Abstract
Importance
High body mass index (BMI) is independently associated with overall survival benefit from immune checkpoint inhibitor therapy in patients with melanoma, yet whether BMI is associated with outcomes in patients with advanced non-small cell lung cancer treated with atezolizumab remains unknown.Objective
To examine whether BMI is associated with survival outcomes and adverse events in patients with non-small cell lung cancer (NSCLC) treated with atezolizumab.Design, setting, and participants
A pooled analysis of individual patient-level data from 4 international, multicenter clinical trials was performed. Two were single-arm phase 2 trials (BIRCH [data cutoff of May 28, 2015] and FIR [data cutoff of January 7, 2015]), and 2 were 2-arm randomized clinical trials (POPLAR [phase 2; data cutoff of May 8, 2015] and OAK [phase 3; data cutoff of July 7, 2016]). Patients with advanced NSCLC previously untreated or treated with at least 1 line of systemic therapy, with measurable disease and good organ function and without contraindications for chemotherapy or immune checkpoint inhibitor therapy, were included in these trials. Data analyses were performed from February 28, 2019, to September 30, 2019.Interventions
The control group was treated with docetaxel once every 3 weeks until disease progression or unacceptable toxic effects occurred in POPLAR and OAK. The experimental group was treated with atezolizumab once every 3 weeks until disease progression or unacceptable toxic effects occurred in all available trials.Main outcomes and measures
Association between BMI and overall survival (OS), progression-free survival (PFS), and treatment-related adverse events. Intention-to-treat analysis was conducted.Results
Adequate data were available for 2110 patients from a total pool of 2261 across 4 trials. Of those 2110, 1434 patients (median age, 64 years [range, 57-70 years]; 890 men [62%]) received atezolizumab and 676 patients (median age, 63 years[range, 57-69 years]; 419 men [62%]) received docetaxel. There was a linear association between increasing BMI and OS in patients treated with atezolizumab. Obesity (BMI ≥30 [calculated as weight in kilograms divided by height in meters squared]) was associated with significantly improved OS in patients treated with atezolizumab, but not in those who received docetaxel after adjusting for confounding variables. The association between BMI and OS/PFS was the strongest in the high PD-L1 expression subgroup. Overall survival for patients with the highest category of PD-L1 expression (≥50% of tumor cells or ≥10% of tumor-infiltrating immune cells; n = 436) had hazard ratios of 0.36 (95% CI, 0.21-0.62) for the group with obesity and 0.69 (95% CI, 0.48-0.98) for the group with overweight. Patients with the highest category of PD-L1 expression had PFS hazard ratios of 0.68 (95% CI, 0.49-0.94) for the group with obesity and 0.72 (95% CI, 0.56-0.92) for the group with overweight. Treatment-related adverse events were not associated with BMI.Conclusions and relevance
High BMI appears to be independently associated with improved survival with atezolizumab in patients with NSCLC, raising the possibility that baseline BMI should be considered as a stratification factor in future immune checkpoint inhibitor therapy trials.Free full text

Association Between Body Mass Index and Overall Survival With Immune Checkpoint Inhibitor Therapy for Advanced Non–Small Cell Lung Cancer
Key Points
Question
Is high body mass index associated with survival outcomes with atezolizumab therapy, an immune checkpoint inhibitor, in patients with non–small cell lung cancer?
Findings
In this pooled analysis of 4 clinical trials that included more than 2261 patients with non–small cell lung cancer, those who had high body mass index had a significant reduction in mortality with atezolizumab, particularly in the presence of high expression of programmed cell death ligand 1.
Meaning
High body mass index appears to be associated with improved overall survival in atezolizumab-treated patients with advanced non–small cell lung cancer.
Abstract
Importance
High body mass index (BMI) is independently associated with overall survival benefit from immune checkpoint inhibitor therapy in patients with melanoma, yet whether BMI is associated with outcomes in patients with advanced non–small cell lung cancer treated with atezolizumab remains unknown.
