05). Nausea was the most prevalent side effect, reported by 29.1% patients. While options for excess weight management in post‐bariatric surgery patients are limited, results of this study suggest that post‐bariatric surgery patients can lose a significant amount of weight while taking liraglutide 3.0 mg regardless of the type of surgery they had. Further, similar to non‐surgical populations, post‐bariatric surgery patients taking liraglutide 3.0 mg may experience gastrointestinal side effects such as nausea and can continue to lose weight up to 1 year.
1. INTRODUCTION
Bariatric surgery is considered the gold standard treatment for weight loss in patients with obesity and complications of obesity, based on superior long‐term weight loss outcomes, and improvements in comorbidities compared to lifestyle and pharmaceutical interventions.1 Despite the dramatic weight loss achieved with bariatric surgery (30%‐45% weight loss2), 10% to 20% of bariatric surgery patients will regain a significant amount of the weight they had lost,3 which can be defined as a patient regaining 10% to 25% of excess weight or total weight lost.4 Regardless, as greater number of patients have surgery, the concern of weight regain post‐surgery has become significant.
In 2015, liraglutide 3.0 mg was approved for the treatment of excess weight in Canada.5 In non‐surgical populations, liraglutide 3.0 mg has been found to be associated with significant reductions in appetite and weight.6 However, as surgical patients were excluded from phase III weight management pharmaceuticals trials, the effectiveness of liraglutide 3.0 mg in this population is unknown. Thus, the objective of the current study was to examine the weight loss associated with the use of liraglutide 3.0 mg in post‐bariatric surgery patients.
2. METHODS
The Wharton Medical Clinic (WMC) are referral‐based secondary care clinics for weight and diabetes management located across southern Ontario. WMC provides care in accordance with the policies in the Canadian Clinical Practice Guidelines for the treatment of obesity in adults and children and National Institutes of Health Clinical Guidelines on the identification, evaluation and treatment of overweight and obesity in adults. All services provided to patients by the WMC are covered by the Ontario Health Insurance Program. Patients were informed that their participation or lack thereof would not hinder their medical treatment and provided written consent for access to their medical records to conduct research. All methods were approved by the York University Institution Review Board in accordance with the Tri‐Council Ethical Conduct for Research Involving Humans, version 2.
There was a total of 649 post‐bariatric surgery patients referred to WMC for weight management and who consented for their data to be used for research. Of these, 174 patients were prescribed liraglutide 3.0 mg for weight management. Patients were excluded if they did not initiate liraglutide 3.0 mg, resulting in 117 patients for analysis. Of the 57 patients who did not initiate liraglutide 3.0 mg, 10 were lost to follow‐up, and three initiated Contrave (an oral weight management medication). The remaining 44 patients participated in the lifestyle intervention at the clinic without adjunctive therapies. Over the 14.7 ±
± 24.0 months these patients attended the clinic, they lost 2.1
24.0 months these patients attended the clinic, they lost 2.1 ±
± 9.0 kg (P
9.0 kg (P >
> .05). The reason for not initiating liraglutide 3.0 mg was recorded for only six patients, and all stated it was due to cost.
.05). The reason for not initiating liraglutide 3.0 mg was recorded for only six patients, and all stated it was due to cost.
2.1. WMC protocol
WMC procedures have been previously discussed in greater detail elsewhere.7 During the initial visit to the clinic, patients complete a comprehensive intake questionnaire on demographics, and medical and weight management history. Anthropometric measurements are done on all patients by trained technicians. Height is measured by a wall‐mounted tape to the nearest 0.1 cm (McArthur Medical Sales, Inc., Ontario), and weight which is measured using a digital scale to the nearest 0.1 kg (Itin Scale Co, Inc., New York). Subsequently, patients attend an introductory educational session about WMC's policies and procedures, followed by an appointment with a bariatric educator and medical doctor. The bariatric educator provides weight management support by establishing dietary changes and exercise regimes. The medical doctor examines and reviews the patient's medical history, discusses pharmaceutical management or surgical intervention where clinically indicated. Patients who choose to initiate liraglutide 3.0 mg are recommended to follow a dosing schedule starting at 0.6 mg that is titrated 0.6 mg/mL until reaching the maximum clinical dosage of 3.0 mg, but this may vary in clinical practice.8 The physician orders blood work, resting metabolic rate test and other relevant tests for each patient.
