Abstract
Free full text

The C-terminal repeat domain of RNA polymerase II largest subunit is essential in vivo but is not required for accurate transcription initiation in vitro.
Abstract
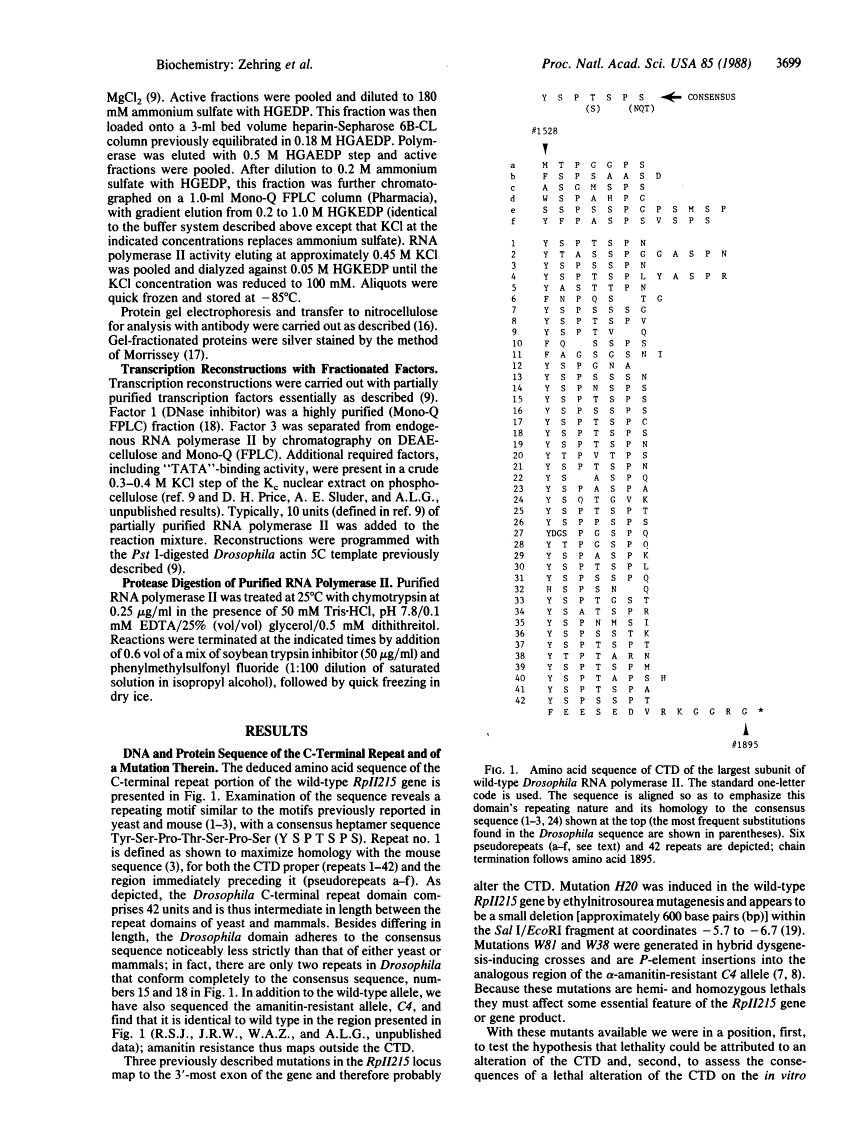
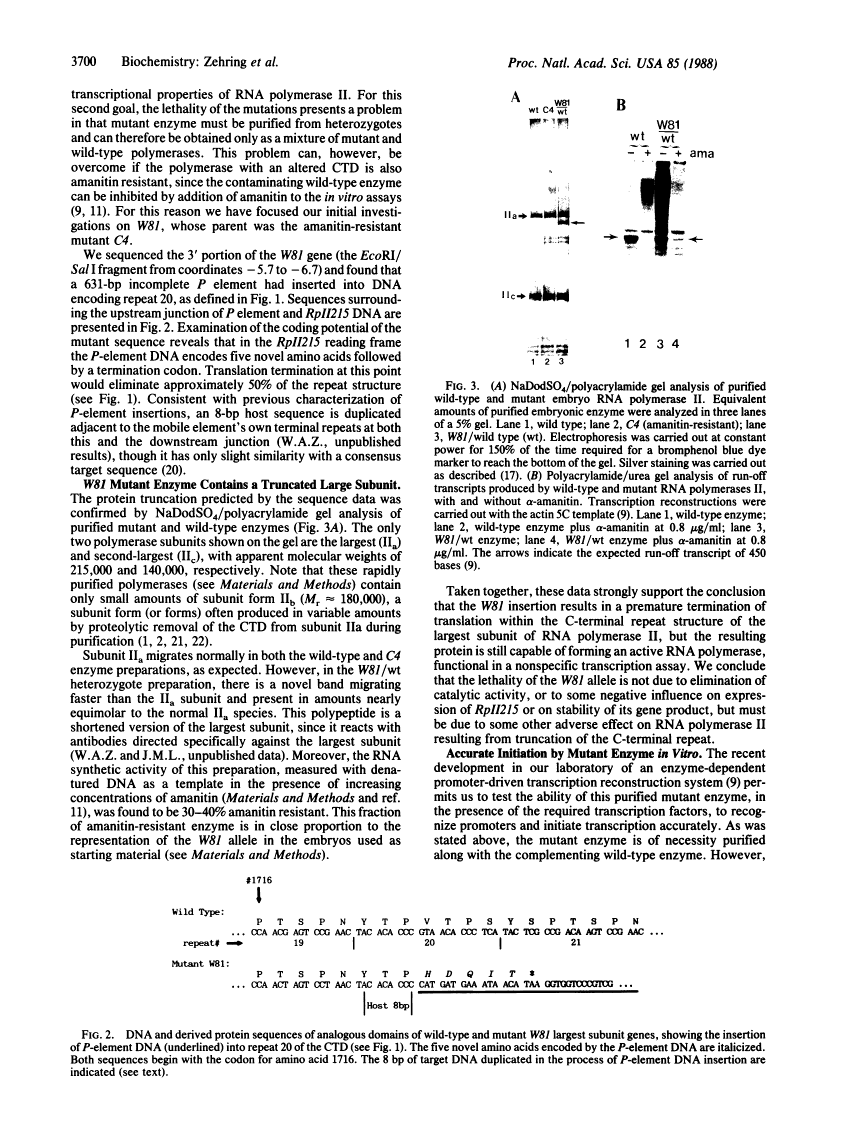
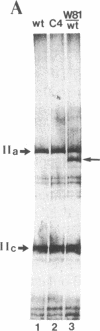
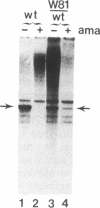
DNA sequence analysis of RpII215, the gene that encodes the Mr215,000 subunit of RNA polymerase II (EC 2.7.7.6) in Drosophila melanogaster, reveals that the 3'-terminal exon includes a region encoding a C-terminal domain composed of 42 repeats of a seven-residue amino acid consensus sequence, Tyr-Ser-Pro-Thr-Ser-Pro-Ser. A hemi- and homozygous lethal P-element insertion into the coding sequence of this domain causes premature translation termination and therefore truncation of the protein, leaving only 20 heptamer repeats. While loss of approximately 50% of the repeat structure in this mutant is a lethal event in vivo, enzyme containing the truncated subunit remains capable of accurate initiation at promoters in vitro. Moreover, treatment of purified intact RNA polymerase II with protease, to remove the entire repeat domain, does not eliminate the enzyme's ability to initiate accurately in vitro. Possible in vivo functions for this unusual protein domain are considered in light of these results.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.2M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison LA, Moyle M, Shales M, Ingles CJ. Extensive homology among the largest subunits of eukaryotic and prokaryotic RNA polymerases. Cell. 1985 Sep;42(2):599–610. [Abstract] [Google Scholar]
- Corden JL, Cadena DL, Ahearn JM, Jr, Dahmus ME. A unique structure at the carboxyl terminus of the largest subunit of eukaryotic RNA polymerase II. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7934–7938. [Europe PMC free article] [Abstract] [Google Scholar]
- Ahearn JM, Jr, Bartolomei MS, West ML, Cisek LJ, Corden JL. Cloning and sequence analysis of the mouse genomic locus encoding the largest subunit of RNA polymerase II. J Biol Chem. 1987 Aug 5;262(22):10695–10705. [Abstract] [Google Scholar]
