Abstract
Free full text

Immunoaffinity purification and neutralization of scrapie prion infectivity.
Abstract
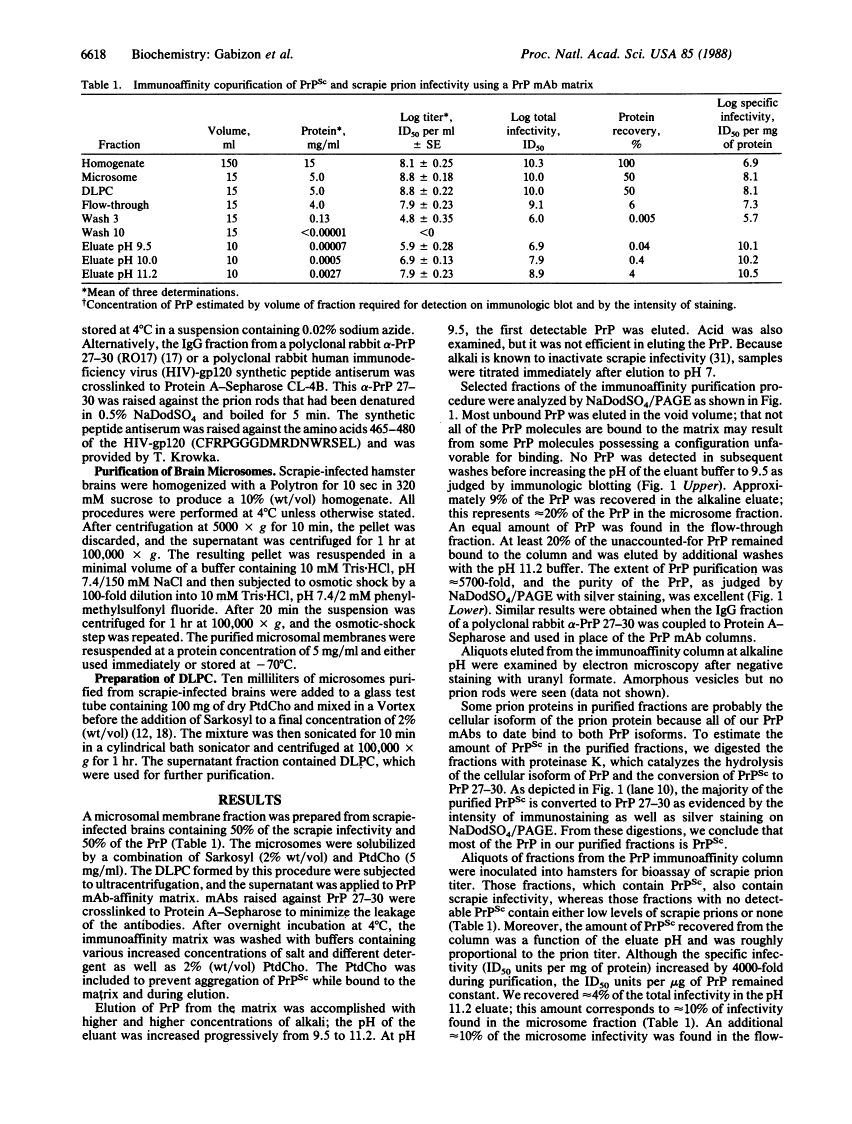
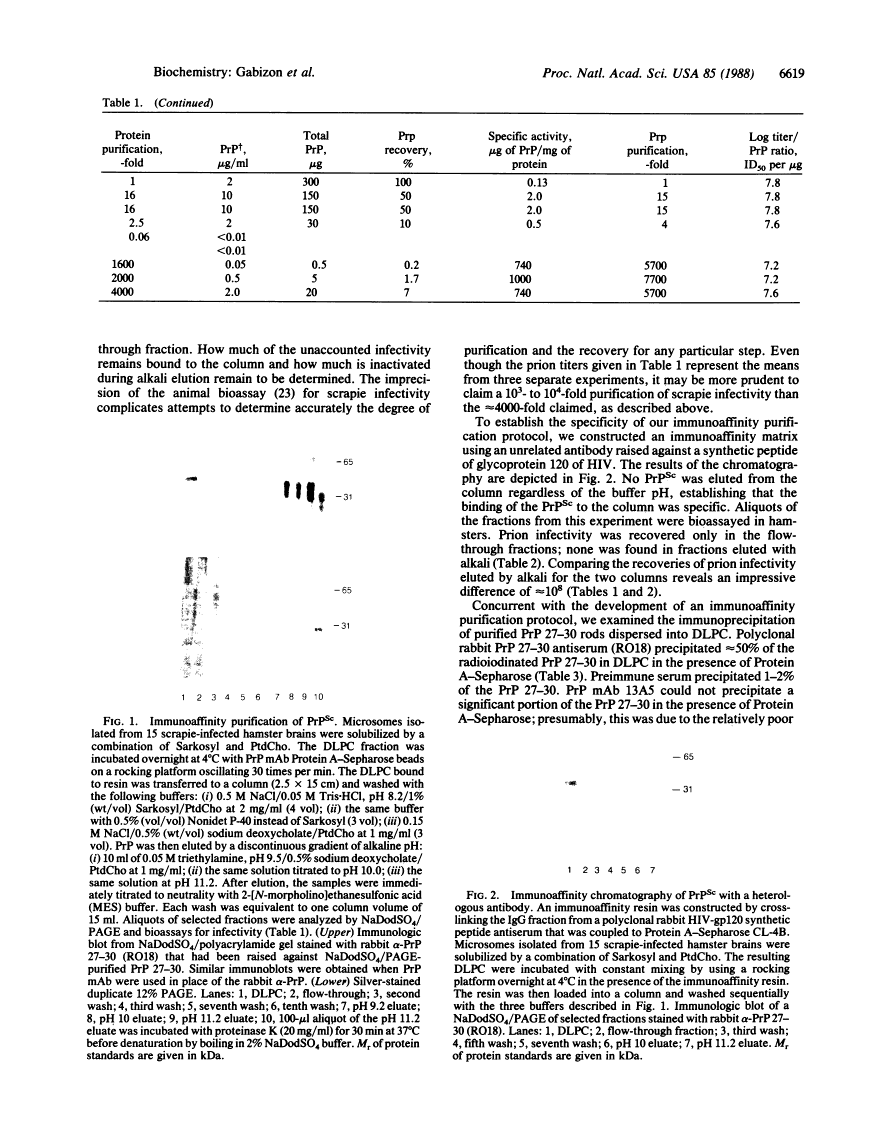
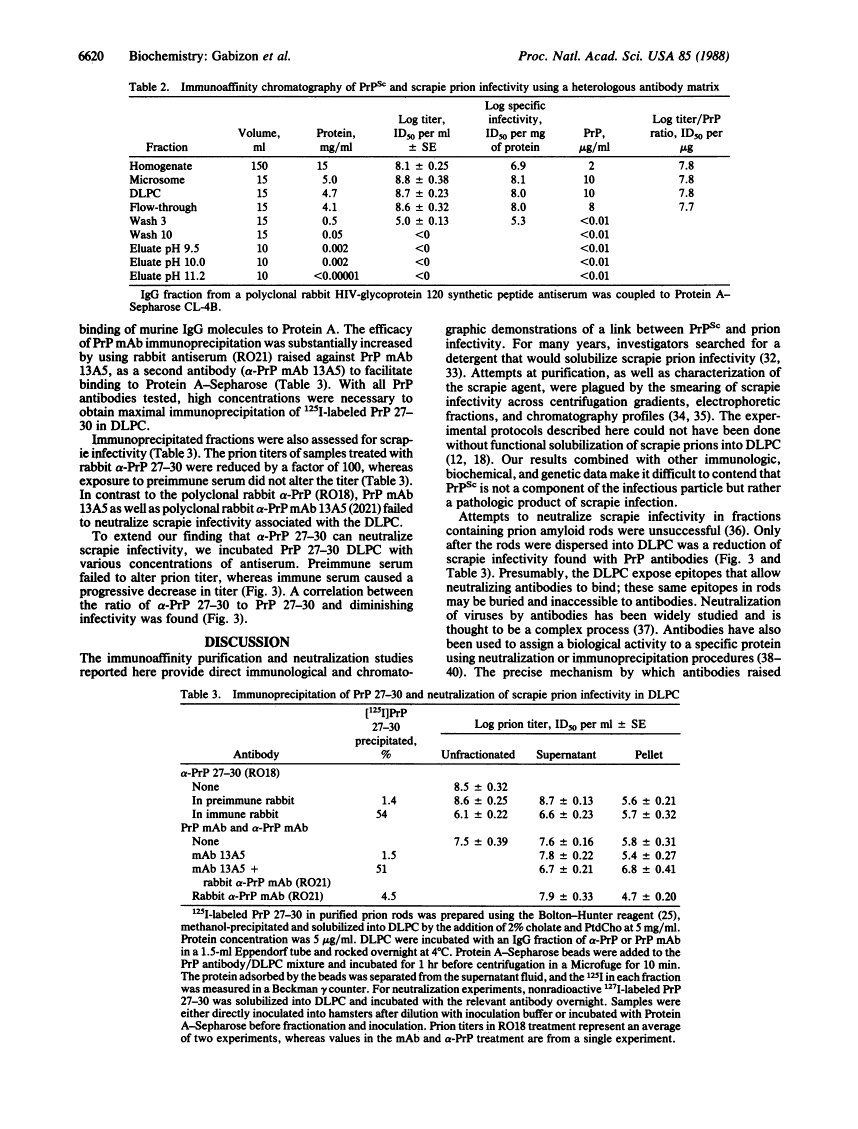
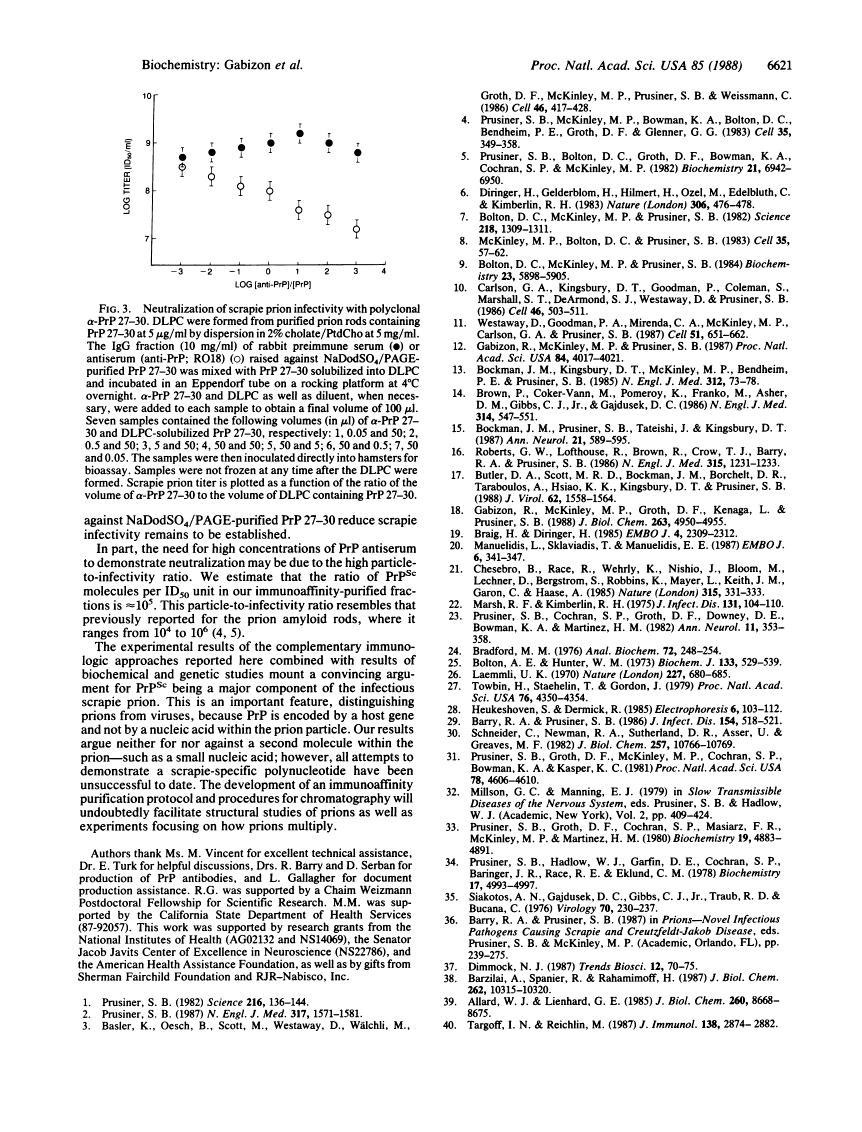
Prions are unusual infectious pathogens causing scrapie of sheep and goats as well as Creutzfeldt-Jakob disease of humans. Biochemical and genetic studies contend that the scrapie isoform of the prion protein (PrPSc) is a major component of the prion. Limited proteinase K digestion of PrPSc produced a protein of 27-30 kDa. After dispersion of brain microsomes isolated from scrapie-infected hamsters into detergent-lipid-protein complexes, copurification of PrPSc and scrapie infectivity was obtained with scrapie prion protein of 27-30 kDa monoclonal antibody-affinity columns. PrPSc was enriched approximately equal to 5700-fold with respect to total brain protein, whereas scrapie prion infectivity was enriched approximately equal to 4000-fold. The ratio of prion titer to PrPSc remained constant throughout purification. Heterologous monoclonal antibody columns failed to bind either PrPSc or scrapie infectivity. Polyclonal rabbit prion protein antiserum raised against NaDodSO4/PAGE-purified scrapie prion protein of 27-30 kDa reduced scrapie infectivity dispersed into detergent-lipid-protein complexes by a factor of 100. These results represent direct immunologic and chromatographic demonstrations of a relationship between PrPSc and prion infectivity as well as providing additional support for the contention that PrPSc is a major component of the infectious scrapie particle. That PrPSc is a host-encoded protein is an important feature distinguishing prions from viruses.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.1M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982 Apr 9;216(4542):136–144. [Abstract] [Google Scholar]
