Abstract
Introduction
This study assessed baseline cardiovascular (CV) risk factors, concomitant CV medication use, risk of major adverse cardiac events-plus (MACE-plus), and bleeding adverse events (AEs) in patients with idiopathic pulmonary fibrosis (IPF) in three randomized, placebo-controlled phase III trials of pirfenidone.Methods
Patients in the pirfenidone phase III trials were included. Patients with unstable or deteriorating cardiac disease within 6 months before enrollment were ineligible. Medical history at baseline and concomitant CV medication use during treatment were reported. A retrospective, blinded review of AE preferred terms was conducted to identify MACE-plus and bleeding events. Subgroup analyses examined the impact of concomitant CV medication use on how pirfenidone treatment affected clinical outcomes.Results
In total, 1247 patients were included [n = 623 pirfenidone (2403 mg/day) and n = 624 placebo]. The median age was 68 years, 74% were male, and 65% were current/former smokers. Commonly reported CV risk factors included hypertension (52%), obesity (44%), hypercholesterolemia (23%), and hyperlipidemia (23%). Pre-existing cardiac disorders included coronary artery disease (16%), myocardial infarction (5%), and atrial fibrillation (5%). Lipid-modifying agents (60%), antithrombotic agents (54%), and renin-angiotensin inhibitors (39%) were commonly used concomitant CV medications. The incidences of MACE-plus and bleeding events were similar between the pirfenidone and placebo groups (1.8% and 2.9% for MACE-plus events and 3.7% and 4.3% for bleeding events, respectively). Except for patients receiving heparin, pirfenidone had a beneficial effect compared with placebo on efficacy outcomes regardless of concomitant CV medications.Conclusions
CV risk factors and comorbidities and use of concomitant CV medications are common in patients with IPF. Pirfenidone did not appear to increase the risk of CV or bleeding events. Use of several concomitant CV medications, including warfarin, did not appear to adversely impact pirfenidone's beneficial effect on efficacy outcomes.Trial registration
NCT00287716, NCT00287729, and NCT01366209.Funding
F. Hoffmann-La Roche Ltd. and Genentech, Inc.Free full text
Cardiovascular Risks, Bleeding Risks, and Clinical Events from 3 Phase III Trials of Pirfenidone in Patients with Idiopathic Pulmonary Fibrosis
Associated Data
Abstract
Introduction
This study assessed baseline cardiovascular (CV) risk factors, concomitant CV medication use, risk of major adverse cardiac events-plus (MACE-plus), and bleeding adverse events (AEs) in patients with idiopathic pulmonary fibrosis (IPF) in three randomized, placebo-controlled phase III trials of pirfenidone.
Methods
Patients in the pirfenidone phase III trials were included. Patients with unstable or deteriorating cardiac disease within 6 months before enrollment were ineligible. Medical history at baseline and concomitant CV medication use during treatment were reported. A retrospective, blinded review of AE preferred terms was conducted to identify MACE-plus and bleeding events. Subgroup analyses examined the impact of concomitant CV medication use on how pirfenidone treatment affected clinical outcomes.
Results
In total, 1247 patients were included [n =
= 623 pirfenidone (2403 mg/day) and n
623 pirfenidone (2403 mg/day) and n =
= 624 placebo]. The median age was 68 years, 74% were male, and 65% were current/former smokers. Commonly reported CV risk factors included hypertension (52%), obesity (44%), hypercholesterolemia (23%), and hyperlipidemia (23%). Pre-existing cardiac disorders included coronary artery disease (16%), myocardial infarction (5%), and atrial fibrillation (5%). Lipid-modifying agents (60%), antithrombotic agents (54%), and renin-angiotensin inhibitors (39%) were commonly used concomitant CV medications. The incidences of MACE-plus and bleeding events were similar between the pirfenidone and placebo groups (1.8% and 2.9% for MACE-plus events and 3.7% and 4.3% for bleeding events, respectively). Except for patients receiving heparin, pirfenidone had a beneficial effect compared with placebo on efficacy outcomes regardless of concomitant CV medications.
624 placebo]. The median age was 68 years, 74% were male, and 65% were current/former smokers. Commonly reported CV risk factors included hypertension (52%), obesity (44%), hypercholesterolemia (23%), and hyperlipidemia (23%). Pre-existing cardiac disorders included coronary artery disease (16%), myocardial infarction (5%), and atrial fibrillation (5%). Lipid-modifying agents (60%), antithrombotic agents (54%), and renin-angiotensin inhibitors (39%) were commonly used concomitant CV medications. The incidences of MACE-plus and bleeding events were similar between the pirfenidone and placebo groups (1.8% and 2.9% for MACE-plus events and 3.7% and 4.3% for bleeding events, respectively). Except for patients receiving heparin, pirfenidone had a beneficial effect compared with placebo on efficacy outcomes regardless of concomitant CV medications.
Conclusions
CV risk factors and comorbidities and use of concomitant CV medications are common in patients with IPF. Pirfenidone did not appear to increase the risk of CV or bleeding events. Use of several concomitant CV medications, including warfarin, did not appear to adversely impact pirfenidone’s beneficial effect on efficacy outcomes.
Trial Registration
NCT00287716, NCT00287729, and NCT01366209.
Funding
F. Hoffmann-La Roche Ltd. and Genentech, Inc.
Electronic supplementary material
The online version of this article (10.1007/s12325-019-01052-y) contains supplementary material, which is available to authorized users.
Introduction
Idiopathic pulmonary fibrosis (IPF) is an irreversible, progressive, unpredictable, and fatal fibrotic lung disease that occurs predominantly in older adults. The prognosis of IPF is poor, with an estimated median survival of 2–5 years from the time of diagnosis and survival rates lower than those reported for many common cancers [1–4]. Until recently, no treatments were available for IPF. In 2014, two drugs, pirfenidone and nintedanib, were approved in the USA to treat IPF. Pirfenidone is an oral small molecule with antifibrotic and antiinflammatory properties that slow fibrosis in various in vivo models [5, 6]. Nintedanib is an oral small molecule that inhibits multiple receptor tyrosine kinases, including platelet-derived growth factor receptor α and β, fibroblast growth factor receptors 1–3, and vascular endothelial growth factor receptors 1–3, which have been implicated in IPF pathogenesis [7].
Cardiovascular (CV) diseases and risk factors constitute an important group of comorbid conditions in patients with IPF, and medications are often used to treat patients with CV risk factors [8–12]. The profile of patients with IPF (> 60 years of age, mostly male, and frequently current or former smokers) is similar to the general profile of patients at higher risk for CV disease. A recent review described a high prevalence of CV disease and CV risk factors, including arrhythmia, atrial fibrillation, ischemic heart disease, cerebrovascular disease, diabetes, hypercholesterolemia, hyperlipidemia, and weight disorders, in patients with IPF [12]. In a claims database analysis, patients with IPF were reported to have a significantly higher prevalence of coronary artery disease, heart failure, atrial fibrillation, and myocardial infarction than matched controls [13]. Patients with IPF have also been reported to be significantly more likely than the general population to have a history of hypertension and diabetes and to have received several CV medications, even prior to IPF diagnosis [8, 11]. A higher prevalence of coronary artery disease has also been reported in patients with IPF compared with matched patients who had chronic obstructive pulmonary disease [9].