Patients are given a meal plan at their third visit which includes a caloric deficit of approximately 500 kcal/day based on their daily energy expenditure. The bariatric educator reviews the meal plan with the patient and discusses food alternatives and snacks. The patient also meets with the physician who examines the patient, reviews the updated anthropometric measurements, and monitors comorbidities and pharmaceuticals.
kcal/day based on their daily energy expenditure. The bariatric educator reviews the meal plan with the patient and discusses food alternatives and snacks. The patient also meets with the physician who examines the patient, reviews the updated anthropometric measurements, and monitors comorbidities and pharmaceuticals.
Following the initial three visits, patients are encouraged to attend monthly appointments at the clinic; however, patients may attend more often if they desire. At all subsequent appointments patients meet with a physician and/or a bariatric educator for continued weight management support.
Weight (kg), height (cm), sex (female/male), age (years), treatment time (months) and type of bariatric surgery (ie, adjustable gastric banding (AGB), Roux‐en‐Y gastric bypass (RNYGB) and gastric sleeve), maximum dose achieved on liraglutide 3.0 mg, side effects, and reason for discontinuation were extracted from electronic medical records. Clinically significant weight loss was defined as >5% decrease in body weight as per clinical guidelines.9, 10 Percent change in weight from lowest weight post‐bariatric surgery to initiating liraglutide 3.0 mg was calculated as ([(maximum weight change post‐bariatric surgery)/(weight change from lowest post‐bariatric surgery weight to initiation of liraglutide 3.0 mg)] × 100). Patients reported more than 1 side effect, thus the percent of adverse events were calculated based on the total number of patients included in the study (ie, [(number of patients reporting the side effect)/117] × 100). Maximum clinical dose was dichotomized as achieving the recommended clinical dose of 3.0 mg (yes/no). All weight variables were measured by trained technicians with the exception of weight prior to bariatric surgery and the maximal weight lost with bariatric surgery which was self‐reported by patients.
2.2. Statistical analysis
Continuous variables are reported as mean ±
± SD and categorical variables as frequency (prevalence). Independent t test was undertaken to compare weight change between patients who did and did not achieve the recommended clinical dose of liraglutide 3.0 mg. One‐way analysis of variance (ANOVA) was used to examine differences in age, body mass index (BMI), treatment time, percent changes in weight, and the side effect nausea and repeated measures ANOVA was used to examine absolute changes in weight based on the type of bariatric surgery patients had underwent. Least‐square difference post‐hoc was used where indicated. Differences in sex and the proportion of individuals achieving ≥5% and ≥10% weight loss based on the type of bariatric surgery were assessed using chi‐square analysis. Changes in weight were compared in 2‐month increments from 1‐month post‐initiation up to 1 year based on the type of bariatric surgery using proc mixed. Changes in weight loss were also compared using proc mixed for patients who were persistent on liraglutide 3.0 mg for ≤2 months, >2 to ≤4 months, and >4 months using proc mixed. All analyses were conducted using SAS version 9.4 (SAS Institute). A P‐value ≤.05 was considered statistically significant.
SD and categorical variables as frequency (prevalence). Independent t test was undertaken to compare weight change between patients who did and did not achieve the recommended clinical dose of liraglutide 3.0 mg. One‐way analysis of variance (ANOVA) was used to examine differences in age, body mass index (BMI), treatment time, percent changes in weight, and the side effect nausea and repeated measures ANOVA was used to examine absolute changes in weight based on the type of bariatric surgery patients had underwent. Least‐square difference post‐hoc was used where indicated. Differences in sex and the proportion of individuals achieving ≥5% and ≥10% weight loss based on the type of bariatric surgery were assessed using chi‐square analysis. Changes in weight were compared in 2‐month increments from 1‐month post‐initiation up to 1 year based on the type of bariatric surgery using proc mixed. Changes in weight loss were also compared using proc mixed for patients who were persistent on liraglutide 3.0 mg for ≤2 months, >2 to ≤4 months, and >4 months using proc mixed. All analyses were conducted using SAS version 9.4 (SAS Institute). A P‐value ≤.05 was considered statistically significant.