- Prusiner SB. Prions and neurodegenerative diseases. N Engl J Med. 1987 Dec 17;317(25):1571–1581. [Abstract] [Google Scholar]
- Basler K, Oesch B, Scott M, Westaway D, Wälchli M, Groth DF, McKinley MP, Prusiner SB, Weissmann C. Scrapie and cellular PrP isoforms are encoded by the same chromosomal gene. Cell. 1986 Aug 1;46(3):417–428. [Abstract] [Google Scholar]
- Prusiner SB, McKinley MP, Bowman KA, Bolton DC, Bendheim PE, Groth DF, Glenner GG. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell. 1983 Dec;35(2 Pt 1):349–358. [Abstract] [Google Scholar]
- Prusiner SB, Bolton DC, Groth DF, Bowman KA, Cochran SP, McKinley MP. Further purification and characterization of scrapie prions. Biochemistry. 1982 Dec 21;21(26):6942–6950. [Abstract] [Google Scholar]
- Diringer H, Gelderblom H, Hilmert H, Ozel M, Edelbluth C, Kimberlin RH. Scrapie infectivity, fibrils and low molecular weight protein. Nature. 1983 Dec 1;306(5942):476–478. [Abstract] [Google Scholar]
- Bolton DC, McKinley MP, Prusiner SB. Identification of a protein that purifies with the scrapie prion. Science. 1982 Dec 24;218(4579):1309–1311. [Abstract] [Google Scholar]
- McKinley MP, Bolton DC, Prusiner SB. A protease-resistant protein is a structural component of the scrapie prion. Cell. 1983 Nov;35(1):57–62. [Abstract] [Google Scholar]
- Bolton DC, McKinley MP, Prusiner SB. Molecular characteristics of the major scrapie prion protein. Biochemistry. 1984 Dec 4;23(25):5898–5906. [Abstract] [Google Scholar]
- Carlson GA, Kingsbury DT, Goodman PA, Coleman S, Marshall ST, DeArmond S, Westaway D, Prusiner SB. Linkage of prion protein and scrapie incubation time genes. Cell. 1986 Aug 15;46(4):503–511. [Abstract] [Google Scholar]