60 years of age, mostly male, and frequently current or former smokers) is similar to the general profile of patients at higher risk for CV disease. A recent review described a high prevalence of CV disease and CV risk factors, including arrhythmia, atrial fibrillation, ischemic heart disease, cerebrovascular disease, diabetes, hypercholesterolemia, hyperlipidemia, and weight disorders, in patients with IPF [12]. In a claims database analysis, patients with IPF were reported to have a significantly higher prevalence of coronary artery disease, heart failure, atrial fibrillation, and myocardial infarction than matched controls [13]. Patients with IPF have also been reported to be significantly more likely than the general population to have a history of hypertension and diabetes and to have received several CV medications, even prior to IPF diagnosis [8, 11]. A higher prevalence of coronary artery disease has also been reported in patients with IPF compared with matched patients who had chronic obstructive pulmonary disease [9].
A recent study of the impact of comorbidities on mortality in patients with IPF showed a significant negative impact of coronary artery disease, arteriosclerosis, and other CV diseases (mainly valvular heart disease, cardiac arrhythmias, and dilated cardiomyopathy) on survival [14]. Of note, the use of statins in patients with IPF was recently reported to be associated with better clinical outcomes, including lower risks of death, all-cause hospitalization, respiratory-related hospitalization, and IPF-related mortality [15]. On the other hand, warfarin as a primary therapy for IPF was shown to be associated with an increased risk for all-cause mortality and combined all-cause mortality (i.e., non-elective, non-bleeding hospitalization, and mortality) in a clinical trial, which was stopped early because of these deleterious outcomes [16]. Furthermore, a post hoc analysis of patients with IPF who were randomized to placebo in the pirfenidone phase III trials suggested that baseline use of anticoagulants (90.6% warfarin) for medical indications (primarily atrial fibrillation, prophylaxis of venous thromboembolism, and deep vein thrombosis) was an independent risk factor for IPF-related mortality [17]. In addition, a retrospective cohort study of patients with IPF suggested worse survival and shorter time to disease progression in patients receiving anticoagulants [18]. There is a general uncertainty about the safety of warfarin in patients with IPF with secondary indications for anticoagulation, such as atrial fibrillation and venous thromboembolic disease [17, 19].
Due to the increased CV risk in the IPF patient population and its correlation with mortality, understanding the impact of IPF treatment on CV and bleeding events in patients with IPF is critical. This analysis examined whether pirfenidone treatment impacted the CV [major adverse cardiac events–plus (MACE-plus)] and bleeding risks in patients with IPF enrolled in the three phase III trials of pirfenidone. In addition, the analysis also assessed whether concomitant CV medication use affected the treatment outcome of pirfenidone. The prevalence of CV risk factors, CV comorbidities, and related concomitant medication use in these three trials is provided. These findings will provide an additional consideration when implementing treatment for IPF, especially in patients with CV risk factors.
Methods
Data Source
All patients randomized to pirfenidone 2403 mg/day or placebo in the phase III paired CAPACITY studies (studies 004 and 006; NCT00287716 and NCT00287729) and the ASCEND study (study 016; NCT01366209) were included [20, 21]. Because 2403 mg/day is the target approved pirfenidone dose for treatment of IPF, 87 patients in the CAPACITY study who received the lower dose of 1197 mg/day were not included in this analysis.
In the two CAPACITY studies, patients were treated for a minimum of 72 weeks and followed up until study closure (maximum, 120 weeks). In the ASCEND study, patients were treated and followed up for 52 weeks. Patients with a history of unstable or deteriorating cardiac or pulmonary disease (other than IPF) within the 6 months before enrollment were ineligible for all three studies. There were no exclusion criteria for patients receiving antithrombotic (antiplatelet or anticoagulation) medications; however, these drugs could not be prescribed as primary therapy for IPF.
A complete medical history at baseline and concomitant medication use during the treatment period were collected and recorded by study investigators. These studies were conducted in full conformance with the Guidelines for Good Clinical Practice and principles of the Declaration of Helsinki. Approval was obtained from all ethics committees/independent review boards at each study site (see Appendix 1, Supporting Information), and all patients provided written informed consent.
Analyses
Patient demographics and clinical characteristics at baseline were reported for the pirfenidone 2403 mg/day and placebo groups. A post hoc, blinded review of treatment-emergent MACE-plus and bleeding AEs that occurred up to 28 days post-treatment was conducted. MACE-plus events were adjudicated by an external, academic cardiologist who was blinded to the assigned treatment.
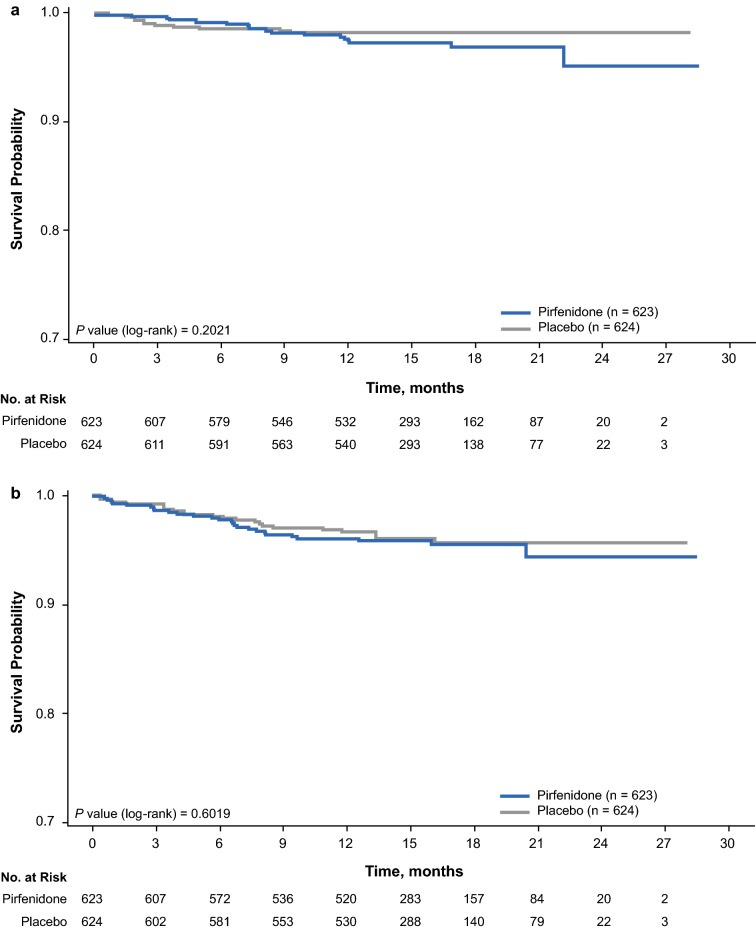
AE preferred terms were used to identify occurrences of MACE-plus and bleeding events. MACE-plus included any CV death [due to myocardial infarction, sudden cardiac death, heart failure, stroke, CV procedures (immediate), and other CV causes (e.g., pulmonary embolism, peripheral arterial disease)], non-fatal myocardial infarction, non-fatal stroke, and acute coronary syndrome [22–24]. Incidences of MACE-plus and bleeding AEs were summarized by treatment group; P values from the Fisher exact test were determined for descriptive purposes. Kaplan-Meier curves were used to graphically display time to first MACE-plus or bleeding event; P values from an unadjusted log-rank test were determined for descriptive purposes.