- Ovchinnikov YuA, Monastyrskaya GS, Gubanov VV, Guryev SO, Salomatina IS, Shuvaeva TM, Lipkin VM, Sverdlov ED. The primary structure of E. coli RNA polymerase, Nucleotide sequence of the rpoC gene and amino acid sequence of the beta'-subunit. Nucleic Acids Res. 1982 Jul 10;10(13):4035–4044. [Europe PMC free article] [Abstract] [Google Scholar]
- Broyles SS, Moss B. Homology between RNA polymerases of poxviruses, prokaryotes, and eukaryotes: nucleotide sequence and transcriptional analysis of vaccinia virus genes encoding 147-kDa and 22-kDa subunits. Proc Natl Acad Sci U S A. 1986 May;83(10):3141–3145. [Europe PMC free article] [Abstract] [Google Scholar]
- Searles LL, Greenleaf AL, Kemp WE, Voelker RA. Sites of P element insertion and structures of P element deletions in the 5' region of Drosophila melanogaster RpII215. Mol Cell Biol. 1986 Oct;6(10):3312–3319. [Europe PMC free article] [Abstract] [Google Scholar]
- Price DH, Sluder AE, Greenleaf AL. Fractionation of transcription factors for RNA polymerase II from Drosophila Kc cell nuclear extracts. J Biol Chem. 1987 Mar 5;262(7):3244–3255. [Abstract] [Google Scholar]
- Greenleaf AL, Borsett LM, Jiamachello PF, Coulter DE. Alpha-amanitin-resistant D. melanogaster with an altered RNA polymerase II. Cell. 1979 Nov;18(3):613–622. [Abstract] [Google Scholar]
- Greenleaf AL, Weeks JR, Voelker RA, Ohnishi S, Dickson B. Genetic and biochemical characterization of mutants at an RNA polymerase II locus in D. melanogaster. Cell. 1980 Oct;21(3):785–792. [Abstract] [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. [Europe PMC free article] [Abstract] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. [Abstract] [Google Scholar]
- Biggs J, Searles LL, Greenleaf AL. Structure of the eukaryotic transcription apparatus: features of the gene for the largest subunit of Drosophila RNA polymerase II. Cell. 1985 Sep;42(2):611–621. [Abstract] [Google Scholar]
- Weeks JR, Coulter DE, Greenleaf AL. Immunological studies of RNA polymerase II using antibodies to subunits of Drosophila and wheat germ enzyme. J Biol Chem. 1982 May 25;257(10):5884–5892. [Abstract] [Google Scholar]
- Morrissey JH. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. [Abstract] [Google Scholar]