- Westaway D, Goodman PA, Mirenda CA, McKinley MP, Carlson GA, Prusiner SB. Distinct prion proteins in short and long scrapie incubation period mice. Cell. 1987 Nov 20;51(4):651–662. [Abstract] [Google Scholar]
- Gabizon R, McKinley MP, Prusiner SB. Purified prion proteins and scrapie infectivity copartition into liposomes. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4017–4021. [Europe PMC free article] [Abstract] [Google Scholar]
- Bockman JM, Kingsbury DT, McKinley MP, Bendheim PE, Prusiner SB. Creutzfeldt-Jakob disease prion proteins in human brains. N Engl J Med. 1985 Jan 10;312(2):73–78. [Abstract] [Google Scholar]
- Brown P, Coker-Vann M, Pomeroy K, Franko M, Asher DM, Gibbs CJ, Jr, Gajdusek DC. Diagnosis of Creutzfeldt-Jakob disease by Western blot identification of marker protein in human brain tissue. N Engl J Med. 1986 Feb 27;314(9):547–551. [Abstract] [Google Scholar]
- Bockman JM, Prusiner SB, Tateishi J, Kingsbury DT. Immunoblotting of Creutzfeldt-Jakob disease prion proteins: host species-specific epitopes. Ann Neurol. 1987 Jun;21(6):589–595. [Abstract] [Google Scholar]
- Roberts GW, Lofthouse R, Brown R, Crow TJ, Barry RA, Prusiner SB. Prion-protein immunoreactivity in human transmissible dementias. N Engl J Med. 1986 Nov 6;315(19):1231–1233. [Abstract] [Google Scholar]
- Butler DA, Scott MR, Bockman JM, Borchelt DR, Taraboulos A, Hsiao KK, Kingsbury DT, Prusiner SB. Scrapie-infected murine neuroblastoma cells produce protease-resistant prion proteins. J Virol. 1988 May;62(5):1558–1564. [Europe PMC free article] [Abstract] [Google Scholar]
- Gabizon R, McKinley MP, Groth DF, Kenaga L, Prusiner SB. Properties of scrapie prion protein liposomes. J Biol Chem. 1988 Apr 5;263(10):4950–4955. [Abstract] [Google Scholar]