Objective
To examine whether BMI is associated with survival outcomes and adverse events in patients with non–small cell lung cancer (NSCLC) treated with atezolizumab.
Design, Setting, and Participants
A pooled analysis of individual patient-level data from 4 international, multicenter clinical trials was performed. Two were single-arm phase 2 trials (BIRCH [data cutoff of May 28, 2015] and FIR [data cutoff of January 7, 2015]), and 2 were 2-arm randomized clinical trials (POPLAR [phase 2; data cutoff of May 8, 2015] and OAK [phase 3; data cutoff of July 7, 2016]). Patients with advanced NSCLC previously untreated or treated with at least 1 line of systemic therapy, with measurable disease and good organ function and without contraindications for chemotherapy or immune checkpoint inhibitor therapy, were included in these trials. Data analyses were performed from February 28, 2019, to September 30, 2019.
Interventions
The control group was treated with docetaxel once every 3 weeks until disease progression or unacceptable toxic effects occurred in POPLAR and OAK. The experimental group was treated with atezolizumab once every 3 weeks until disease progression or unacceptable toxic effects occurred in all available trials.
Main Outcomes and Measures
Association between BMI and overall survival (OS), progression-free survival (PFS), and treatment-related adverse events. Intention-to-treat analysis was conducted.
Results
Adequate data were available for 2110 patients from a total pool of 2261 across 4 trials. Of those 2110, 1434 patients (median age, 64 years [range, 57-70 years]; 890 men [62%]) received atezolizumab and 676 patients (median age, 63 years[range, 57-69 years]; 419 men [62%]) received docetaxel. There was a linear association between increasing BMI and OS in patients treated with atezolizumab. Obesity (BMI ≥30 [calculated as weight in kilograms divided by height in meters squared]) was associated with significantly improved OS in patients treated with atezolizumab, but not in those who received docetaxel after adjusting for confounding variables. The association between BMI and OS/PFS was the strongest in the high PD-L1 expression subgroup. Overall survival for patients with the highest category of PD-L1 expression (≥50% of tumor cells or ≥10% of tumor-infiltrating immune cells; n =
= 436) had hazard ratios of 0.36 (95% CI, 0.21-0.62) for the group with obesity and 0.69 (95% CI, 0.48-0.98) for the group with overweight. Patients with the highest category of PD-L1 expression had PFS hazard ratios of 0.68 (95% CI, 0.49-0.94) for the group with obesity and 0.72 (95% CI, 0.56-0.92) for the group with overweight. Treatment-related adverse events were not associated with BMI.
436) had hazard ratios of 0.36 (95% CI, 0.21-0.62) for the group with obesity and 0.69 (95% CI, 0.48-0.98) for the group with overweight. Patients with the highest category of PD-L1 expression had PFS hazard ratios of 0.68 (95% CI, 0.49-0.94) for the group with obesity and 0.72 (95% CI, 0.56-0.92) for the group with overweight. Treatment-related adverse events were not associated with BMI.
Conclusions and Relevance
High BMI appears to be independently associated with improved survival with atezolizumab in patients with NSCLC, raising the possibility that baseline BMI should be considered as a stratification factor in future immune checkpoint inhibitor therapy trials.
Introduction
The treatment options for non–small cell lung cancer (NSCLC) have rapidly evolved over the past 2 decades with the availability of chemotherapy, molecularly targeted drugs, and immune checkpoint inhibitors. Immune checkpoint inhibitors that target programmed cell death 1 (PD-1) or its ligand 1 (PD-L1) monoclonal antibodies, such as atezolizumab, durvalumab, nivolumab, and pembrolizumab, are increasingly used for the treatment of both early and advanced NSCLC.1 Although durable responses were noted in advanced cancers, only a limited proportion of patients benefit from immune checkpoint inhibitors. Moreover, attempts to increase response using combination strategies incorporating multiple immune checkpoint inhibitors have a high incidence of immune-mediated adverse events (irAEs) resulting in early discontinuation. Predictive biomarkers for immune checkpoint inhibitor therapy response are required to identify patients who benefit from or have adverse events associated with immune checkpoint inhibitors.