- Sluder AE, Price DH, Greenleaf AL. An activity necessary for in vitro transcription is a DNase inhibitor. Biochimie. 1987 Nov-Dec;69(11-12):1199–1205. [Abstract] [Google Scholar]
- Lacy LR, Eisenberg MT, Osgood CJ. Molecular analysis of chemically-induced mutations at the RpII215 locus of Drosophila melanogaster. Mutat Res. 1986 Aug;162(1):47–54. [Abstract] [Google Scholar]
- O'Hare K, Rubin GM. Structures of P transposable elements and their sites of insertion and excision in the Drosophila melanogaster genome. Cell. 1983 Aug;34(1):25–35. [Abstract] [Google Scholar]
- Greenleaf AL, Haars R, Bautz EK. In vitro proteolysis of a large subunit of Drosophila melanogaster RNA polymerase B. FEBS Lett. 1976 Dec 1;71(2):205–208. [Abstract] [Google Scholar]
- Dezélée S, Wyers F, Sentenac A, Fromageot P. Two forms of RNA polymerase B in yeast. Proteolytic conversion in vitro of enzyme BI into BII. Eur J Biochem. 1976 Jun 1;65(2):543–552. [Abstract] [Google Scholar]
- Nonet M, Sweetser D, Young RA. Functional redundancy and structural polymorphism in the large subunit of RNA polymerase II. Cell. 1987 Sep 11;50(6):909–915. [Abstract] [Google Scholar]
- Bartolomei MS, Halden NF, Cullen CR, Corden JL. Genetic analysis of the repetitive carboxyl-terminal domain of the largest subunit of mouse RNA polymerase II. Mol Cell Biol. 1988 Jan;8(1):330–339. [Europe PMC free article] [Abstract] [Google Scholar]
- Dahmus ME, Kedinger C. Transcription of adenovirus-2 major late promoter inhibited by monoclonal antibody directed against RNA polymerases IIO and IIA. J Biol Chem. 1983 Feb 25;258(4):2303–2307. [Abstract] [Google Scholar]
- Bartholomew B, Dahmus ME, Meares CF. RNA contacts subunits IIo and IIc in HeLa RNA polymerase II transcription complexes. J Biol Chem. 1986 Oct 25;261(30):14226–14231. [Abstract] [Google Scholar]
- Cadena DL, Dahmus ME. Messenger RNA synthesis in mammalian cells is catalyzed by the phosphorylated form of RNA polymerase II. J Biol Chem. 1987 Sep 15;262(26):12468–12474. [Abstract] [Google Scholar]
- Allison LA, Wong JK, Fitzpatrick VD, Moyle M, Ingles CJ. The C-terminal domain of the largest subunit of RNA polymerase II of Saccharomyces cerevisiae, Drosophila melanogaster, and mammals: a conserved structure with an essential function. Mol Cell Biol. 1988 Jan;8(1):321–329. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.85.11.3698