3. RESULTS
One hundred seventeen patients were included in the analysis. Patients were primarily female (87.2%) and were 51.2 ± 9.4 years of age. The majority of patients underwent RNYGB (45.3%) or AGB (42.7%). Prior to initiating bariatric surgery, patients had a BMI of 49.7 ± 12.1 kg/m2 and lost 40.7 ±
12.1 kg/m2 and lost 40.7 ± 25.0 kg (28.8%) with surgery. Prior to initiating liraglutide 3.0 mg, patients had regained weight 21.2 ±
25.0 kg (28.8%) with surgery. Prior to initiating liraglutide 3.0 mg, patients had regained weight 21.2 ± 16.9 kg (58.6%) of their weight loss with an average BMI of 42.5 ± 9.6 kg/m2. Patients initiated liraglutide 3.0 mg approximately 8 years (7.8 ± 5.7 years, interquartile range: 4‐10 years) post‐bariatric surgery. Over 7.6 ± 7.1 months taking liraglutide 3.0 mg, patients lost 5.5 ± 6.2% (6.3 ± 7.7 kg, P <
16.9 kg (58.6%) of their weight loss with an average BMI of 42.5 ± 9.6 kg/m2. Patients initiated liraglutide 3.0 mg approximately 8 years (7.8 ± 5.7 years, interquartile range: 4‐10 years) post‐bariatric surgery. Over 7.6 ± 7.1 months taking liraglutide 3.0 mg, patients lost 5.5 ± 6.2% (6.3 ± 7.7 kg, P < .05) of their body weight.
.05) of their body weight.
Patients who had undergone RNYGB had a lower BMI prior to initiating liraglutide 3.0 mg than patients who had received a AGB or sleeve gastrectomy (P < .05, Table Table1).1). Regardless of the surgery, patients who took liraglutide 3.0 mg lost a similar amount of weight (P >
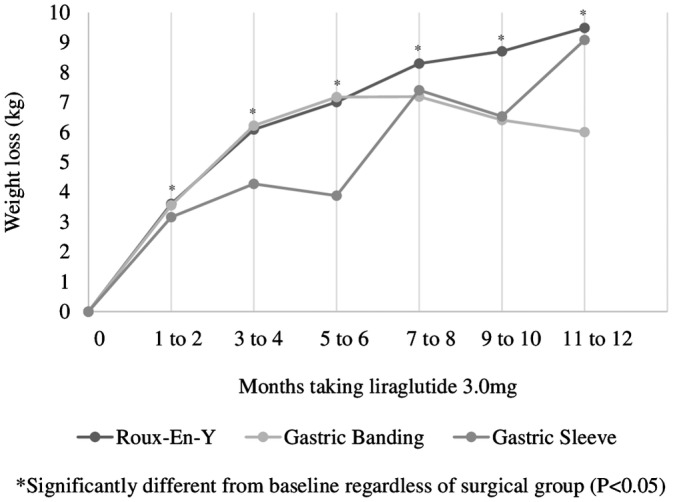
.05, Table Table1).1). Regardless of the surgery, patients who took liraglutide 3.0 mg lost a similar amount of weight (P > .05). Patients experienced a significant weight loss as early as 1 to 2 months post‐initiation of liraglutide 3.0 mg, which remained significant up to 1 year of taking the medication (P <
.05). Patients experienced a significant weight loss as early as 1 to 2 months post‐initiation of liraglutide 3.0 mg, which remained significant up to 1 year of taking the medication (P < .05) regardless of the type of bariatric surgery they had (P >
.05) regardless of the type of bariatric surgery they had (P > .05, Figure Figure1).1). Further, on average those persistent on liraglutide 3.0 mg lost significantly more weight by 11 to 12
.05, Figure Figure1).1). Further, on average those persistent on liraglutide 3.0 mg lost significantly more weight by 11 to 12 months post‐initiation than at 5 to 6 months (2.0 ± 7.4 kg, P =
months post‐initiation than at 5 to 6 months (2.0 ± 7.4 kg, P = .004). As expected, patients who discontinued liraglutide 3.0 mg within 4 months lost less weight early in the treatment as compared to patients who remained on the drug for greater than 4 months (data not shown, P <
.004). As expected, patients who discontinued liraglutide 3.0 mg within 4 months lost less weight early in the treatment as compared to patients who remained on the drug for greater than 4 months (data not shown, P < .05).