Available predictive biomarkers for response, such as tumor mutation burden, PD-L1 expression, and microsatellite instability, are generally focused on cancer and its associated tumor-infiltrating lymphocytes. Because the patients who receive immune checkpoint inhibitor therapies are highly heterogeneous and tumor-based biomarkers are resource intensive and not validated, several simple clinical and demographic characteristics are also being evaluated to estimate the probability of response.2 One such characteristic is obesity.
The association between obesity and its surrogate noninvasive measure of body fat, high body mass index (BMI), and cancer is complicated, with increased incidence, rapid disease progression, recurrence after treatment, and mortality for some cancers but protection from other cancers (obesity paradox).3 Previous studies suggested that high BMI was associated with a lower incidence of lung cancers and lower cancer-specific mortality.4,5,6,7 Moreover, high BMI is an independent positive prognostic factor for survival among patients treated with surgery in early-stage NSCLC, paclitaxel plus carboplatin chemotherapy for advanced disease and radiotherapy for bone metastases.8,9,10,11 However, it is unclear whether high BMI might also be a factor in the association between immune checkpoint inhibitor treatment and cancer outcomes.
In a recent retrospective study, McQuade et al12 reported that, in patients with advanced melanoma treated with immune checkpoint inhibitors and targeted therapies, obesity (BMI ≥30 [calculated as weight in kilograms divided by height in meters squared]) was associated with improved progression-free survival (PFS) and overall survival (OS), but no such association was noted in patients treated with chemotherapy. Cortellini et al13 reported that, for patients with advanced cancers treated with immune checkpoint inhibitors, PFS and OS were significantly longer for patients who were overweight or obese (BMI ≥25) compared with those who were not overweight (BMI <25). Similarly, Richtig et al14 reported a higher response rate with immune checkpoint inhibitors and longer survival in patients with obesity and melanoma but not in patients with normal body weight. Although the pathophysiologic factors behind the positive association between obesity with survival after immune checkpoint inhibitor therapy is unclear, leptin-mediated T-cell dysfunction may be a factor.15
In the present study, we explored the association between high BMI and survival in patients with advanced NSCLC treated with immune checkpoint inhibitors. The main objectives were to investigate the association between BMI and survival outcomes of patients initiating atezolizumab or docetaxel and examine the association between BMI and the incidence of treatment-related adverse events (TRAEs) and irAEs in the same cohort.
Methods
Patients
A pooled post hoc analysis of individual participant data from the clinical trials OAK (NCT02008227; July 7, 2016, data cutoff),16 POPLAR (NCT01903993; May 8, 2015, data cutoff),17 BIRCH (NCT02031458; May 28, 2015, data cutoff),18 and FIR (NCT01846416; January 7, 2015, data cutoff)19 was conducted. Results for the primary analyses of data from all 4 trials were previously published.16,17,18,19 Secondary analysis of trial data was deemed to be negligible risk and exempt by the Southern Adelaide Clinical Human Research Ethics Committee. Deidentified data were accessed according to Roche’s policy and process for clinical study data sharing.20 Data were analyzed from February 28, 2019, to September 30, 2019.
OAK (phase 3) and POPLAR (phase 2) were randomized clinical trials of atezolizumab, 1200 mg, vs docetaxel, 75 mg/m2, with both administered intravenously every 3 weeks for patients with advanced NSCLC in whom platinum-containing therapy had failed.16,17 BIRCH and FIR were single-arm, phase 2 trials in patients with PD-L1–positive tumors who were either receiving first-line treatment or later lines of therapy with atezolizumab.18,19 Pooled analyses of OAK, POPLAR, BIRCH, and FIR were used to demonstrate consistency of identified associations within an expanded cohort of patients treated with atezolizumab. Programmed death ligand 1–positive tumors were defined by PD-L1 expression on 5% or more of tumor cells or tumor-infiltrating cells based on an PDL-1 immunohistochemistry assay (Ventana PDL-1 SP142; Ventana Medical Systems Inc).