Subgroup analyses were performed to examine whether the pirfenidone benefit observed in the pooled analyses of the phase III trials persisted when patients also received concomitant cardiovascular medications. Clinical outcomes up to month 12 were compared between treatment arms in each medication subgroup of patients with or without concomitant use of selected CV medication classes during the first year (start date on or before day 365) to assess the impact of these medications on the benefit of pirfenidone. The following outcomes were evaluated: death from any cause; any respiratory-related hospitalization; ≥
≥ 10% absolute decline in percent predicted forced vital capacity (% predicted FVC) or death from any cause; and
10% absolute decline in percent predicted forced vital capacity (% predicted FVC) or death from any cause; and ≥
≥ 10% absolute decline in % predicted FVC or any respiratory-related hospitalization or death from any cause. To be consistent with the primary efficacy analysis from the individual studies, patients with a missing % predicted FVC value for a reason other than death had the value imputed using the sum of squared differences (SSD) method [20, 21]. The percentage of patients with data imputed using the SSD method was low (month 12: 6.9% pirfenidone, 5.4% placebo); sensitivity analyses were performed to examine the primary analysis results using other imputation methods and the findings remained consistent [20, 21, 25]. For cases when the % predicted FVC was imputed, the corresponding FVC assessment date was imputed as the targeted visit date or the last assessment date, whichever came first. Medication classes included any antithrombotic agent, any anticoagulant agent, warfarin, heparin (including low-molecular-weight heparins), any antiplatelet agent, acetylsalicylic acid, and any statin. Within each medication class, the hazard ratio (95% confidence interval) for the time to first event between pirfenidone and placebo groups was estimated using the Cox proportional hazards model, with individual studies as a stratification factor and no additional covariate adjustments. The proportional hazards assumption was evaluated using the supremum test [26].
10% absolute decline in % predicted FVC or any respiratory-related hospitalization or death from any cause. To be consistent with the primary efficacy analysis from the individual studies, patients with a missing % predicted FVC value for a reason other than death had the value imputed using the sum of squared differences (SSD) method [20, 21]. The percentage of patients with data imputed using the SSD method was low (month 12: 6.9% pirfenidone, 5.4% placebo); sensitivity analyses were performed to examine the primary analysis results using other imputation methods and the findings remained consistent [20, 21, 25]. For cases when the % predicted FVC was imputed, the corresponding FVC assessment date was imputed as the targeted visit date or the last assessment date, whichever came first. Medication classes included any antithrombotic agent, any anticoagulant agent, warfarin, heparin (including low-molecular-weight heparins), any antiplatelet agent, acetylsalicylic acid, and any statin. Within each medication class, the hazard ratio (95% confidence interval) for the time to first event between pirfenidone and placebo groups was estimated using the Cox proportional hazards model, with individual studies as a stratification factor and no additional covariate adjustments. The proportional hazards assumption was evaluated using the supremum test [26].
As a reference, real-world CV comorbidity rates were also provided based on data from the prospective, real-world pirfenidone registry, PASSPORT, and similar registries of patients with IPF, INSIGHTS-IPF and IPF-PRO [27–29].
Results
Baseline Demographics of Patients in the Phase III Trials of Pirfenidone
A total of 1247 patients (555 from ASCEND and 692 from CAPACITY) were included. Among these patients, 623 received pirfenidone 2403 mg/day and 624 received placebo.
Baseline demographics and clinical characteristics were similar between the pirfenidone and placebo groups (Table 1). Overall, the median age was 68.0 years, and 74.4% of patients were male. Upon evaluation of smoking status, 34.6% of patients had never smoked, 63.4% had previously smoked, and 2.0% were current smokers (the ASCEND study did not enroll current smokers).
Table 1
Baseline demographics and clinical characteristics
| Pirfenidone 2403 mg/day (n  = = 623) 623) | Placebo (n  = = 624) 624) | Total (N  = = 1247) 1247) | |
|---|---|---|---|
| Age, median (range), (years) | 68.0 (45–80) | 68.0 (40–80) | 68.0 (40–80) |
| Male, (%) | 74.3 | 74.5 | 74.4 |
| White, (%) | 95.0 | 94.6 | 94.8 |
| Body weight, median (range), (kg) | |||
| Male | 89.8 (50–168) | 88.9 (53–147) | 89.5 (50–168) |
| Female | 74.0 (40–120) | 73.0 (40–115) | 73.0 (40–120) |
| BMI, median (range), (kg/m2) | |||
| Male | 29.5 (19–44) | 29.3 (20–48) | 29.4 (19–48) |
| Female | 29.8 (19–47) | 29.3 (15–44) | 29.5 (15–47) |
Never smoked (< 100 cigarettes in life), (%) 100 cigarettes in life), (%) | 33.5 | 35.7 | 34.6 |
Previously smoked (≥ 100 cigarettes in life), (%) 100 cigarettes in life), (%) | 65.2 | 61.5 | 63.4 |
| Packs per day, mean (SD)a | 1.03 (0.62) | 1.08 (0.59) | 1.05 (0.60) |
| Current smoker, (%)b | 1.3 | 2.7 | 2.0 |
| Packs per day, mean (SD) | 0.65 (0.47) | 1.03 (0.46) | 0.91 (0.49) |
BMI body mass index
aData on packs per day were collected only in the CAPACITY studies in 692 patients (345 for pirfenidone and 347 for placebo)
bCurrent smokers were excluded from the ASCEND study
Cardiovascular Comorbidities and Concomitant Medication Profile
Aside from older age and male sex, the most common CV risk factors at baseline were smoking, hypertension, obesity, hypercholesterolemia, and hyperlipidemia (Table 2). Of the 1247 patients in the clinical trials, approximately half (52.0%) reported hypertension at baseline. Obesity, hypercholesterolemia, hyperlipidemia, diabetes (type 1 or 2), and sleep apnea were reported in 44.3%, 23.4%, 22.6%, 20.4%, and 14.9% of patients at baseline, respectively.