- Braig HR, Diringer H. Scrapie: concept of a virus-induced amyloidosis of the brain. EMBO J. 1985 Sep;4(9):2309–2312. [Europe PMC free article] [Abstract] [Google Scholar]
- Manuelidis L, Sklaviadis T, Manuelidis EE. Evidence suggesting that PrP is not the infectious agent in Creutzfeldt-Jakob disease. EMBO J. 1987 Feb;6(2):341–347. [Europe PMC free article] [Abstract] [Google Scholar]
- Chesebro B, Race R, Wehrly K, Nishio J, Bloom M, Lechner D, Bergstrom S, Robbins K, Mayer L, Keith JM, et al. Identification of scrapie prion protein-specific mRNA in scrapie-infected and uninfected brain. Nature. 1985 May 23;315(6017):331–333. [Abstract] [Google Scholar]
- Marsh RF, Kimberlin RH. Comparison of scrapie and transmissible mink encephalopathy in hamsters. II. Clinical signs, pathology, and pathogenesis. J Infect Dis. 1975 Feb;131(2):104–110. [Abstract] [Google Scholar]
- Prusiner SB, Cochran SP, Groth DF, Downey DE, Bowman KA, Martinez HM. Measurement of the scrapie agent using an incubation time interval assay. Ann Neurol. 1982 Apr;11(4):353–358. [Abstract] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. [Abstract] [Google Scholar]
- Bolton AE, Hunter WM. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. [Europe PMC free article] [Abstract] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. [Abstract] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. [Europe PMC free article] [Abstract] [Google Scholar]
- Barry RA, Prusiner SB. Monoclonal antibodies to the cellular and scrapie prion proteins. J Infect Dis. 1986 Sep;154(3):518–521. [Abstract] [Google Scholar]
- Schneider C, Newman RA, Sutherland DR, Asser U, Greaves MF. A one-step purification of membrane proteins using a high efficiency immunomatrix. J Biol Chem. 1982 Sep 25;257(18):10766–10769. [Abstract] [Google Scholar]