Predictor and Outcome Definitions
The primary outcome assessed was OS. The secondary outcomes were PFS and TRAEs and irAEs (any grade and grade 3 or 4 TRAEs/irAEs, using National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0).21 Progression-free survival was assessed by the investigator for POPLAR and OAK and defined by Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1).16,17 An independent review facility assessed PFS via RECIST, version 1.1 for BIRCH,18 whereas, in FIR, the PFS was investigator assessed as per modified RECIST.19 Adverse events reported as associated with the treatment interventions were considered as TRAEs and those reported as immune mediated were considered irAEs.
Baseline BMI was calculated and recorded at study enrollment or on the first day of treatment.22 Body mass index was categorized by World Health Organization criteria: underweight (<18.5), normal weight (18.5-24.9), overweight (25-29.9), and obese (≥30).22 Patients with missing height and/or weight information for the calculation of BMI and those within the underweight category of BMI were excluded from the analysis. As in McQuade et al,12 patients who were underweight were excluded from analyses because of low prevalence (<5%) and the focus was on comparing overweight and obese BMI categories with the normal-weight BMI category.
Clinically relevant confounding factors evaluated included patient’s age, sex, race (white/Asian/other), Eastern Cooperative Oncology Group performance status (scale ranges from 0 to 4, with 0 being fully functional and asymptomatic, and 4 being bedridden), smoker status (current, previous, or never), tumor histologic type (squamous or nonsquamous), number of tumor sites (<3 or ≥3), number of prior treatments in the advanced setting, PD-L1 expression (positive or negative), serum lactate dehydrogenase level (less than or greater than or equal to the
greater than or equal to the upper limit of normal), blood C-reactive protein level, and blood neutrophil to lymphocyte ratio (<3 or ≥3).
upper limit of normal), blood C-reactive protein level, and blood neutrophil to lymphocyte ratio (<3 or ≥3).
Statistical Analysis
Associations between BMI and OS and PFS were modeled using Cox proportional hazards regression models and are reported as hazard ratios (HRs) with 95% CIs. Associations between BMI and TRAEs were modeled using logistic regression and are reported as HRs (95% CIs). All regression analyses were stratified by study. Survival curves for each category of BMI were estimated using the Kaplan-Meier method.
Adjustment for potential confounding variables was undertaken by multivariable regression adjustment. Whether the association between BMI group and survival differed between men and women and between PD-L1–positive and PD-L1–negative tumors was assessed using an interaction term in the Cox proportional hazards regression model. Differences between BMI groups for the incidence of adverse events (both TRAEs and irAEs) were also evaluated using Cox proportional hazards regression.
Evaluation of treatment benefit (atezolizumab vs docetaxel) by BMI subgroups was undertaken based on the intention-to-treat (ITT) populations of the 2 randomized trials: OAK and POPLAR. A treatment-by-BMI statistical interaction was evaluated in a Cox proportional hazards regression model stratified by study.
All analyses were conducted in R, version 3.4.3 (R Foundation for Statistical Computing), using the survival package.23 Statistical tests were 2-sided and a P value <.05 was considered statistically significant.
Results
BMI and Outcomes in Atezolizumab-Treated Patients
A total of 2261 patients were treated with atezolizumab or docetaxel across 4 trials. Of the 1548 participants who received atezolizumab, 114 (BMI unavailable in 40 participants and 74 underweight [BMI <18.5] were excluded from further analysis, leaving 1434 participants (median age, 64 years [range, 57-70 years]; 890 men [62%]). Of these, 705 participants (49%) were normal weight, 490 participants (34%) were overweight, and 239 participants (7%) were obese (eTable 1 in the Supplement). Compared with patients who were not obese, a larger proportion of patients with obesity were white, male, previous smokers, had a neutrophil to lymphocyte ratio less than 3, and lower C-reactive protein concentrations (eTable 1 in the Supplement).