.05).
Table 1
Patient characteristics by type of bariatric surgery
| Variable | Roux‐en‐Y bypass | Gastric band | Gastric sleeve |
|---|---|---|---|
| Sample size (n) | 53 | 50 | 14 |
| Age (y) | 49.9 ± ± 9.1 9.1 | 52.5 ± ± 9.5 9.5 | 51.4 ± ± 10.3 10.3 |
| Men (n, %) | 3 (5.7)a | 8 (16.0) | 4 (28.6) |
| Pre‐bariatric surgery BMI (kg/m2) | 50.8 ± ± 11.2 11.2 | 47.6 ± ± 13.1e 13.1e
| 52.2 ± ± 11.9 11.9 |
| Maximum weight change post‐bariatric surgery (kg) | −51.6 ± ± 23.5a
,
b 23.5a
,
b
| −29.8 ± ± 23.3e 23.3e
| −34.7 ± ± 19.5 19.5 |
| Weight change from lowest post‐bariatric surgery weight to initiation of liraglutide 3.0 mg (kg) | 19.0 ± ± 13.5 13.5 | 25.4 ± ± 20.4e 20.4e
| 15.8 ± ± 14.1 14.1 |
| Weight change from lowest post‐bariatric surgery weight to initiation of liraglutide 3.0 mg (%)c | 44.8 ± ± 54.9a 54.9a
| 80.0 ± ± 79.7f 79.7f
| 48.4 ± ± 31.7g 31.7g
|
| Preliraglutide BMI (kg/m2) | 39.0 ± ± 7.0a
,
b 7.0a
,
b
| 45.4 ± ± 11.0 11.0 | 45.4 ± ± 9.6 9.6 |
| Weight change on liraglutide 3.0 mg (kg) | −7.1 ± ± 8.7d 8.7d
| −6.0 ± ± 7.2d 7.2d
| −4.5 ± ± 4.5d 4.5d
|
| Weight change on liraglutide 3.0 mg (%) | −6.6 ± ± 7.1 7.1 | −4.9 ± ± 5.6 5.6 | −3.6 ± ± 3.0 3.0 |
| Attained 5% weight loss (n, %) | 25 (47.2) | 19 (38.0) | 5 (35.7) |
| Attained 10% weight loss (n, %) | 13 (24.5)a | 6 (12.0) | 0 (0.0) |
| Treatment time (mo) | 8.0 ± ± 7.6 7.6 | 6.8 ± ± 6.7 6.7 | 8.6 ± ± 7.3 7.3 |
| Reported nausea with liraglutide 3.0 mg (n, %) | 15 (28.3) | 12 (24.0) | 5 (35.7) |
 .05).
.05). .05).
.05). .05).
.05).More than half of the participants (n = 73 of 117, 62.4%) achieved the recommended maximum clinical dose of 3.0 mg. Of the participants (n = 94 of 117, 80%) that had taken liraglutide 3.0 mg for long enough (ie, >1 month) to titrate to the recommended maximum clinical dose, 25.5% (n = 24 of 94) did not achieve the recommended maximum clinical dose. The proportion of patients who did not achieve the recommended maximum clinical dose of 3.0 mg was similar regardless of type of bariatric surgery (P = .29). Patients who were persistent on liraglutide 3.0 mg for more than 1 month (n = 94 of 117) lost a similar amount of weight whether or not they achieved the recommended maximum clinical dose (7.4 ± 6.9 vs 6.8 ±
.29). Patients who were persistent on liraglutide 3.0 mg for more than 1 month (n = 94 of 117) lost a similar amount of weight whether or not they achieved the recommended maximum clinical dose (7.4 ± 6.9 vs 6.8 ± 11.4, P =
11.4, P = .81).