Table 2
Cardiovascular and bleeding risk factors at baseline
| Disease or disorder, n (%) | Pirfenidone 2403 mg/day (n  = = 623) 623) | Placebo (n  = = 624) 624) | Total (N  = = 1247) 1247) |
|---|---|---|---|
| Baseline CV risk factorsa | 574 (92.1) | 576 (92.3) | 1150 (92.2) |
Patients with ≥ ≥ 3 CV risk factorsa 3 CV risk factorsa | 286 (45.9) | 280 (44.9) | 566 (45.4) |
| Smoking (former or current)b | 414 (66.5) | 401 (64.2) | 815 (65.3) |
| Hypertension | 308 (49.4) | 340 (54.5) | 648 (52.0) |
| Obesityc | 285 (45.7) | 268 (42.9) | 553 (44.3) |
Class I (BMI, ≥ ≥ 30 to 30 to < < 35) 35) | 208 (33.4) | 197 (31.6) | 405 (32.5) |
Class II (BMI, ≥ ≥ 35 to 35 to < < 40) 40) | 57 (9.1) | 59 (9.5) | 116 (9.3) |
Class III (BMI, ≥ ≥ 40) 40) | 20 (3.2) | 12 (1.9) | 32 (2.6) |
| Hypercholesterolemia | 152 (24.4) | 140 (22.4) | 292 (23.4) |
| Hyperlipidemia | 144 (23.1) | 138 (22.1) | 282 (22.6) |
| Diabetes, type 1 or 2 | 134 (21.5) | 120 (19.2) | 254 (20.4) |
| Sleep apnea | 91 (14.6) | 95 (15.2) | 186 (14.9) |
| Baseline cardiac disorders | 216 (34.7) | 234 (37.5) | 450 (36.1) |
| Coronary artery disease | 97 (15.6) | 98 (15.7) | 195 (15.6) |
| Myocardial infarction | 32 (5.1) | 33 (5.3) | 65 (5.2) |
| Atrial fibrillation | 29 (4.7) | 29 (4.6) | 58 (4.7) |
| Angina pectoris | 19 (3.0) | 12 (1.9) | 31 (2.5) |
| Deep vein thrombosis | 13 (2.1) | 14 (2.2) | 27 (2.2) |
| Pulmonary embolism | 8 (1.3) | 6 (1.0) | 14 (1.1) |
BMI body mass index, CV cardiovascular
aCV risk factors, not including age or sex, were defined as smoking, hypertension, hypercholesterolemia, hyperlipidemia, diabetes, sleep apnea, and obesity (BMI ≥
≥ 30)
30)
bThe ASCEND study did not enroll current smokers
cObesity was based on the World Health Organization classification of BMI [38]
Cardiac disorders as a system organ class were reported in 36.1% of patients at baseline (Table 2). Among these, the most commonly reported conditions were coronary artery disease (15.6%), myocardial infarction (5.2%), and atrial fibrillation (4.7%). Overall, 566 patients (45.4%) had ≥
≥ 3 CV risk factors (excluding age or sex) [30–32]. The CV-related medical history profiles at baseline were generally similar between the pirfenidone and placebo groups. History of deep vein thrombosis and pulmonary embolism at baseline was reported in 2.2% and 1.1% of all patients, respectively (Table 2).
3 CV risk factors (excluding age or sex) [30–32]. The CV-related medical history profiles at baseline were generally similar between the pirfenidone and placebo groups. History of deep vein thrombosis and pulmonary embolism at baseline was reported in 2.2% and 1.1% of all patients, respectively (Table 2).
During the treatment period of the pirfenidone phase III trials, the most common concomitant CV medications were lipid-modifying agents (60.3%), antithrombotic agents (54.2%), inhibitors of the renin-angiotensin–aldosterone system (38.7%), and beta-blockers (25.0%) (Table 3). Concomitant use of angiotensin-converting enzyme inhibitors, warfarin, heparin, and statins was permitted in the pirfenidone trials for treatment of a non-IPF indication if no other clinically acceptable therapy for the same indication was available. Almost half of all patients (48.9%) were receiving antiplatelet agents, and 12.2% were receiving anticoagulation agents. Overall, the use of various CV-related medications was generally similar between the pirfenidone and placebo groups.
Table 3
Most common concomitant cardiovascular medications used
| Medication, n (%) | Pirfenidone 2403 mg/day (n  = = 623)a 623)a | Placebo (n  = = 624)a 624)a | Total (N  = = 1247) 1247) |
|---|---|---|---|
| Lipid-modifying agents | 378 (60.7) | 374 (59.9) | 752 (60.3) |
| Statins | 314 (50.4) | 314 (50.3) | 628 (50.4) |
| Fish oil | 84 (13.5) | 104 (16.7) | 188 (15.1) |
| Nicotinic acid | 27 (4.3) | 26 (4.2) | 53 (4.3) |
| Antithrombotic agents | 319 (51.2) | 357 (57.2) | 676 (54.2) |
| Antiplatelet agents | 294 (47.2) | 316 (50.6) | 610 (48.9) |
| ASA (all doses) | 286 (45.9) | 305 (48.9) | 591 (47.4) |
| Any non-ASA antiplatelet agent | 44 (7.1) | 54 (8.7) | 98 (7.9) |
| Anticoagulation agents | 66 (10.6) | 86 (13.8) | 152 (12.2) |
| Heparin (including low-molecular-weight heparins) | 35 (5.6) | 51 (8.2) | 86 (6.9) |
| Warfarin | 34 (5.5) | 42 (6.7) | 76 (6.1) |
| Inhibitors of the RAAS | 217 (34.8) | 266 (42.6) | 483 (38.7) |
| Lisinopril | 54 (8.7) | 70 (11.2) | 124 (9.9) |
| Losartan | 36 (5.8) | 45 (7.2) | 81 (6.5) |
| Valsartan | 33 (5.3) | 45 (7.2) | 78 (6.3) |
| Beta-blockers | 154 (24.7) | 158 (25.3) | 312 (25.0) |
| Metoprolol | 89 (14.3) | 78 (12.5) | 167 (13.4) |
| Atenolol | 37 (5.9) | 41 (6.6) | 78 (6.3) |
| Carvedilol | 16 (2.6) | 16 (2.6) | 32 (2.6) |
| Diuretics | 130 (20.9) | 140 (22.4) | 270 (21.7) |
| Furosemide | 53 (8.5) | 69 (11.1) | 122 (9.8) |
| Hydrochlorothiazide | 52 (8.3) | 46 (7.4) | 98 (7.9) |
| Hydrochlorothiazide and triamterene | 18 (2.9) | 10 (1.6) | 28 (2.2) |
| Antidiabetes agents | 131 (21.0) | 127 (20.4) | 258 (20.7) |
| Metformin | 91 (14.6) | 84 (13.5) | 175 (14.0) |
| Insulin | 27 (4.3) | 33 (5.3) | 60 (4.8) |
| Glibenclamide | 22 (3.5) | 14 (2.2) | 36 (2.9) |
| Calcium-channel blockers | 107 (17.2) | 112 (17.9) | 219 (17.6) |
| Amlodipine | 53 (8.5) | 64 (10.3) | 117 (9.4) |
| Diltiazem | 36 (5.8) | 31 (5.0) | 67 (5.4) |
| Verapamil | 12 (1.9) | 13 (2.1) | 25 (2.0) |
| Other antihypertensive agents | 15 (2.4) | 16 (2.6) | 31 (2.5) |
| Doxazosin | 9 (1.4) | 3 (0.5) | 12 (1.0) |
| Clonidine | 1 (0.2) | 7 (1.1) | 8 (0.6) |
| Terazosin | 2 (0.3) | 3 (0.5) | 5 (0.4) |
ASA acetylsalicylic acid, RAAS renin-angiotensin-aldosterone system
aThe maximum study durations of treatment with pirfenidone and placebo in the pooled clinical trials were 27 and 28 months, respectively. All concomitant medication use during those periods has been included
CV and Bleeding Events
In this pooled analysis, the median (range) durations of exposure to pirfenidone 2403 mg/day and placebo were 12.3 months (> 0–27 months) and 12.5 months (>
0–27 months) and 12.5 months (> 0–28 months), respectively. MACE-plus events were reported in 2.3% of patients overall. CV death occurred in 0.6% of patients (due to myocardial infarction in 2 patients and acute myocardial infarction, acute cor pulmonale, arrhythmia, cardiac arrest, hemorrhagic stroke, and sudden cardiac death in 1 patient each), and non-fatal myocardial infarction in 0.6%, non-fatal stroke in 0.8%, and acute coronary syndrome in 0.2% of patients (Table 4).