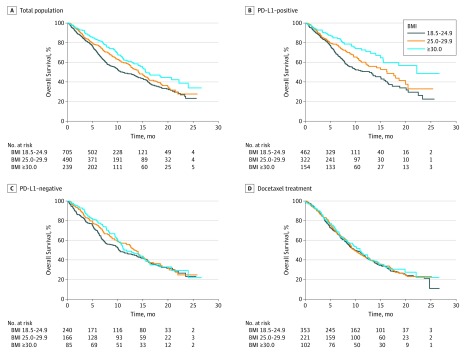
Overall survival differed significantly between the patients with normal weight, overweight, and obesity treated with atezolizumab (P <
< .001), with improved OS for patients with obesity (HR, 0.64; 95% CI, 0.51-0.81) and overweight (HR, 0.81; 95% CI, 0.68-0.95) compared with patients with normal BMI (Table and Figure 1A). This association remained significant after adjustment for potentially confounding variables (eg, for obesity, HR, 0.69; 95% CI, 0.54-0.87) (eTable 2 in the Supplement). The association between BMI groups and OS was consistent for men and women (P value for interaction
.001), with improved OS for patients with obesity (HR, 0.64; 95% CI, 0.51-0.81) and overweight (HR, 0.81; 95% CI, 0.68-0.95) compared with patients with normal BMI (Table and Figure 1A). This association remained significant after adjustment for potentially confounding variables (eg, for obesity, HR, 0.69; 95% CI, 0.54-0.87) (eTable 2 in the Supplement). The association between BMI groups and OS was consistent for men and women (P value for interaction =
= .76) (eTable 3 in the Supplement) but was significantly different between PD-L1–positive and PD-L–negative tumors (P value for interaction
.76) (eTable 3 in the Supplement) but was significantly different between PD-L1–positive and PD-L–negative tumors (P value for interaction =
= .02). Specifically, the survival advantage associated with the groups with BMI classified as overweight and obese was larger for PD-L1–positive tumors (overweight: HR, 0.73; 95% CI, 0.58-0.91 vs obese: HR, 0.48; 95% CI, 0.34-0.66) than PD-L1–negative tumors (Table and Figure 1B, C). Furthermore, OS for patients with the highest PD-L1 expression (≥50% of tumor cells or ≥10% of tumor-infiltrating immune cells; n
.02). Specifically, the survival advantage associated with the groups with BMI classified as overweight and obese was larger for PD-L1–positive tumors (overweight: HR, 0.73; 95% CI, 0.58-0.91 vs obese: HR, 0.48; 95% CI, 0.34-0.66) than PD-L1–negative tumors (Table and Figure 1B, C). Furthermore, OS for patients with the highest PD-L1 expression (≥50% of tumor cells or ≥10% of tumor-infiltrating immune cells; n =
= 436) had HRs of 0.36 (95% CI, 0.21-0.62) for patients with obesity and 0.69 (95% CI, 0.48-0.98) for those who were overweight. Non–cancer-related deaths were similar across the trials and BMI categories (eTable 4 and eTable 5 in the Supplement).
436) had HRs of 0.36 (95% CI, 0.21-0.62) for patients with obesity and 0.69 (95% CI, 0.48-0.98) for those who were overweight. Non–cancer-related deaths were similar across the trials and BMI categories (eTable 4 and eTable 5 in the Supplement).
Table.
| BMI Group | HR (95% CI) | ||
|---|---|---|---|
| All Patients | PD-L1 | ||
| Positive | Negative | ||
| Atezolizumab-Treated Patients | |||
| Overall survival | |||
| 18.5-24.9 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 25.0-29.9 | 0.81 (0.68-0.95) | 0.73 (0.58-0.91) | 0.91 (0.71-1.16) |
| ≥30.0 | 0.64 (0.51-0.81) | 0.48 (0.34-0.66) | 0.90 (0.66-1.22) |
| P value | <.001 | <.001 | .68 |
| Progression-free survival | |||
| 18.5-24.9 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 25.0-29.9 | 0.89 (0.78-1.01) | 0.86 (0.72-1.01) | 0.93 (0.75-1.14) |
| ≥30.0 | 0.86 (0.73-1.01) | 0.78 (0.62-0.96) | 1.01 (0.78-1.31) |
| P value | .09 | .04 | .73 |
| Docetaxel-Treated Patients | |||
| Overall survival | |||
| 18.5-24.9 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 25.0-29.9 | 0.96 (0.78-1.18) | 1.18 (0.83-1.69) | 0.89 (0.72-1.11) |
| ≥30.0 | 0.92 (0.70-1.21) | 0.90 (0.55-1.45) | 1.03 (0.77-1.37) |
| P value | .82 | .48 | .51 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HR, hazard ratio; PD-L1, programmed cell death ligand 1.