.81).
A total of 92 side effects were reported among 59 of 117 patients (50.4%, Table Table2).2). Gastrointestinal side effects were the most prevalent, with the top three most frequently reported side effects being nausea (n = 34 of 117, 37.0%), constipation (n = 13 of 177, 14.1%) and diarrhoea (n = 8 of 117, 8.7%). A similar proportion of patients reported nausea as a side effect from taking liraglutide 3.0 mg regardless of the type of surgery they had undergone (P = .96). Other side effects were rare and were generally mild with the exception of one patient developing pancreatitis (0.9%).
.96). Other side effects were rare and were generally mild with the exception of one patient developing pancreatitis (0.9%).
Table 2
Side effects for liraglutide 3.0 mg
| Symptom | n (%)a |
|---|---|
| Nausea | 34 (29.1%) |
| Constipation | 13 (11.1%) |
| Diarrhoea | 8 (6.8%) |
| Fatigue | 7 (6.0%) |
| Headache | 4 (3.4%) |
| Rash | 4 (3.4%) |
| Indigestion | 3 (2.6%) |
| Vomiting | 3 (2.6%) |
| Dry mouth | 3 (2.6%) |
| Bloating | 2 (1.7%) |
| Sweating | 2 (1.7%) |
| Otherb | 9 (7.7%) |
Over the observation period, just over one‐third of patients were persistent on liraglutide 3.0 mg (n = 43, 36.8%). Of the additional 74 patients, 62.2% (n = 46) of patients were lost to follow‐up, and therefore we cannot ascertain whether or not they discontinued taking liraglutide 3.0 mg. Twenty‐eight participants reported discontinuing liraglutide 3.0 mg, and details regarding the reason for discontinuation was captured for 20 participants. The most commonly reported (n = 10 of 20, 50%) reason for discontinuing liraglutide 3.0 mg was lack of effectiveness for weight management. Other reasons for discontinuing liraglutide 3.0 mg include cost (n = 7 of 20, 35%) and having an adverse event (n = 3 of 20, 15%), which include an allergic reaction (n = 2 of 3), and pancreatitis (n = 1 of 3).
4. DISCUSSION
Options for the management of excessive weight regain or insufficient weight loss post‐bariatric surgery are limited, and with the increase in the number of patients receiving bariatric surgery, there is a pressing need for additional treatment options. Fortunately, results of this study suggest that post‐bariatric surgery patients taking liraglutide 3.0 mg can lose a significant amount of weight. Indeed, significant reductions in weight were observed shortly after initiating liraglutide 3.0 mg and up to 1‐year post‐initiation of the medication regardless of the type of bariatric surgery patients had. Similar to what is observed in non‐post‐surgical populations, post‐bariatric surgery patients report side effects from the medication that are primarily gastrointestinal in nature and can continue to lose weight up to 1‐year post‐initiation of liraglutide 3.0 mg.
Treatment options for patients with insufficient weight loss or excessive weight regain following bariatric surgery are limited to repair of post‐surgical issues, conversion (typically from a restrictive procedure to one that incorporates a greater degree of malabsorption), endoscopic therapies, and/or reintroduction of lifestyle management techniques. However, revision and conversion surgeries have higher rates of complications than the initial procedure itself.11 Thus, pharmacotherapy may provide a safer alternative for weight management in this patient population. Currently, there is no weight management pharmaceutical approved for use in patients who have had bariatric surgery as having had bariatric surgery is often an exclusion for non‐surgical weight loss trials. However, research on the off‐labelled prescription of weight management pharmaceuticals in post‐bariatric surgery patients have reported significant decrease in weight post‐initiation of these agents.12, 13, 14 Consistent with these findings, post‐bariatric surgery patients in the current study taking liraglutide 3.0 mg lost a significant amount of weight. This may suggest that the use of weight management pharmaceuticals may be an effective treatment to address excessive weight regain or insufficient weight loss in post‐bariatric surgery patients.
