Abstract
Free full text

Postural Orthostatic Tachycardia Syndrome Is Associated With Elevated G‐Protein Coupled Receptor Autoantibodies
Abstract
Background
The etiology of postural orthostatic tachycardia syndrome (POTS) is yet to be established. The disorder is often misdiagnosed as chronic anxiety or a panic disorder because the autonomic failure in these patients is not severe. A growing body of evidence suggests that POTS may be an autoimmune disorder. Antinuclear antibodies and elevations of ganglionic, adrenergic, and muscarinic acetylcholine receptor antibodies have all been reported.
Methods and Results
We collected detailed clinical symptoms of 55 patients diagnosed with POTS. We also evaluated serum levels of autoantibodies against 4 subtypes of G‐protein coupled adrenergic receptors and 5 subtypes of G‐protein coupled muscarinic acetylcholine receptors by ELISA. Our patients had a multitude of comorbidities, were predominantly young females, and reported viral‐like symptoms preceding episodes of syncope. We detected a significant number of patients with elevated levels of autoantibodies against the adrenergic alpha 1 receptor (89%) and against the muscarinic acetylcholine M4 receptor (53%). Surprisingly, elevations of muscarinic receptor autoantibodies appeared to be dependent upon elevation of autoantibodies against the A1 adrenergic receptor! Four patients had elevations of G‐protein coupled autoantibodies against all 9 receptor subtypes measured in our study. Five POTS patients had no elevation of any autoantibody; similarly, controls were also negative for autoantibody elevations. There was a weak correlation of clinical symptom severity with G‐protein coupled autoantibodies.
Conclusions
Our observations provide further evidence that, in most cases, POTS patients have at least 1 elevated G‐protein coupled adrenergic autoantibody and, in some instances, both adrenergic and muscarinic autoantibodies, supporting the hypothesis that POTS may be an autoimmune disorder.
Postural orthostatic tachycardia syndrome (POTS) is a disorder affecting as many as 3 million people in the United States,1 predominantly young women of childbearing age, having a spectrum of clinical manifestations.2, 3 The syndrome was first described by Schondorf and Low in 1993, that included a heterogeneous group of conditions/disorders previously reported in the literature, having similar clinical physiological presentations.4, 5 The disorder can be extremely debilitating, and the diagnosis requires the presence of chronic orthostatic intolerance associated with an increased heart rate of ≥30 beats per minute from the supine or sitting basal rate or a rate that exceeds 120 beats per minute when standing or by an upright tilt test that occurs within 10 minutes.6, 7 An inability of the peripheral vasculature to maintain adequate resistance related to orthostatic stress is thought to lead to excessive pooling of blood in the more‐dependent areas of the body.8, 9, 10 Yet, the variety of comorbidities identified in affected patients illustrates the potential for a variety of etiologies for the development of POTS.11, 12, 13, 14, 15, 16 There have been numerous postulates to explain the mechanisms related to the etiology of POTS, with strong evidence that a predisposing viral infection, celiac disease, thyroiditis, and joint hypermobility may be triggers.7, 11
Postural orthostatic tachycardia syndrome may be classified as either a primary (or idiopathic) or secondary condition, and to date the etiology of POTS is complex with no known specific basis that could be utilized to diagnose the disorder with a laboratory test. Clinical history, physical findings, and head‐upright tilt test are, at present, the best diagnostic tools. Primary forms of POTS are idiopathic and are not associated with other diseases, and the most common primary form is referred to as “partial dysautonomic” or “neurogenic” POTS that is usually reported as having a 5:1 female‐to‐male ratio.17, 18, 19 Secondary POTS is associated with a known disease or syndrome; chronic diabetes mellitus is the most common disease related to POTS. Other associated diseases include amyloidosis, sarcoidosis, alcoholism, Lupus, Sjogren's syndrome, heavy metal intoxication, and following chemotherapy (especially from vinca alkaloids).20, 21
There is a growing body of evidence that the etiology of POTS may have an immune‐mediated pathogenesis. Some of the earliest reports have identified autoantibodies against ganglionic acetylcholine receptors in patients diagnosed with dysautonomia.22, 23, 24 Vernino et al24 described their evaluation of 157 patients with a variety of dysautonomias that included 6 of 67 patients (9%) with POTS who were seropositive for antibodies specific to nicotinic acetylcholine receptors. More recently, investigators have reported both β‐adrenergic and muscarinic cholinergic receptor (mAChR) autoantibodies in patients with significant orthostatic hypotension and have postulated that these autoantibodies serve as vasodilators as a novel mechanism inducing or exacerbating orthostatic hypotension.25, 26, 27, 28, 29
The purpose of this study was to evaluate individuals diagnosed with primary POTS for elevations of G‐protein coupled adrenergic and mAChR antibodies. In essence, this was a proof‐of‐concept study to determine whether POTS patients had elevations of these receptor autoantibodies. With the growing body of literature, our hypothesis was that a significant number of our patients would have elevated autoantibodies to G‐protein coupled adrenergic or muscarinic receptors.