BMI indicates body mass index (calculated as weight in kilograms divided by height in meters squared).
The PFS for the obese and overweight groups did not reach statistical significance when analyzed as separate groups (overweight: HR, 0.89; 95% CI, 0.78-1.01 vs obese: HR, 0.86; 95% CI, 0.73-1.01; P =
= .09) (Table). The groups with overweight and obesity had similar PFS outcomes and in an exploratory analysis the group with combined BMI classified as overweight or obesity demonstrated improved PFS compared with the group with normal BMI (HR, 0.88; 95% CI, 0.78-0.99; P
.09) (Table). The groups with overweight and obesity had similar PFS outcomes and in an exploratory analysis the group with combined BMI classified as overweight or obesity demonstrated improved PFS compared with the group with normal BMI (HR, 0.88; 95% CI, 0.78-0.99; P =
= .03). The association between BMI and PFS was most apparent for the PD-L1–positive tumors (overweight: HR, 0.86; 95% CI, 0.72-1.01 vs obese: HR, 0.78; 95% CI, 0.62-0.96; P
.03). The association between BMI and PFS was most apparent for the PD-L1–positive tumors (overweight: HR, 0.86; 95% CI, 0.72-1.01 vs obese: HR, 0.78; 95% CI, 0.62-0.96; P =
= .03) (PD-L1 expression on ≥5% of tumor cells or tumor-infiltrating immune cells), and there was little indication of association for PD-L1–negative tumors (overweight: HR, 0.93; 95% CI, 0.75-1.14 vs obese: HR, 1.01; 95% CI, 0.78-1.31; P
.03) (PD-L1 expression on ≥5% of tumor cells or tumor-infiltrating immune cells), and there was little indication of association for PD-L1–negative tumors (overweight: HR, 0.93; 95% CI, 0.75-1.14 vs obese: HR, 1.01; 95% CI, 0.78-1.31; P =
= .73) (Table). Patients with the highest category of PD-L1 expression (on ≥50% of tumor cells or ≥10% of tumor-infiltrating immune cells; n
.73) (Table). Patients with the highest category of PD-L1 expression (on ≥50% of tumor cells or ≥10% of tumor-infiltrating immune cells; n =
= 436) had PFS HRs of 0.68 (95% CI, 0.49-0.94) for the group with obesity and 0.72 (95% CI, 0.56-0.92) for the group with overweight.
436) had PFS HRs of 0.68 (95% CI, 0.49-0.94) for the group with obesity and 0.72 (95% CI, 0.56-0.92) for the group with overweight.
The incidence of TRAEs was not significantly different between the BMI categories (all grades: 65% [normal], 64% [overweight], and 65% [obese]; P =
= .92, and grade 3-5: 12% [normal], 14% [overweight], and 12% [obese]; P
.92, and grade 3-5: 12% [normal], 14% [overweight], and 12% [obese]; P =
= .66). Similarly, no significant differences were seen in the frequency of irAEs across BMI categories except for skin-related irAEs (eg, HR, 1.47; 95% CI, 1.2-2.0 for overweight) (eTable 6 and eFigure 1 in the Supplement).
.66). Similarly, no significant differences were seen in the frequency of irAEs across BMI categories except for skin-related irAEs (eg, HR, 1.47; 95% CI, 1.2-2.0 for overweight) (eTable 6 and eFigure 1 in the Supplement).