- Prusiner SB, Groth DF, McKinley MP, Cochran SP, Bowman KA, Kasper KC. Thiocyanate and hydroxyl ions inactivate the scrapie agent. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4606–4610. [Europe PMC free article] [Abstract] [Google Scholar]
- Prusiner SB, Groth DF, Cochran SP, Masiarz FR, McKinley MP, Martinez HM. Molecular properties, partial purification, and assay by incubation period measurements of the hamster scrapie agent. Biochemistry. 1980 Oct 14;19(21):4883–4891. [Abstract] [Google Scholar]
- Prusiner SB, Hadlow WJ, Garfin DE, Cochran SP, Baringer JR, Race RE, Eklund CM. Partial purification and evidence for multiple molecular forms of the scrapie agent. Biochemistry. 1978 Nov 14;17(23):4993–4999. [Abstract] [Google Scholar]
- Siakotos AN, Gajdusek DC, Gibbs CJ, Jr, Traub RD, Bucana C. Partial purification of the scrapie agent from mouse brain by pressure disruption and zonal centrifugation in sucrose-sodium chloride gradients. Virology. 1976 Mar;70(1):230–237. [Abstract] [Google Scholar]
- Barzilai A, Spanier R, Rahamimoff H. Immunological identification of the synaptic plasma membrane Na+-Ca2+ exchanger. J Biol Chem. 1987 Jul 25;262(21):10315–10320. [Abstract] [Google Scholar]
- Allard WJ, Lienhard GE. Monoclonal antibodies to the glucose transporter from human erythrocytes. Identification of the transporter as a Mr = 55,000 protein. J Biol Chem. 1985 Jul 25;260(15):8668–8675. [Abstract] [Google Scholar]
- Targoff IN, Reichlin M. Measurement of antibody to Jo-1 by ELISA and comparison to enzyme inhibitory activity. J Immunol. 1987 May 1;138(9):2874–2882. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.85.18.6617
Read article for free, from open access legal sources, via Unpaywall:
http://www.pnas.org/content/85/18/6617.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1073/pnas.85.18.6617
Article citations
Creutzfeldt-Jakob disease and other prion diseases.
Nat Rev Dis Primers, 10(1):14, 29 Feb 2024
Cited by: 7 articles | PMID: 38424082
Review
PMCA for ultrasensitive detection of prions and to study disease biology.
Cell Tissue Res, 392(1):307-321, 26 Dec 2022
Cited by: 5 articles | PMID: 36567368 | PMCID: PMC9790818
Review Free full text in Europe PMC
Toward Therapy of Human Prion Diseases.
Annu Rev Pharmacol Toxicol, 58:331-351, 27 Sep 2017
Cited by: 40 articles | PMID: 28961066
Review
Proteomics applications in prion biology and structure.
Expert Rev Proteomics, 12(2):171-184, 01 Apr 2015
Cited by: 4 articles | PMID: 25795148
Review
Molecular dynamics studies on the NMR and X-ray structures of rabbit prion proteins.
J Theor Biol, 342:70-82, 31 Oct 2013
Cited by: 10 articles | PMID: 24184221
Go to all (91) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Immunoaffinity purification and neutralization of scrapie prions.
Prog Clin Biol Res, 317:583-600, 01 Jan 1989
Cited by: 2 articles | PMID: 2574871
Review
Scrapie-infected murine neuroblastoma cells produce protease-resistant prion proteins.
J Virol, 62(5):1558-1564, 01 May 1988
Cited by: 235 articles | PMID: 3282080 | PMCID: PMC253182
Prion diseases of the central nervous system.
Monogr Pathol, (32):86-122, 01 Jan 1990
Cited by: 4 articles | PMID: 2192281
Review
Scrapie prion rod formation in vitro requires both detergent extraction and limited proteolysis.
J Virol, 65(3):1340-1351, 01 Mar 1991
Cited by: 119 articles | PMID: 1704926 | PMCID: PMC239910
Funding
Funders who supported this work.
NIA NIH HHS (1)
Grant ID: AG02132
NINDS NIH HHS (2)
Grant ID: NS14069
Grant ID: NS22786