Methods
The authors declare that all supporting data are available within the article.
Patients
This study was performed under protocols approved by the Institutional Review Board of The University of Toledo Medical Center. Twenty‐six patients were recruited with informed consent for a prospective evaluation of autoantibodies against G‐protein coupled receptor antibodies, including 4 anti–adrenergic receptor (AdrR) and 5 anti‐mAChR epitopes. An additional 29 subjects were included retrospectively utilizing stored samples of plasma acquired from blood submitted for evaluation of platelet‐dense granule deficiency12 for a total of 55 subjects in this study. All patients had been diagnosed with primary POTS in our Syncope and Autonomic Disorders clinic utilizing clinical history, physical examination, and head‐upright tilt table analysis in the fasting state to be eligible for inclusion. All had a history of orthostatic intolerance manifested by orthostatic tachycardia, weakness, lightheadedness, fatigue, and near syncope for at least 6 months or longer. As part of the clinical workup, each patient had undergone a thorough history and physical examination as well as detailed blood chemistry analysis and thyroid profile analysis.
We utilized a clinical checklist that could be scored as either positive or negative (1 or 0) to be able to calculate an arbitrary numerical severity value of clinical symptoms described by our patients. A total of 135 symptom descriptors were utilized during the patient history and physical review of medical systems with emphasis upon general health (fatigue), head (severe headache), eyes (vision change), heart (palpitations, chest pressure), lungs (shortness of breath, problems sleeping), gastrointestinal (cramping, constipation, or nausea), skeletal (muscle aches, hyperflexibility, or joint pain), psychiatric (anxiety, depression), and blood (easy bruising, frequent nose bleeds). At the time of acquisition of blood samples, we assessed hyperflexibility using the Brighton criteria.30
Patients were excluded from this study if the following criteria were identified: (1) patients on chronic antihypertensive, diuretic, anticholinergic, or antidepressant medications; (2) those with diabetic neuropathy or multisystem disease of any etiology; and (3) any patients immobile for prolonged periods.
ELISA Kits for Detection of G‐Protein Coupled Receptor Antibodies
ELISA kits were purchased from CellTrend GmbH (Luckenwalde, Germany) to detect antibodies against 9 different G‐protein coupled receptor antibodies, including 4 anti–human AdrR epitopes and 5 anti–human mAChR epitopes. These kits have been validated by the manufacturer and used successfully in a recent report identifying autoantibodies to beta adrenergic and muscarinic cholinergic receptors in chronic fatigue syndrome, of which many symptoms overlap with POTS.31 These kits were as follows: AdrR autoantibodies, Alpha 1 (cat. no. 12400), Alpha 2 (cat. no. 12500), Beta 1 (cat. no. 12600), and Beta 2 (cat. no. 12700); and mAChR autoantibodies, M1 (cat. no.15100), M2 (cat. no. 15200), M3 (cat. no. 15300), M4 (cat. no. 15400), and M5 (cat. no. 15500).
Sample Preparation
Blood samples obtained in our prospective study were centrifuged to obtain platelet‐poor plasma that was stored in a freezer for up to a month to have sufficient number of samples for batch processing with the ELISA kits. Samples obtained retrospectively had been stored frozen for up to 2 years. All procedures followed the manufacturer's instructions for each kit and included standards and controls for incubation with test samples. Cut‐off values utilized for determination of elevated antibody titers were established by the manufacturer for each kit. In general, each assay utilized 100 μL of sample, standard, or control for 2 hours of incubation at 4°C and followed by a wash step, a 1‐hour incubation at room temperature for the detection antibodies, another wash step, a substrate incubation for 20 to 30 minutes at room temperature, and finally the addition of a stop solution before spectrophotometry.
Statistical Analysis
Unless otherwise stated, data are presented as mean+1 SD. The Pearson correlation test was used to compare our clinical symptom severity scores with autoantibody concentrations. All statistical analyses were performed and graphed using SigmaPlot software (SSPS, Inc., Chicago, IL).