0–28 months), respectively. MACE-plus events were reported in 2.3% of patients overall. CV death occurred in 0.6% of patients (due to myocardial infarction in 2 patients and acute myocardial infarction, acute cor pulmonale, arrhythmia, cardiac arrest, hemorrhagic stroke, and sudden cardiac death in 1 patient each), and non-fatal myocardial infarction in 0.6%, non-fatal stroke in 0.8%, and acute coronary syndrome in 0.2% of patients (Table 4).
Table 4
Treatment-emergent major adverse cardiac events and bleeding events
| Pirfenidone 2403 mg/day (n  = = 623) 623) | Placebo (n  = = 624) 624) | Total (N  = = 1247) 1247) | ||||
|---|---|---|---|---|---|---|
| n (%) | No. of events | n (%) | No. of events | n (%) | No. of events | |
| MACE-plusa | 11 (1.8) | 13 | 18 (2.9) | 19 | 29 (2.3) | 32 |
| CV death | 3 (0.5) | 3 | 5 (0.8) | 5 | 8 (0.6) | 8 |
| Myocardial infarction | 1 (0.2) | 1 | 1 (0.2) | 1 | 2 (0.2) | 2 |
| Acute myocardial infarction | 0 | 0 | 1 (0.2) | 1 | 1 (0.1) | 1 |
| Acute cor pulmonale | 0 | 0 | 1 (0.2) | 1 | 1 (0.1) | 1 |
| Arrhythmia | 1 (0.2) | 1 | 0 | 0 | 1 (0.1) | 1 |
| Cardiac arrest | 1 (0.2) | 1 | 0 | 0 | 1 (0.1) | 1 |
| Hemorrhagic stroke | 0 | 0 | 1 (0.2) | 1 | 1 (0.1) | 1 |
| Sudden cardiac death | 0 | 0 | 1 (0.2) | 1 | 1 (0.1) | 1 |
| Non-fatal myocardial infarction | 3 (0.5) | 4 | 5 (0.8) | 6 | 8 (0.6) | 10 |
| Acute myocardial infarction | 2 (0.3) | 3 | 1 (0.2) | 2 | 3 (0.2) | 5 |
| Myocardial infarction | 1 (0.2) | 1 | 4 (0.6) | 4 | 5 (0.4) | 5 |
| Non-fatal stroke | 4 (0.6) | 4 | 6 (1.0) | 6 | 10 (0.8) | 10 |
| Cerebrovascular accident | 2 (0.3) | 2 | 2 (0.3) | 2 | 4 (0.3) | 4 |
| Cerebral artery occlusion | 1 (0.2) | 1 | 0 | 0 | 1 (0.1) | 1 |
| Hemorrhagic stroke | 0 | 0 | 1 (0.2) | 1 | 1 (0.1) | 1 |
| Ischemic stroke | 1 (0.2) | 1 | 0 | 0 | 1 (0.1) | 1 |
| Lacunar infarction | 0 | 0 | 1 (0.2) | 1 | 1 (0.1) | 1 |
| Thrombotic stroke | 0 | 0 | 1 (0.2) | 1 | 1 (0.1) | 1 |
| Transient ischemic attack | 0 | 0 | 1 (0.2) | 1 | 1 (0.1) | 1 |
| Acute coronary syndrome | 1 (0.2) | 2 | 2 (0.3) | 2 | 3 (0.2) | 4 |
| Acute coronary syndrome | 1 (0.2) | 1 | 0 | 0 | 1 (0.1) | 1 |
| Coronary artery occlusion | 0 | 0 | 1 (0.2) | 1 | 1 (0.1) | 1 |
| Stent occlusion | 1 (0.2) | 1 | 0 | 0 | 1 (0.1) | 1 |
| Unstable angina | 0 | 0 | 1 (0.2) | 1 | 1 (0.1) | 1 |
| Bleeding eventb | 23 (3.7) | 26 | 27 (4.3) | 38 | 50 (4.0) | 64 |
| Hemoptysis | 10 (1.6) | 12 | 10 (1.6) | 14 | 20 (1.6) | 26 |
| Hematochezia | 4 (0.6) | 4 | 6 (1.0) | 9 | 10 (0.8) | 13 |
| Rectal hemorrhage | 2 (0.3) | 2 | 3 (0.5) | 4 | 5 (0.4) | 6 |
| Gastrointestinal hemorrhage | 2 (0.3) | 2 | 1 (0.2) | 1 | 3 (0.2) | 3 |
| Hemorrhagic stroke | 0 | 0 | 2 (0.3) | 2 | 2 (0.2) | 2 |
| Pulmonary alveolar hemorrhage | 0 | 0 | 2 (0.3) | 3 | 2 (0.2) | 3 |
| Vaginal hemorrhage | 1 (0.2) | 1 | 1 (0.2) | 1 | 2 (0.2) | 2 |
| Catheter-site hemorrhage | 1 (0.2) | 1 | 0 | 0 | 1 (0.1) | 1 |
| Extravasation blood | 0 | 0 | 1 (0.2) | 1 | 1 (0.1) | 1 |
| Urinary tract hemorrhage | 1 (0.2) | 1 | 0 | 0 | 1 (0.1) | 1 |
| Hemorrhoidal hemorrhage | 0 | 0 | 1 (0.2) | 1 | 1 (0.1) | 1 |
| Injection-site hemorrhage | 0 | 0 | 1 (0.2) | 1 | 1 (0.1) | 1 |
| Large intestinal hemorrhage | 0 | 0 | 1 (0.2) | 1 | 1 (0.1) | 1 |
| Peptic ulcer hemorrhage | 1 (0.2) | 1 | 0 | 0 | 1 (0.1) | 1 |
| Post-menopausal hemorrhage | 1 (0.2) | 1 | 0 | 0 | 1 (0.1) | 1 |
| Pulmonary hemorrhage | 1 (0.2) | 1 | 0 | 0 | 1 (0.1) | 1 |
CV cardiovascular, MACE major adverse cardiovascular event
aSome patients had >
> 1 type of MACE-plus event
1 type of MACE-plus event
bSome patients had >
> 1 type of bleeding event
1 type of bleeding event
No difference was observed in the incidence of MACE-plus events between patients in the pirfenidone and placebo groups; MACE-plus events were reported in 1.8% of patients in the pirfenidone group and 2.9% of patients in the placebo group (P =
= 0.2593) (Fig. 1a, Table 4). CV death was reported in 0.5% of patients in the pirfenidone group and 0.8% of patients in the placebo group. Non-fatal myocardial infarction was reported in 0.5% of patients in the pirfenidone group and 0.8% of patients in the placebo group, and non-fatal stroke was reported in 0.6% of patients in the pirfenidone group and 1.0% of patients in the placebo group. Acute coronary syndrome events were reported in 0.2% of patients in the pirfenidone group and 0.3% of patients in the placebo group.