Read article for free, from open access legal sources, via Unpaywall:
http://www.pnas.org/content/85/11/3698.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
The role of O-GlcNAcylation in RNA polymerase II transcription.
J Biol Chem, 300(3):105705, 02 Feb 2024
Cited by: 2 articles | PMID: 38311176 | PMCID: PMC10906531
Review Free full text in Europe PMC
Mechanisms and Functions of the RNA Polymerase II General Transcription Machinery during the Transcription Cycle.
Biomolecules, 14(2):176, 01 Feb 2024
Cited by: 1 article | PMID: 38397413 | PMCID: PMC10886972
Review Free full text in Europe PMC
Assessment of the roles of Spt5-nucleic acid contacts in promoter proximal pausing of RNA polymerase II.
J Biol Chem, 299(9):105106, 28 Jul 2023
Cited by: 3 articles | PMID: 37517697 | PMCID: PMC10482750
Causes and consequences of RNA polymerase II stalling during transcript elongation.
Nat Rev Mol Cell Biol, 22(1):3-21, 18 Nov 2020
Cited by: 84 articles | PMID: 33208928
Review
The CTD Is Not Essential for the Post-Initiation Control of RNA Polymerase II Activity.
J Mol Biol, 432(19):5489-5498, 21 Jul 2020
Cited by: 6 articles | PMID: 32707132 | PMCID: PMC7502503
Go to all (96) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Analysis of the gene encoding the largest subunit of RNA polymerase II in Drosophila.
Mol Gen Genet, 215(2):266-275, 01 Jan 1989
Cited by: 133 articles | PMID: 2496296
The C-terminal domain of the largest subunit of RNA polymerase II of Saccharomyces cerevisiae, Drosophila melanogaster, and mammals: a conserved structure with an essential function.
Mol Cell Biol, 8(1):321-329, 01 Jan 1988
Cited by: 151 articles | PMID: 3122024 | PMCID: PMC443572
A unique structure at the carboxyl terminus of the largest subunit of eukaryotic RNA polymerase II.
Proc Natl Acad Sci U S A, 82(23):7934-7938, 01 Dec 1985
Cited by: 192 articles | PMID: 2999785 | PMCID: PMC390884
Genetic analysis of the repetitive carboxyl-terminal domain of the largest subunit of mouse RNA polymerase II.
Mol Cell Biol, 8(1):330-339, 01 Jan 1988
Cited by: 134 articles | PMID: 3275873 | PMCID: PMC363128