Results
As stated previously, all patients included in the study had been diagnosed with POTS by head‐upright tilt table analysis. Patient demographics and common clinical symptoms are itemized in Table. Briefly, a number of characteristics and comorbidities appear to be common for the postural orthostatic tachycardia patients included in our study. Fifty‐two (94.5%) of the study subjects were female, with an average age of 29.9±11.2. A number of common complaints were described, with the most common being fatigue (95%), aching muscles and/or palpitations (84%), and severe headache or migraine headache (78%).
Table 1
Patient Demographics
| Study Subjects | |
|---|---|
| n | 55 |
| Age, y | 29.9±11.2 |
| Females | 52 (94.5%) |
| Clinical Symptoms | No. Affected |
|---|---|
| Fatigue | 52 (94.5%) |
| Aching muscles | 46 (83.6%) |
| Palpitations | 46 (83.6%) |
| Severe headache/migraine | 43 (78.2%) |
| Shortness of breath/anxiety | 42 (76.4%) |
| Joint hyperflexibility | 40 (72.7%) |
| Nausea | 39 (70.9%) |
| Easy bruising | 35 (63.6%) |
| Cognitive issues | 35 (63.6%) |
| Irritable bowel syndrome/gastritis | 30 (54.5%) |
| Anemia | 30 (54.5%) |
| Raynaud's syndrome | 27 (49.1%) |
| Epistaxis | 26 (47.3%) |
| Depression | 19 (34.5%) |
| Arthralgia | 12 (21.8%) |
The arbitrary symptom score average was 44.9 from a potential 135 inquiry items. The range of “positive” symptom checks was a low of 12 to a high of 88. There was a tendency that lower symptoms scores correlated with having no autoantibodies or a low titer of autoantibody detected. However, 3 patients, with no elevation of any of the 9 receptors assayed, had scores of 41, 43, and 53 respectfully. Using an arbitrary method of calculating a relative symptom score for determination of symptom severity, a weak correlation with all 9 receptor subtypes of autoantibody concentration was found (Figure 1, AdrR A1 plot, for example). The Pearson correlation coefficients (r) and P values for AdrR autoantibody subtypes were: A1: r=0.31, P=0.02; A2: r=0.33, P=0.01; B1: r=0.29, P=0.03; and B2: r=0.21, P=0.13. The r and P values for mAChR autoantibodies were: M1: r=0.23, P=0.09; M2: r=0.23, P=0.09; M3: r=0.23, P=0.09; M4: r=0.25, P=0.06; and M5: r=0.17, P=0.21. Symptoms such as frequent chest colds, blood in urine, heart murmurs, and vomiting were rarely noted whereas heart palpitations, nausea, and easy bruising were very common.
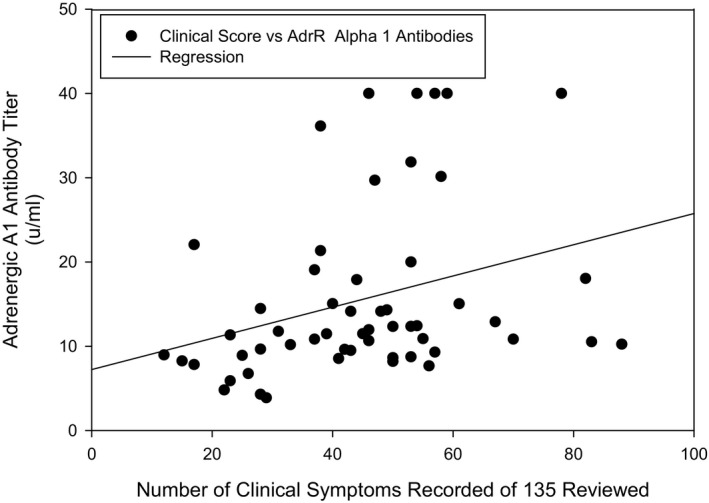
Clinical symptoms reported vs concentration of autoantibodies against adrenergic Alpha1. Receptors weak correlations for both adrenergic and muscarinic receptor autoantibodies detected in POTS patients sera was found with the number of clinical symptoms reported. This graph of adrenergic A1 autoantibody concentration vs symptom number serves as an example. AdrR indicates adrenergic receptor; POTS, postural orthostatic tachycardia syndrome.