BMI and Outcomes for Docetaxel-Treated Patients
Of the 713 participants treated with docetaxel in the OAK and POPLAR trials, 676 individuals (median age, 63 years [range, 57-69 years]; 419 men [62%]) were included for further analysis. Of those 676 individuals, BMI was unavailable in 9, and 28 were underweight. Characteristics of this cohort by BMI category are summarized in eTable 7 in the Supplement.
For patients treated with docetaxel, there was no significant association between BMI and OS (overweight: HR, 0.96; 95% CI, 0.78-1.18 vs obese: HR, 0.92; 95% CI, 0.70-1.21; P =
= .82) (Table and Figure 1D) or PFS (P
.82) (Table and Figure 1D) or PFS (P =
= .36). In addition, the association between BMI and OS did not differ significantly between PD-L1–positive and PD-L1–negative tumors (P value for interaction
.36). In addition, the association between BMI and OS did not differ significantly between PD-L1–positive and PD-L1–negative tumors (P value for interaction =
= .41) (Table).
.41) (Table).
BMI and Atezolizumab Efficacy
Prior analyses evaluated BMI as a prognostic marker of survival: the association between BMI and survival for patients treated with a specific treatment. Herein we report the evaluation of treatment effect modification in randomized clinical trials. In contrast to evaluating whether BMI is a prognostic marker of survival, we evaluate whether BMI is a predictive marker of treatment benefit, which indicates the degree to which atezolizumab improves survival over docetaxel.
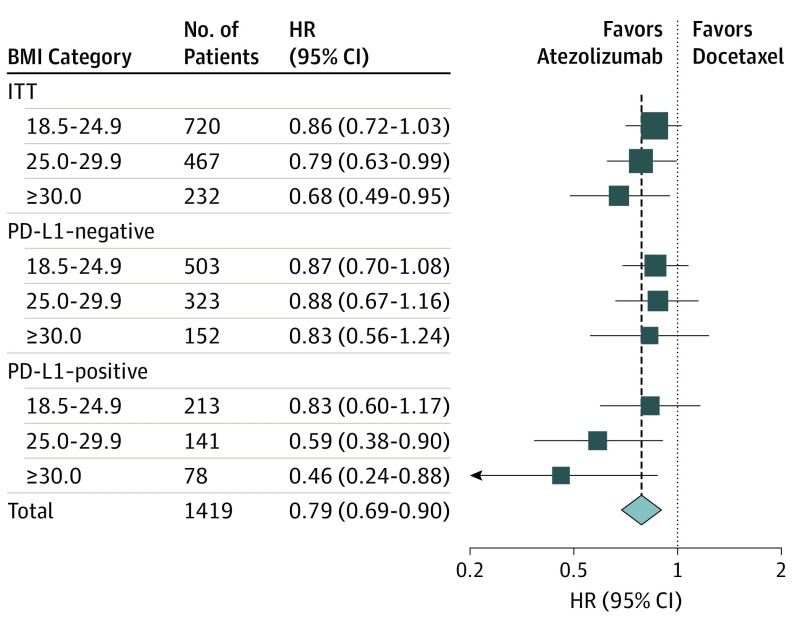
Exploratory analysis of atezolizumab treatment benefit vs docetaxel for BMI subgroups was restricted to the ITT populations of the OAK and POPLAR randomized clinical trials (ie, excluding the single-arm studies BIRCH and FIR). The pooled ITT populations of OAK and POPLAR included 1512 patients. However, because BMI was unavailable for 24 patients and 69 were underweight, 93 individuals were excluded, leaving 1419 participants (707 randomly allocated to atezolizumab treatment and 712 randomly allocated to docetaxel treatment) in the ITT analysis population. Baseline characteristics were well balanced between the 2 treatment arms in the ITT analysis population (eTable 8 in the Supplement). The median OS value was 13.2 months for atezolizumab and 9.8 months for docetaxel with a treatment efficacy HR of 0.79 (95% CI, 0.69-0.90, P <
< .001).
.001).