0.2593) (Fig. 1a, Table 4). CV death was reported in 0.5% of patients in the pirfenidone group and 0.8% of patients in the placebo group. Non-fatal myocardial infarction was reported in 0.5% of patients in the pirfenidone group and 0.8% of patients in the placebo group, and non-fatal stroke was reported in 0.6% of patients in the pirfenidone group and 1.0% of patients in the placebo group. Acute coronary syndrome events were reported in 0.2% of patients in the pirfenidone group and 0.3% of patients in the placebo group.
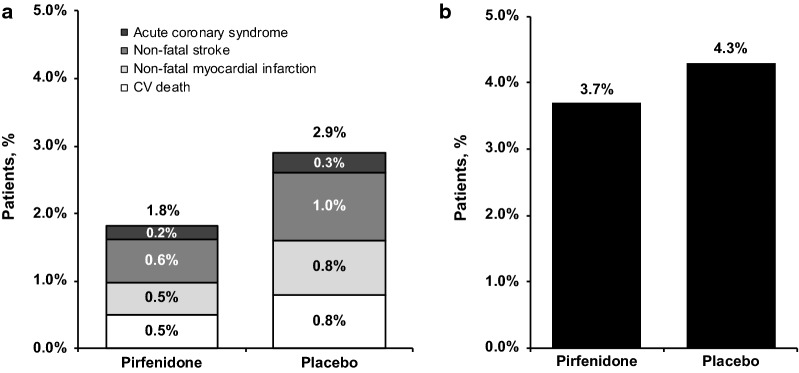
Treatment-emergent major adverse cardiovascular events–plus (a) and treatment-emergent bleeding events (b)
Bleeding events were reported in 4.0% of patients overall, the most common being hemoptysis in 1.6% of patients (Table 4). The incidence of bleeding events was not different between the pirfenidone and placebo groups, occurring in 3.7% of patients in the pirfenidone group and 4.3% of patients in the placebo group (P =
= 0.6654) (Fig. 1b).
0.6654) (Fig. 1b).
No difference was observed in the time to first MACE-plus or bleeding event between the pirfenidone and placebo groups (Fig. 2).
IPF Outcomes in Patients Receiving Concomitant CV Medications
The effect of pirfenidone on the outcomes of death, respiratory-related hospitalization, a composite of ≥
≥ 10% absolute decline in % predicted FVC or death, and a composite of
10% absolute decline in % predicted FVC or death, and a composite of ≥
≥ 10% absolute decline in % predicted FVC, respiratory-related hospitalization, or death from baseline to month 12 is shown in Fig. 3 and S1 Table, according to the use or non-use of selected concomitant CV medications. Except for patients receiving heparin, pirfenidone had a beneficial effect on outcomes among both users and non-users of all concomitant CV medications analyzed. The benefit of pirfenidone over placebo on the outcomes of death and respiratory-related hospitalizations was greater among statin users than non-users. The benefit of pirfenidone compared with placebo was evident in both users and non-users of warfarin.
10% absolute decline in % predicted FVC, respiratory-related hospitalization, or death from baseline to month 12 is shown in Fig. 3 and S1 Table, according to the use or non-use of selected concomitant CV medications. Except for patients receiving heparin, pirfenidone had a beneficial effect on outcomes among both users and non-users of all concomitant CV medications analyzed. The benefit of pirfenidone over placebo on the outcomes of death and respiratory-related hospitalizations was greater among statin users than non-users. The benefit of pirfenidone compared with placebo was evident in both users and non-users of warfarin.
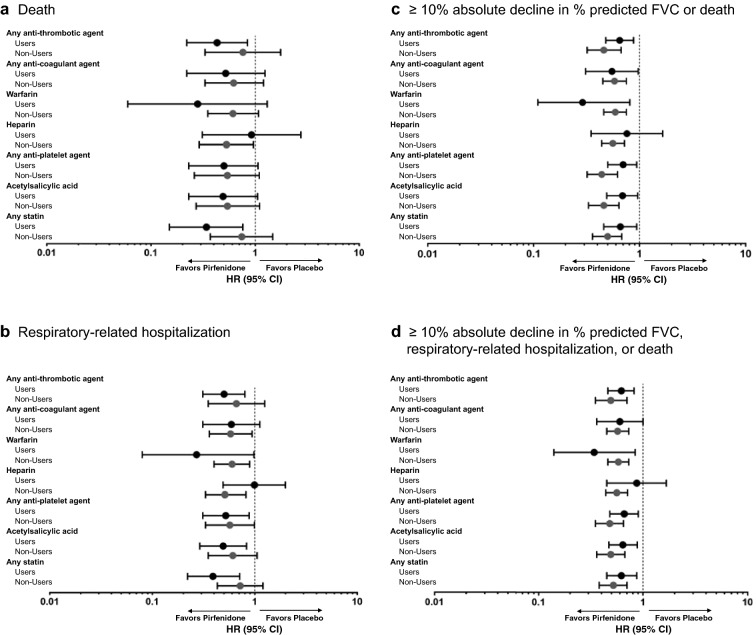
Hazard ratios for death (a), respiratory-related hospitalization (b), ≥
≥ 10% absolute decline in % predicted FVC or death (c), and ≥
10% absolute decline in % predicted FVC or death (c), and ≥ 10% absolute decline in % predicted FVC, respiratory-related hospitalization, or death (d) among users and non-users of concomitant CV medications over 12 months*. CV cardiovascular, FVC forced vital capacity, HR hazard ratio. *Only medications with a start date on or before day 365 were included in this analysis, which evaluated outcomes for 12 months. Heparin includes low-molecular-weight heparins. Subgroup n and HR values can be found in S1 Table
10% absolute decline in % predicted FVC, respiratory-related hospitalization, or death (d) among users and non-users of concomitant CV medications over 12 months*. CV cardiovascular, FVC forced vital capacity, HR hazard ratio. *Only medications with a start date on or before day 365 were included in this analysis, which evaluated outcomes for 12 months. Heparin includes low-molecular-weight heparins. Subgroup n and HR values can be found in S1 Table
Discussion
Given the older age and high reported prevalence of CV-related comorbidities in patients with IPF, the purpose of this analysis was to describe the frequency of baseline CV risk factors, CV comorbidities, and related concomitant medication use and to assess CV and bleeding events in patients with IPF from the pirfenidone phase III clinical trials. The impact of specific CV medication use concomitant with pirfenidone on clinical outcomes was also examined. Consistent with previous reports and with findings in real-world observational registries, CV risk factors and comorbidities were highly prevalent in patients with IPF enrolled in the pirfenidone phase III trial population [8–10, 27–29]. Current or former smoking, hypertension, obesity, hypercholesterolemia, and hyperlipidemia were the most common risk factors, reported in 65%, 52%, 44%, 23%, and 23% of patients, respectively. The percentages of patients with CV risk factors in the pirfenidone phase III trials were consistent with data from prospective real-world registries, including PASSPORT (patients using pirfenidone) and INSIGHTS-IPF and IPF-PRO (patients with IPF), although actual rates varied, and the low number of patients in IPF-PRO limits comparisons (S1 Fig) [27–29]. In PASSPORT, hypertension and coronary artery disease were reported in 42% and 11% of patients at baseline, respectively. In INSIGHTS-IPF, coronary artery disease was reported in 25% of patients at baseline; in IPF-PRO, sleep apnea and coronary artery disease were reported in 29% and 31% of patients at baseline, respectively.