G‐Protein Coupled Receptor Autoantibodies
Anti‐AdrR antibodies
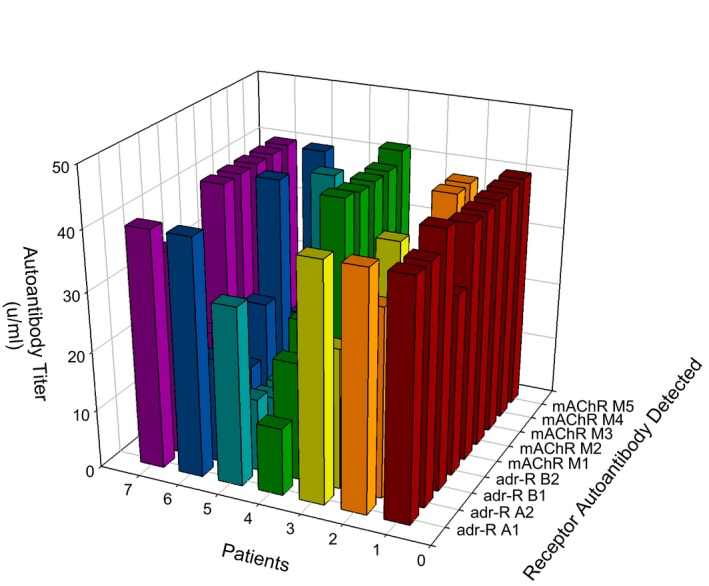
We detected elevated autoantibodies against all four G‐protein coupled AdrRs (Figures 2 and and3),3), with the most significant being an elevation of the alpha 1 receptor autoantibody in 89% (49 of 55) of the POTS patients in this study. Thirty‐one percent (17 of 55) of subjects were found to have only 1 AdrR autoantibody, and in all instances, the antibody was against the alpha 1 receptor. Ten subjects (18%) were found to have 2 or more elevated adrenergic AdrR antibodies, and 4 (7%; 4 of 55) had elevation of all autoantibodies (both adrenergic and muscarinic acetylcholine receptors; Figure 4); 2 additional subjects (4%) had 8 of 9, and 1 patient had 5 of 9 elevated autoantibodies (Figure 4). Five POTS patients (9%) had no evidence of any elevation of autoantibodies nor did our controls.
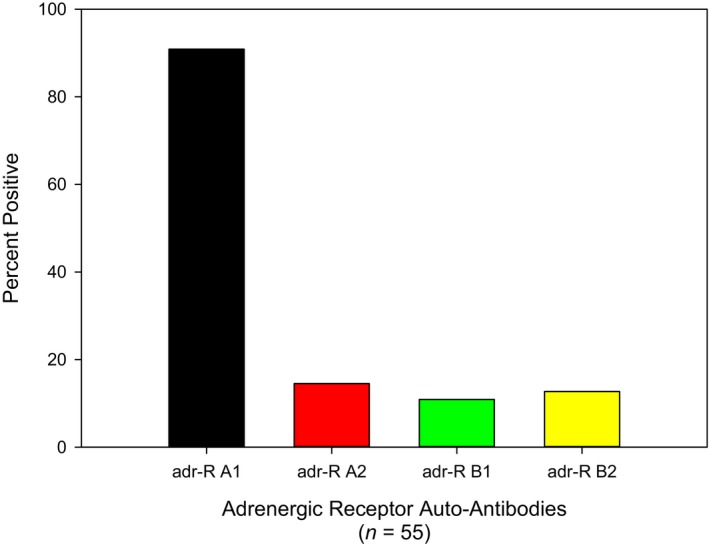
Elevated autoantibodies against adrenergic receptors in patients diagnosed with postural orthostatic tachycardia syndrome. Autoantibodies against the adrenergic alpha 1 receptor was the most common elevation of detected. Adr‐R indicates adrenergic receptor.
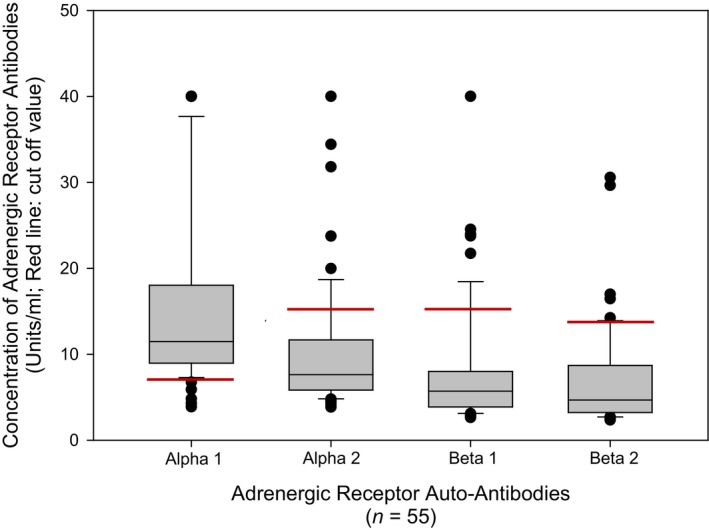
Concentration of autoantibodies against adrenergic receptors in patients diagnosed with postural orthostatic tachycardia syndrome. Adrenergic alpha 1 receptor antibodies had the highest mean concentration in serum for the study group with a mean of 15.5±10.3 units/mL. Some patients had antibodies against all 4 receptor subtypes and some had elevations exceeding the assay maximum concentration cutoff. Five patients had AdrR A1 antibody values that exceed the chart, and 1 patient each had antibodies >40 units/mL for AdrR A2 and B1; no one had antibodies >40 units/mL for B2; these patient values are graphed in Figure 4.