Surgeries that remove a portion of the stomach (eg, RNYGB and gastric sleeve) are associated with increase in glucagon‐like peptide‐1 (GLP‐1) levels, which have been thought to in part contribute to sustained weight loss post‐surgery.15, 16 Despite these well‐known differences due to the type of bariatric surgery, few papers have examined the differences in weight loss by surgery type for those taking weight management pharmaceuticals. The studies which have primarily examined the use of phentermine, phentermine/topiramate, and naltrexone/bupropion, and report no differences for patients who had undergone RNYGB and AGB. However, individuals who had undergone gastric sleeve lost less weight than those who had undergone RNYGB and AGB.13, 17 Consistent with previous literature, we did observe similar reductions in weight while taking liraglutide 3.0 mg for patients who had undergone RNYGB or AGB. This is surprising given GLP‐1 levels are known to increase post‐surgery in RNYGB patients but not in AGB patients, and as a GLP‐1 analogue, liraglutide 3.0 mg temporarily increase the circulating levels of GLP‐1 in the body. In contrast to previous research we did not observe less weight loss for patients who had had a gastric sleeve. In fact, patients had similar reductions in weight as of 1‐month post‐initiation of liraglutide 3.0 mg and up to 1 year regardless of the type of surgery they had. Discrepancies in our findings may be due to differences in weight management pharmaceuticals or could be due to power issues given the relatively small number of patients who had undergone sleeve gastrectomy and had data on liraglutide 3.0 mg use for 1 year. Nonetheless, this may suggest that liraglutide 3.0 mg is especially beneficial for patients who have undergone gastric sleeve. As we are the first to study differences in weight loss by type of surgery for patients taking liraglutide 3.0 mg, more research is still necessary to substantiate this theory.
Current guidelines for the use of liraglutide 3.0 mg as a weight management pharmaceutical state that patients should discontinue use of the medication if they have not achieved a weight loss of 5% or more after 4 months of use5, 18 as patients are unlikely to achieve greater weight loss with continued use. Consistent with these findings, approximately 45% of patients in the current study who discontinued taking liraglutide 3.0 mg by 4 months loss less weight than those who persisted taking the medication. In addition, patients discontinued at a similar rate regardless of the type of surgery they had undergone. This further supports the notion that liraglutide 3.0 mg is equally beneficial for weight loss in patients who have undergone any of the three examined bariatric surgery types. In most lifestyle and pharmaceutical weight loss interventions, maximum weight loss is achieved by 6 months.19 However, results of the liraglutide 3.0 mg phase III trial observed continued weight loss post 6 months in subjects who had not had bariatric surgery.6 Consistent with these results, patients in the current study continued to lose weight up to 1‐year post‐initiation of liraglutide 3.0 mg. This may suggest that post‐bariatric surgery patients can experience sustained weight reduction with the use of this medication.
According to the liraglutide 3.0 mg monograph, gastrointestinal symptoms are prevalent side effects for patients who have not had bariatric surgery.5 Consistent with the product monograph,5 and research in post‐bariatric surgery patients,14 patients in the current study reported side effects that were primarily gastrointestinal in nature, with nausea being the most commonly reported side effect. Overall, the medication appeared to be well tolerated, with only three patients reporting discontinuing due to adverse events (ie, two patients due to an allergic reaction, and one patient due to pancreatitis). Pancreatitis is a known potential side effect of liraglutide 3.0 mg and may occur in 0.1% to 0.3% of patients.5 In the current study, the proportion was slightly higher but this could be attributed to our smaller sample size. Nonetheless, these results suggest that the side effects experienced by patients with bariatric surgery are similar to those reported in non‐bariatric surgery patients taking liraglutide 3.0 mg and supports the notion that this medication may be a safe option to manage excess weight in post‐bariatric surgery patients.