In this IPF clinical trial population, the use of concomitant CV and antithrombotic medications was frequent, with approximately half of all patients receiving statins (50.4%) and/or antiplatelet agents (48.9%). The percentage of patients who experienced CV or bleeding events was similar between groups receiving pirfenidone 2403 mg/day or placebo.
The beneficial effect of pirfenidone on the month 12 outcomes of death, respiratory-related hospitalization, and composites (including ≥
≥ 10% absolute decline in % predicted FVC) was generally apparent in both users and non-users of CV and antithrombotic medications. The overall benefit of pirfenidone on these outcomes among patients receiving warfarin is encouraging considering current international guidelines, which strongly recommend against the use of warfarin in patients with IPF [33]. This recommendation is based on the results of the ACE-IPF study, which evaluated warfarin as a primary therapy for IPF [16]. Not only did warfarin fail to exhibit efficacy in ACE-IPF, but patients with IPF receiving warfarin had increased mortality [16]. In a previous analysis, patients in the phase III trials who were randomized to placebo and received anticoagulants had an increased risk of IPF-related mortality vs non-users of anticoagulants, supporting the results in the ACE-IPF study [17, 18]. In contrast, our findings suggest that warfarin can be safely administered with pirfenidone and retain the beneficial effect of pirfenidone without detriment to the patient. Importantly, most patients receiving warfarin did so for the entire study period. However, because of the small number of patients treated with both pirfenidone and warfarin during the first year (n
10% absolute decline in % predicted FVC) was generally apparent in both users and non-users of CV and antithrombotic medications. The overall benefit of pirfenidone on these outcomes among patients receiving warfarin is encouraging considering current international guidelines, which strongly recommend against the use of warfarin in patients with IPF [33]. This recommendation is based on the results of the ACE-IPF study, which evaluated warfarin as a primary therapy for IPF [16]. Not only did warfarin fail to exhibit efficacy in ACE-IPF, but patients with IPF receiving warfarin had increased mortality [16]. In a previous analysis, patients in the phase III trials who were randomized to placebo and received anticoagulants had an increased risk of IPF-related mortality vs non-users of anticoagulants, supporting the results in the ACE-IPF study [17, 18]. In contrast, our findings suggest that warfarin can be safely administered with pirfenidone and retain the beneficial effect of pirfenidone without detriment to the patient. Importantly, most patients receiving warfarin did so for the entire study period. However, because of the small number of patients treated with both pirfenidone and warfarin during the first year (n =
= 28; S1 Table), these findings must be interpreted with caution. No apparent difference was observed in outcomes between pirfenidone and placebo among heparin users; this finding should also be interpreted with caution due to small sample size (n
28; S1 Table), these findings must be interpreted with caution. No apparent difference was observed in outcomes between pirfenidone and placebo among heparin users; this finding should also be interpreted with caution due to small sample size (n =
= 25 for pirfenidone; n
25 for pirfenidone; n =
= 40 for placebo; S1 Table) and limited duration of use during the study period (most patients received heparin for
40 for placebo; S1 Table) and limited duration of use during the study period (most patients received heparin for ≤
≤ 30 days). To examine the effects of pirfenidone on clinical efficacy outcomes, larger real-world studies of patients receiving anticoagulants for treatment of medical indications will need to be analyzed.
30 days). To examine the effects of pirfenidone on clinical efficacy outcomes, larger real-world studies of patients receiving anticoagulants for treatment of medical indications will need to be analyzed.
Among users of statins, treatment with pirfenidone was associated with a beneficial effect at month 12 on death, respiratory-related hospitalizations, and the composite endpoints, including ≥
≥ 10% absolute decline in % predicted FVC. Statin use in patients with IPF has shown potential clinical benefits. A post hoc analysis of the use of statins in patients in the placebo arms of the pirfenidone phase III trials found that statin use was associated with improved disease-related outcomes, including IPF-related mortality and all-cause and respiratory-related hospitalization [15].
10% absolute decline in % predicted FVC. Statin use in patients with IPF has shown potential clinical benefits. A post hoc analysis of the use of statins in patients in the placebo arms of the pirfenidone phase III trials found that statin use was associated with improved disease-related outcomes, including IPF-related mortality and all-cause and respiratory-related hospitalization [15].
Previous integrated analyses of the clinical trials of pirfenidone demonstrated its favorable long-term safety [34, 35]. Given the older age and high reported prevalence of CV risk factors and comorbidities in patients with IPF, it is important to understand the impact of IPF treatment specifically on CV and bleeding risks. MACE-plus and bleeding events occurred in 2.3% and 4.0% of the clinical trial population, respectively, over a median duration of exposure to study drug of approximately 12 months. The incidences of MACE-plus and bleeding events were similar between the pirfenidone and placebo groups, suggesting that pirfenidone did not increase the risk of CV or bleeding events compared with placebo.
Although many factors such as lifestyle and convenience contribute to the choice of treatment in IPF, given the potential to affect comorbid conditions and interact with concomitant medications, a drug’s mechanism of action is also an important consideration. Although the exact mechanism of action of pirfenidone is unclear, this analysis suggests that pirfenidone treatment does not affect CV or bleeding risk in patients with IPF, many of whom have increased numbers of CV risk factors and comorbidities. Interestingly, in a rat model of cardiac fibrosis, pirfenidone demonstrated a protective effect, which may be partially controlled by a feedback loop of the angiotensin II type 1 receptor/p38 mitogen-activated kinase/renin-angiotensin system axis via liver X receptor-α activation [36]. Furthermore, the use of several concomitant CV medications, including warfarin, did not appear to adversely impact the benefit of pirfenidone on efficacy outcomes. Considering the high prevalence of patients with CV risk factors in the IPF population as confirmed in this analysis, in addition to the recent study showing the association of CV disease with increased mortality in patients with IPF, the findings here provide an additional consideration when implementing treatment for IPF, especially in patients with CV risk factors [14]. Of note, use of nintedanib, presumably because of one of its mechanisms of action (inhibition of vascular endothelial growth factor receptor), is associated with bleeding events and arterial thromboembolic events, which are listed among the warnings and precautions in the prescribing information [37].