Anti–muscarinic receptor antibodies
Elevation of muscarinic mAChR autoantibodies was dependent upon elevation of AdrR antibodies! All POTS patients with elevated mAChR autoantibodies had at least 1 elevated AdrR antibody. Thirty‐one POTS patients (56%) were found to have an elevation of at least 1 mAChR autoantibody (Figures 5 and and6),6), with the most common elevation of antibodies against the M4 receptor in 29 of the patients (53%). Seventeen subjects (31%) were found to have 2 or more mAChR autoantibodies.
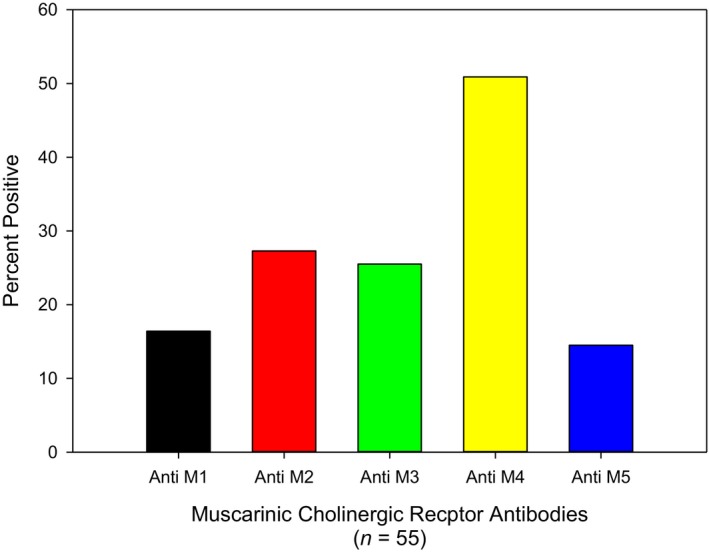
Elevated autoantibodies against muscarinic cholinergic receptors in patients with postural orthostatic tachycardia syndrome. The most common muscarinic acetylcholine receptor autoantibody was against the M4 subtype.
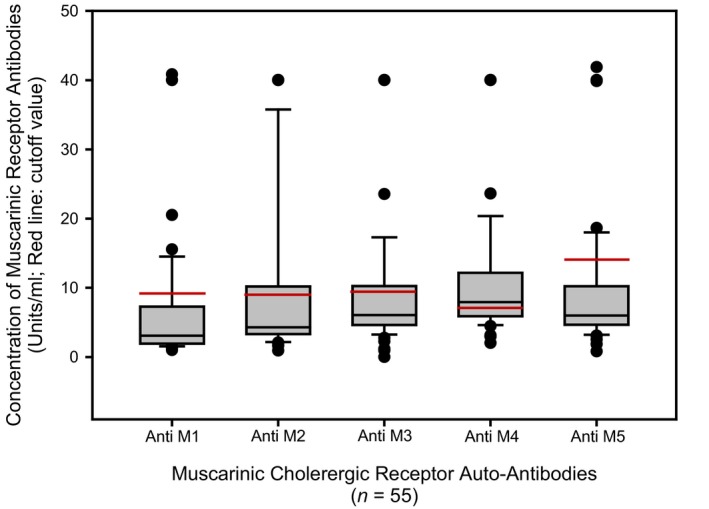
Concentration of autoantibodies against muscarinic cholinergic receptors in patients with postural orthostatic tachycardia syndrome. The mean concentration of muscarinic acetylcholine receptor autoantibodies was lower than the cut‐off value, except for the M4 subtype with a mean of 11.0±9.3 units/mL. Some patients had autoantibodies against all 5 receptor subtypes that exceeded the maximum cut‐off value. Three patients had mAChR M1 antibody values that exceed the chart, 5 patients had antibodies >40 units/mL for mAChR M2, and 4 patients each had antibodies against mAChR M3, M4, and M5 >40 units/mL; these patient values are graphed in Figure 4.