Our study has several strengths and limitations that warrant mentioning. A strength of this study includes being one of the few studies to examine the use of liraglutide 3.0 mg, one of only three available weight loss pharmaceuticals in Canada, in a post‐bariatric surgery population. In addition, this is the first to examine differences in weight loss based on the type of bariatric surgery for patients taking liraglutide 3.0
mg, one of only three available weight loss pharmaceuticals in Canada, in a post‐bariatric surgery population. In addition, this is the first to examine differences in weight loss based on the type of bariatric surgery for patients taking liraglutide 3.0 mg. Patients were also recruited from a publically funded weight management program with a large catchment area increasing the diversity of the sample and the generalization of these results to populations with obesity. Limitations include the retrospective collection of data making us unable to establish a control group. Additionally, we were unable to assess patient medication adherence or dose titration. Currently, 3.0
mg. Patients were also recruited from a publically funded weight management program with a large catchment area increasing the diversity of the sample and the generalization of these results to populations with obesity. Limitations include the retrospective collection of data making us unable to establish a control group. Additionally, we were unable to assess patient medication adherence or dose titration. Currently, 3.0 mg is the only recommended clinical dose of liraglutide for weight management, thus, differences in dose titration or lack of adherence to medication delivery schedule could have resulted in variability in weight loss. Patients date of initiating liraglutide 3.0 mg are based on self‐report at a follow‐up appointment. Current guidelines for the use of liraglutide for weight management recommend discontinuing treatment if the patient is unable to achieve a ≥5% weight loss after 16
mg is the only recommended clinical dose of liraglutide for weight management, thus, differences in dose titration or lack of adherence to medication delivery schedule could have resulted in variability in weight loss. Patients date of initiating liraglutide 3.0 mg are based on self‐report at a follow‐up appointment. Current guidelines for the use of liraglutide for weight management recommend discontinuing treatment if the patient is unable to achieve a ≥5% weight loss after 16 weeks of use.5, 18 As 47% (n = 55) of the patients in this study had taken liraglutide for 4 months or less, our sample may have included patients who are not responsive to liraglutide. Indeed, when patients who have taken liraglutide for 4 months or less are excluded, the proportion of patients who achieve clinically significant weight loss increases from 41.9% to 59.4%. Thus, the inclusion of these patients likely biased our results to the null. Our length of time of follow‐up was variable, we could only verify that 10 of 53 patients had discontinued taking liraglutide 3.0 mg by 4 months.
weeks of use.5, 18 As 47% (n = 55) of the patients in this study had taken liraglutide for 4 months or less, our sample may have included patients who are not responsive to liraglutide. Indeed, when patients who have taken liraglutide for 4 months or less are excluded, the proportion of patients who achieve clinically significant weight loss increases from 41.9% to 59.4%. Thus, the inclusion of these patients likely biased our results to the null. Our length of time of follow‐up was variable, we could only verify that 10 of 53 patients had discontinued taking liraglutide 3.0 mg by 4 months.
In summary, in our centre, we found that post‐bariatric surgery patients with insufficient weight loss or excessive weight regain who use liraglutide 3.0 mg were able to achieve a statistically significant weight loss, regardless of the type of bariatric surgery they had undergone. In addition, post‐bariatric surgery patients taking liraglutide 3.0
mg were able to achieve a statistically significant weight loss, regardless of the type of bariatric surgery they had undergone. In addition, post‐bariatric surgery patients taking liraglutide 3.0 mg continue to lose weight at 1 year. Further, post‐bariatric surgery patients taking liraglutide 3.0
mg continue to lose weight at 1 year. Further, post‐bariatric surgery patients taking liraglutide 3.0 mg have a similar side effect profile as what is observed in patients without bariatric surgery, with the exception being potentially more severe adverse events reported. These results are promising as current methods for weight management in post‐bariatric surgical patients are limited, and often have greater risks to the patient than the initial procedure itself. More research, using a more rigorous study design, is still necessary to determine the reproducibility of these results and whether certain weight management pharmaceuticals may be more efficacious for post‐bariatric surgery patients than others.
mg have a similar side effect profile as what is observed in patients without bariatric surgery, with the exception being potentially more severe adverse events reported. These results are promising as current methods for weight management in post‐bariatric surgical patients are limited, and often have greater risks to the patient than the initial procedure itself. More research, using a more rigorous study design, is still necessary to determine the reproducibility of these results and whether certain weight management pharmaceuticals may be more efficacious for post‐bariatric surgery patients than others.