Study Limitations
This analysis is limited to a carefully selected IPF clinical trial population; patients with unstable or deteriorating cardiac disease within the previous 6 months were excluded from the pirfenidone clinical trials. Despite this limitation, CV comorbidities and CV medication use were common, and CV events were found to be relatively infrequent over the limited duration of the studies. The occurrence of CV events might be higher in broader groups of patients with IPF. This reasoning underscores the importance of comorbidity awareness in patients with IPF by all providers because pulmonologists typically rely on close collaboration with primary care providers in the comprehensive management of these complicated cases. In addition, this was a post hoc analysis of studies in which MACE-plus and bleeding events were included as reported by the investigator. Concomitant use of some CV medications of interest (e.g., anticoagulants such as warfarin and heparin) was limited in this cohort; therefore, these findings need to be interpreted with caution.
Conclusions
Awareness of CV risk factors, CV comorbidities, and use of concomitant medications is an important consideration in the management and treatment of patients with IPF. In this large, controlled study of patients with IPF, our findings confirm that CV risk factors and comorbidities are highly prevalent in patients with IPF and suggest that pirfenidone does not increase the risk of MACE-plus or bleeding events in these patients. The benefit of pirfenidone on clinical outcomes did not appear to be adversely impacted by use of several concomitant CV medication classes, including warfarin, antiplatelet agents, and statins. Compared with non-users of statins, patients receiving statins displayed a greater benefit of pirfenidone compared with placebo on the outcomes of death and respiratory-related hospitalization. No deleterious effects of treatment with pirfenidone on mortality or other clinical endpoints were observed in patients receiving warfarin; however, because of the small number of patients treated, no firm conclusions can be drawn. Larger studies are needed to investigate the effect of pirfenidone treatment on clinically important outcomes in patients receiving warfarin.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the participants in our study. Portions of these data were presented at the 2016 American Thoracic Society Annual Meeting. The authors thank Karina Raimundo, Bann-Mo Day, and Connie Lew for their contributions to this study.
Funding
Sponsorship for study and article processing chargers were funded by F. Hoffmann-La Roche Ltd. and Genentech, Inc. All authors had full access to the data and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing and/or Editorial Assistance
Support for third-party writing assistance, furnished by Christine Gould, PhD, CMPP, of Health Interactions, Inc., was provided by F. Hoffmann-La Roche Ltd.
Authorship
All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this article, take responsibility for integrity of the work as a whole and have provided their approval for this version to be published.
Disclosures
Marilyn K. Glassberg was a member of the ASCEND study steering committee; has served as a consultant for Bellerophon, Boehringer Ingelheim, InterMune, and Roche and has received research funding from Genentech/Roche. Steven D. Nathan was a member of the ASCEND study steering committee; has served on a scientific advisory board for and received research funding from InterMune; has received research funding from and served as a consultant for Boehringer Ingelheim, Gilead, and Roche/Genentech; and is on a speakers bureau for Roche/Genentech; his institution has received research funding from Boehringer Ingelheim and Roche/Genentech. Chin-Yu Lin is an employee of Genentech, Inc. Elizabeth A. Morgenthien is an employee of Genentech, Inc. John L. Stauffer is an employee of Genentech, Inc. Willis Chou was an employee of Genentech, Inc., at the time of the manuscript preparation and is currently an employee of FibroGen, Inc. Paul W. Noble was a member of the ASCEND study steering committee and the CAPACITY study steering committee and has served as a consultant for Boehringer Ingelheim, Bristol-Myers Squibb, InterMune, Moerae Matrix, Roche and Takeda.
Compliance with Ethics Guidelines
These studies were conducted in full conformance with the Guidelines for Good Clinical Practice and principles of the Declaration of Helsinki. Approval was obtained from all ethics committees/independent review boards at each study site (see Appendix 1, Supporting Information), and all patients provided written informed consent.
Data Sharing
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. The clinical trial data were collected by InterMune (a wholly owned subsidiary of Roche) and analyzed by statisticians at Genentech/Roche. The analyzed data were then shared with the authors upon request. Qualified researchers may request access to individual patient-level data through the clinical study data request platform (http://www.clinicalstudydatarequest.com). Further details on Roche’s criteria for eligible studies are available here (https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Roche.aspx). For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.9205154.
Change history
9/9/2019
In the Original Publication the colors of Figure 2 have been switched. The correct figures are given below.
References
Full text links
Read article at publisher's site: https://doi.org/10.1007/s12325-019-01052-y
Read article for free, from open access legal sources, via Unpaywall:
https://link.springer.com/content/pdf/10.1007/s12325-019-01052-y.pdf
Citations & impact
Impact metrics
Article citations
Pirfenidone in Idiopathic Pulmonary Fibrosis: Real-World Observation on Efficacy and Safety, Focus on Patients Undergoing Antithrombotic and Anticoagulant.
Pharmaceuticals (Basel), 17(7):930, 11 Jul 2024
Cited by: 1 article | PMID: 39065780 | PMCID: PMC11280355
Pirfenidone use in fibrotic diseases: What do we know so far?
Immun Inflamm Dis, 12(7):e1335, 01 Jul 2024
Cited by: 1 article | PMID: 38967367 | PMCID: PMC11225083
Review Free full text in Europe PMC
Endocrine and metabolic factors and the risk of idiopathic pulmonary fibrosis: a Mendelian randomization study.
Front Endocrinol (Lausanne), 14:1321576, 08 Jan 2024
Cited by: 7 articles | PMID: 38260151 | PMCID: PMC10801027
Current and Future Treatment Landscape for Idiopathic Pulmonary Fibrosis.
Drugs, 83(17):1581-1593, 26 Oct 2023
Cited by: 8 articles | PMID: 37882943 | PMCID: PMC10693523
Review Free full text in Europe PMC
Fibrotic Signaling in Cardiac Fibroblasts and Vascular Smooth Muscle Cells: The Dual Roles of Fibrosis in HFpEF and CAD.
Cells, 11(10):1657, 17 May 2022
Cited by: 5 articles | PMID: 35626694 | PMCID: PMC9139546
Review Free full text in Europe PMC
Go to all (8) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials (3)
- (1 citation) ClinicalTrials.gov - NCT01366209
- (1 citation) ClinicalTrials.gov - NCT00287716
- (1 citation) ClinicalTrials.gov - NCT00287729
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Pirfenidone in patients with idiopathic pulmonary fibrosis and more advanced lung function impairment.
Respir Med, 153:44-51, 24 Apr 2019
Cited by: 37 articles | PMID: 31153107
Safety and tolerability of acetylcysteine and pirfenidone combination therapy in idiopathic pulmonary fibrosis: a randomised, double-blind, placebo-controlled, phase 2 trial.
Lancet Respir Med, 4(6):445-453, 05 May 2016
Cited by: 49 articles | PMID: 27161257
Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials.
Lancet, 377(9779):1760-1769, 13 May 2011
Cited by: 1058 articles | PMID: 21571362
Systematic Review and Network Meta-analysis of Idiopathic Pulmonary Fibrosis Treatments.
J Manag Care Spec Pharm, 23(3-b suppl):S5-S16, 01 Mar 2017
Cited by: 27 articles | PMID: 28287346 | PMCID: PMC10410677
Review Free full text in Europe PMC
Funding
Funders who supported this work.

 1
1