Discussion
The aim of this study was to detect both the presence and prevalence of G‐protein coupled adrenergic and cholinergic receptor antibodies in patients with postural orthostatic tachycardia syndrome. Although our working hypothesis was that autoantibodies to these autonomic system receptors would be found in some POTS patients, the results or our investigation were surprising and unexpected. Of the 55 POTS subjects evaluated, 49 (89%) were found to have elevated antibodies against the Alpha 1 adrenergic receptor and 28 subjects (51%) had elevations of antibodies against the muscarinic cholinergic receptor, M4. Even more striking was that mAChR antibodies were elevated only if patients had an elevation of an AdrR antibody. Similarly, although we detected autoantibodies against all 4 subtypes of the G‐protein coupled adrenergic receptor, elevation of antibodies against Alpha 2, Beta 1, or Beta 2 receptors were present only if antibodies were also elevated against the A1 adrenergic receptor. Five patients had no elevation of autoantibodies against any of the 9 receptors evaluated.
The female/male ratio of 18:1 and mean age (29.9) in our study is similar to one of our previous reports,12 but significantly greater than usually described for POTS having a female‐to‐male ration of between 5:1 and 3:1 and a median age of 23.32 Clinical symptoms of our subjects were consistent with those described in the literature, including 94.5% complaining of fatigue and 72.7% with hyperflexibility, for example (Table). Of the many symptoms we recorded (Table), clinical comorbidities were essentially identical to those we have previously described, including easy bruising (63.6%) and frequent epistaxis (47.3%) in a POTS study that found platelet delta granule storage pool deficiency in 81% of our patients (146 of 181).12 These symptoms may be described as 3 broad‐reaching categories of symptoms related to (1) connective tissue, joints, and the heart and vasculature; (2), neurophysiological manifestation of headaches, cognitive issues, and depression; and (3) symptoms suggestive of platelet dysfunction, specifically delta granule storage pool deficiency.12 The constellation of clinical presentations demonstrates a significant heterogeneity, consistent with that described in the literature.
The symptom of hyperflexibility was not evaluated using the current diagnostic criteria to categorize hypermobile Elhers‐Danlos syndrome.33 Our experience using the Beighton 9‐point scoring system for joint hypermobility is much lower, with 20% to 30% of our POTS patients having scores of 6 or more. Given that we did not include the criteria to diagnose Elhers‐Danlos syndrome in this study, any correlation with detection of autoantibodies was therefore not evaluated. With the heterogeneity of symptoms associated with POTS, a variety of proposed etiologies have been suggested, yet no conclusive basis or mechanism has been established.7, 20, 21, 34, 35 The less‐frequent primary form of POTS (hyperadrenergic POTS) has been described as a form of β‐adrenergic receptor hypersensitivity with many of these patients experiencing true migraine headaches, including onset with photophobia and nausea.36 A secondary form of POTS resulting from peripheral autonomic deinnervation, but with maintained cardiac innervation, is usually associated with underlying disease states with diabetes mellitus the most common. Hypermobile Elhers‐Danlos syndrome has also been considered as secondary form of POTS. Our results support the hypothesis that POTS may be an autoimmune disorder affecting the autonomic nervous system.
Some of the earliest reports of orthostatic intolerance with elevated antibodies against autonomic nervous system antibodies focused upon ganglionic acetylcholine receptors.24, 37, 38, 39 More recently, reports of a significant relationship with orthostatic intolerance and muscarinic receptor autoantibodies28, 29, 40, 41 and adrenergic receptor autoantibodies have been published.26, 28, 29, 40 Thus, autoantibodies against both the sympathetic and the parasympathetic autonomic nervous system have been implicated as potential etiologies of POTS.