CONFLICTS OF INTEREST
S.W. is the Medical Director of the Wharton Medical Clinic, and an internal medicine specialist with privileges at Toronto East General Hospital, and Hamilton Health Sciences. S.W. has received payment in the past from Novo Nordisk, Eli Lilly, Janssen, and Astra Zeneca for advisory work. J.L.K. has received funding in the past in the form of research grants from CIHR (#131594). E.K. is currently employed as the Research Coordinator at the Wharton Medical Clinic. R.A.G.C. was previously employed as the Research Coordinator at the Wharton Medical Clinic and is working with Novo Nordisk on research publications. M.L. has no conflicts of interests to report.
ACKNOWLEDGEMENTS
Our sincere thanks to Wali Mir and Maral Dehlehhosseinzadeh for their work in abstracting data for the analyses. In addition, we would also like thank the patients at the Wharton Medical Clinic who consented for their data to be used, and the staff who worked with the patients and collected the data, without which it would not have been possible to conduct this research.
Notes
Wharton S, Kuk JL, Luszczynski M, Kamran E, Christensen RAG. Liraglutide 3.0 mg for the management of insufficient weight loss or excessive weight regain post‐bariatric surgery. Clin Obes. 2019;9:e12323. 10.1111/cob.12323 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
The copyright line for this article was changed on 23 August 2019 after original online publication.
REFERENCES
 mg for Weight Management NDA 206‐321 Briefing Document; 2014.
https://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/endocrinologicandmetabolicdrugsadvisorycommittee/ucm413318.pdf. Accessed May 15, 2017.
mg for Weight Management NDA 206‐321 Briefing Document; 2014.
https://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/endocrinologicandmetabolicdrugsadvisorycommittee/ucm413318.pdf. Accessed May 15, 2017.Full text links
Read article at publisher's site: https://doi.org/10.1111/cob.12323
Read article for free, from open access legal sources, via Unpaywall:
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/cob.12323
Citations & impact
Impact metrics
Article citations
Multidisciplinary combined treatment based on bariatric surgery for metabolic syndrome: a review article.
Int J Surg, 110(6):3666-3679, 01 Jun 2024
Cited by: 0 articles | PMID: 38489549
Review
Pharmacotherapy as an Augmentation to Bariatric Surgery for Obesity.
Drugs, 84(8):933-952, 06 Jul 2024
Cited by: 1 article | PMID: 38970626 | PMCID: PMC11343883
Review Free full text in Europe PMC
Semaglutide for weight loss: unanswered questions.
Front Endocrinol (Lausanne), 15:1382814, 05 Jun 2024
Cited by: 1 article | PMID: 38904050 | PMCID: PMC11188346
Adjuvant and Neo-Adjuvant Anti-Obesity Medications and Bariatric Surgery: A Scoping Review.
Curr Obes Rep, 13(2):377-402, 20 Mar 2024
Cited by: 0 articles | PMID: 38507194
Review
Weight Regain after Metabolic Surgery: Beyond the Surgical Failure.
J Clin Med, 13(4):1143, 18 Feb 2024
Cited by: 1 article | PMID: 38398456 | PMCID: PMC10888585
Review Free full text in Europe PMC
Go to all (50) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Efficacy of liraglutide 3.0 mg treatment on weight loss in patients with weight regain after bariatric surgery.
Eat Weight Disord, 27(7):2775-2781, 28 Jun 2022
Cited by: 10 articles | PMID: 35763245 | PMCID: PMC9556416
Early metabolomic, lipid and lipoprotein changes in response to medical and surgical therapeutic approaches to obesity.
Metabolism, 138:155346, 12 Nov 2022
Cited by: 15 articles | PMID: 36375643
Efficacy of High-Dose Liraglutide as an Adjunct for Weight Loss in Patients with Prior Bariatric Surgery.
Obes Surg, 28(11):3553-3558, 01 Nov 2018
Cited by: 38 articles | PMID: 30022424
Surgery for weight loss in adults.
Cochrane Database Syst Rev, (8):CD003641, 08 Aug 2014
Cited by: 495 articles | PMID: 25105982 | PMCID: PMC9028049
Review Free full text in Europe PMC

 1
1