The ELISA kits used in our investigation, although not reported by others in the evaluation of autoantibodies in POTS, have been utilized in a variety of studies, including a large study of elderly patients with cardiac insufficiency.42 The CellTrend GmbH ELISA kits have been validated and utilized in studies of chronic heart failure patients.43, 44 Autoantibodies against both adrenergic and muscarinic cholinergic receptors have been associated with a variety of cardiovascular diseases, including ischemic heart disease, Chagas disease, and idiopathic dilated cardiomyopathy, and have been associated with biomarkers of inflammation and myocardial damage.44, 45 In addition to these cardiovascular diseases, elevations of G‐coupled protein receptor autoantibodies have been found in a number of autoimmune diseases, including systemic sclerosis and Sjögren's syndrome, and in other conditions such as Alzheimer's disease and complex regional pain syndrome.45, 46 A common denominator could be a predisposing inflammatory or ischemic stress process. Our patients were all referrals from other institutions, and an ability to evaluate the onset of POTS, the severity of symptoms, and consideration of G‐coupled protein receptor autoantibody titer concentration could not be evaluated to determine whether autoantibodies preceded or followed POTS. Our specific aim of this project was for proof of concept to evaluate the growing body of evidence that POTS patients have elevated G‐coupled protein receptor autoantibodies, and the disorder's etiology is potentially autoimmune.46
The significance of autoantibodies against the adrenergic alpha 1 receptor in 89% of our patients is unknown. One might postulate that a viral infection could stimulate molecular mimicry and that the A1 receptor might have stoichiometry similar to an unknown antigenic epitope in infection and/or inflammation. A significant number of our patients have described Epstein–Barr virus infections and gastrointestinal pain that could be related to an enteric viral infection preceding the onset of symptoms and ultimately the development of POTS and its diagnosis. A well‐documented association with vaccination preceding the development of POTS is known, and a number of studies have reported viral infections and molecular mimicry associated with autoimmune diseases.47, 48, 49, 50
Autoantibodies reported to be associated with POTS target G‐coupled (guanine‐nucleotide–binding) proteins. The cholinergic autoantibodies are directed against nicotinic and muscarinic acetylcholine receptors. Nicotinic acetylcholine receptors are ionotropic, acting as cationic channels, and are principally found in the central and peripheral nervous system and the primary receptor in the motor end plate of skeletal muscle.39, 40 Muscarinic receptors function in both the central and peripheral nervous systems and are the primary end receptor in the parasympathetic nervous system.51 Muscarinic have been subtyped into 5 different classifications and function through a second messenger with either stimulatory (Gi; M2, M4; messengers diacyl glycerol and inositol triphosphate) or inhibitory function (Gq; M1, M3, and M5, inhibits cAMP).52 The inhibitory G‐coupled acetylcholine M2 receptors are expressed in both heart and smooth muscle and many other tissues whereas M4 receptors are not, but both appear to modulate the heart through sympathetic neurotransmitter release in the atria.52, 53 The stimulatory M1 and M3 receptors are reported to play a role in both vasodilation and ‐constriction of the vasculature whereas the M5 receptor is yet to be understood.52, 53
In 2012, Li et al29 reported autoantibodies to beta adrenergic (β2) and muscarinic (M3) receptors by ELISA in 75% of patients with significant orthostatic hypotension. Subsequently, antibodies to both adrenergic Alpha 1 and Beta 1 receptors have been reported in POTS patients,25, 28, 42 and angiotension II type 1 autoantibodies have also been found in POTS.54 In a recent review of POTS, Bryarly et al55 state that although the varied reports of serum autoantibodies against both adrenergic and cholinergic receptors were detected in POTS patients, an association is yet to be proven in terms of pathophysiology or diagnostic relevance. Our results provide further evidence of a potential etiology for POTS as an autoimmune disorder.
We report that both adrenergic and muscarinic receptor autoantibodies may be detected by ELISA in POTS patients. The most prevalent elevation of autoantibody in our investigation was antiadrenergic A1 receptor, and detection of other adrenergic and muscarinic receptor autoantibodies were not detected without elevation of autoantibodies against the A1 receptor. We do not understand the significance of this observation. These results may support the postulate by Fedorowski et al25 that autoantibodies against the A1 receptor may have a negative modulatory effect; in other words, the A1 adrenergic receptor functions as a vasoconstrictor, and antibodies specific to this G‐coupled protein receptor would therefore cause an ineffective response to stimulus resulting in hypotension.
POTS affects as many as 3 million people in the United States1 and is the least severe of the dysautonomias.1 Yet POTS tends to be more severe with numerous associated symptoms than observed in typical cases of neurocardiogenic syncope.18, 56 With a variety of subtle clinical symptoms, including fatigue, lightheadedness, nausea, headache, near syncope, and exercise intolerance, the disorder is often misdiagnosed as chronic anxiety or a panic disorder because the autonomic failure in these patients is not severe.18, 56 We have previously reported a comorbidity in POTS that could explain many of these clinical symptoms; platelet delta granule storage pool deficiency with diminished serotonin levels appears to be frequent in our experience.12 We have not proposed delta granule storage pool deficiency as an etiology of POTS; however, the deficiency might be acquired by autoantibodies, perhaps against enteric cells that produce serotonin in the gut that is stored in platelet granules. Regardless, it may be prudent to consider including an investigation of autoantibodies against the alpha 1 adrenergic receptor during diagnostic workup of the orthostatic hypotension patient.
Weaknesses of our study include that it is a descriptive study, not a case‐control investigation. At the onset of our investigation, we found no elevation of antibodies against adrenergic or muscarinic receptors of any subtype in 3 control subjects. Thus, solely for financial reasons, we decided to evaluate as many POTS patients as possible with our limited resources, and each ELISA kit included controls to establish a standardized curve for determination of antibody concentration in POTS patients’ sample sera. All test samples were appropriately assayed with both positive kit standards and negative controls. Another potential weakness might be that we did not consider evaluating serum for angiotensin II type 1 antibodies; the report of autoantibodies to this G‐protein coupled receptor had not yet been reported at the time we initiated our study.
In conclusion, our findings support the growing body of evidence suggesting that POTS may be an autoimmune disorder. It is very interesting that both sympathetic and parasympathetic nervous system receptors are immune‐mediated targets. Of particular interest is the apparent tendency that subtypes of both adrenergic (A2, B1, and B2) and muscarinic receptor antibodies were not detected in the serum of POTS patients, unless autoantibodies were expressed against the alpha 1 adrenergic receptor subtype. This phenomenon requires further investigation.
Sources of Funding
This study could not have been conducted without research grant funded by The Dysautonomia Advocacy Foundation (Charleston, SC) and a gift by Life as a Zebra Foundation (Lansing, MI).
Disclosures
None.
Notes
(J Am Heart Assoc. 2019;8:e013602 DOI: 10.1161/JAHA.119.013602.) [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
References
Articles from Journal of the American Heart Association: Cardiovascular and Cerebrovascular Disease are provided here courtesy of Wiley
Full text links
Read article at publisher's site: https://doi.org/10.1161/jaha.119.013602
Read article for free, from open access legal sources, via Unpaywall:
https://www.ahajournals.org/doi/pdf/10.1161/JAHA.119.013602
Citations & impact
Impact metrics
Article citations
Postural Orthostatic Tachycardia Syndrome Associated with COVID-19: A Narrative Review.
Medicina (Kaunas), 60(8):1325, 15 Aug 2024
Cited by: 0 articles | PMID: 39202605 | PMCID: PMC11356245
Review Free full text in Europe PMC
Long-Term POTS Outcomes Survey: Diagnosis, Therapy, and Clinical Outcomes.
J Am Heart Assoc, 13(14):e033485, 03 Jul 2024
Cited by: 0 articles | PMID: 38958137 | PMCID: PMC11292765
Joint Hypermobility, Autonomic Dysfunction, Gastrointestinal Dysfunction, and Autoimmune Markers: Clinical Associations and Response to Intravenous Immunoglobulin Therapy.
Am J Gastroenterol, 119(11):2298-2306, 24 Jun 2024
Cited by: 2 articles | PMID: 38912927 | PMCID: PMC11524627
Autoimmunity against Nucleus Ambiguous Is Putatively Possible in Both Long-COVID-19 and Vaccinated Subjects: Scientific Evidence and Working Hypothesis.
Biology (Basel), 13(6):359, 21 May 2024
Cited by: 0 articles | PMID: 38927239
Autoimmunity in Syndromes of Orthostatic Intolerance: An Updated Review.
J Pers Med, 14(4):435, 20 Apr 2024
Cited by: 1 article | PMID: 38673062 | PMCID: PMC11051445
Review Free full text in Europe PMC
Go to all (65) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Inflammatory Biomarkers in Postural Orthostatic Tachycardia Syndrome with Elevated G-Protein-Coupled Receptor Autoantibodies.
J Clin Med, 10(4):623, 06 Feb 2021
Cited by: 15 articles | PMID: 33562074 | PMCID: PMC7914580
Serum Activity Against G Protein-Coupled Receptors and Severity of Orthostatic Symptoms in Postural Orthostatic Tachycardia Syndrome.
J Am Heart Assoc, 9(15):e015989, 30 Jul 2020
Cited by: 26 articles | PMID: 32750291 | PMCID: PMC7792263
Autoimmune basis for postural tachycardia syndrome.
J Am Heart Assoc, 3(1):e000755, 26 Feb 2014
Cited by: 141 articles | PMID: 24572257 | PMCID: PMC3959717
G protein-coupled receptors related to autoimmunity in postural orthostatic tachycardia syndrome.
Immunol Med, 1-8, 20 Jun 2024
Cited by: 0 articles | PMID: 38900132
Review

 1
1