The estimated OS benefit of atezolizumab treatment compared with docetaxel treatment differed numerically between BMI groups (Figure 2; eFigure 2 in the Supplement), with HR values of 0.86 (95% CI, 0.72-1.03) for normal weight, 0.79 (95% CI, 0.63-0.99) for overweight, and 0.68 (95% CI, 0.49-0.95) for obesity. Atezolizumab survival benefit differences between BMI subgroups was most pronounced for participants with PD-L1–positive tumors (n =
= 432), with treatment efficacy HR of 0.83 for normal weight, 0.59 for overweight, and 0.46 for obesity, and no OS differences were noted for the PD-L1–negative subgroup (n
432), with treatment efficacy HR of 0.83 for normal weight, 0.59 for overweight, and 0.46 for obesity, and no OS differences were noted for the PD-L1–negative subgroup (n =
= 978) (Figure 2). However, the test for statistical interaction between BMI subgroups and atezolizumab OS benefit over docetaxel did not reach statistical significance for the ITT analysis population (P for interaction
978) (Figure 2). However, the test for statistical interaction between BMI subgroups and atezolizumab OS benefit over docetaxel did not reach statistical significance for the ITT analysis population (P for interaction =
= .10) or the subset of PD-L1–positive tumors (P for interaction
.10) or the subset of PD-L1–positive tumors (P for interaction =
= .10). The HR values numerically favored atezolizumab in all BMI subgroups (Figure 2).
.10). The HR values numerically favored atezolizumab in all BMI subgroups (Figure 2).
Discussion
To our knowledge, the present analyses, which pooled data from multiple prospectively conducted clinical trials of atezolizumab, is the largest study to evaluate the association between obesity and immune checkpoint inhibitor therapy outcomes. The findings suggest that high BMI was associated with improved OS in patients with advanced NSCLC. We believe we have identified for the first time that there may be a nearly linear relationship between BMI and OS with atezolizumab therapy when normal, overweight, and obese categories were compared. The association between BMI and OS remained significant after adjustment for trial-specific stratification factors and several clinically relevant confounders. The strength of the association was further increased by the presence of PD-L1 in the tumor and immune cells. Although the present study is a post hoc analysis of data from clinical trials, the results are consistent with those of prior studies suggesting that high BMI is associated with improved survival outcomes with immune checkpoint inhibitors across cancer types, such as melanoma.12,13,15
The present study adds to the emerging evidence that high BMI may be associated with cancer survival following immunotherapy. However, the biological basis of the association is just beginning to be understood. It is possible that obesity may induce a low-grade systemic meta-inflammation and impaired immune response. Moreover, obesity induces T-cell dysfunction and increases the exhausted PD-1–positive T-cell phenotype in fat and tumor microenvironment through leptin production, which may be the link between obesity and immune response.15,24 The identified association between high BMI and OS with atezolizumab appeared to be particularly strong in the PD-L1–positive population, lending further support to the presence of a T-cell dysfunction state in patients with obesity. Atezolizumab, through its mechanism of action of PD-1/PD-L1 axis inhibition on T-cells, might induce a favorable response in patients with obesity with an established T-cell exhausted state.
The association between obesity and cancer prognosis is complicated. Although obesity increases the risks of development of certain types of cancers, such as breast cancers, obesity protects against worse outcomes in patients with advanced cancers, such as lung cancers that are associated with wasting.25 Obesity’s association with improved survival in patients with lung cancer may not be specific to immune checkpoint inhibitor therapy. Previous observations suggested that high BMI is associated with better outcomes with surgery, radiotherapy, and some types of chemotherapy in patients with early and advanced NSCLC.8,9,10,11 In contrast, high BMI was not associated with survival benefit from chemotherapy with docetaxel. It appears that obesity may have a varying influence across the spectrum of treatment interventions for lung cancer.
The ITT comparison of atezolizumab vs docetaxel for BMI subgroups is novel. The observed signal of atezolizumab OS benefit between BMI subgroups in PD-L1–positive tumors should be reevaluated in future studies with larger data sets of immune checkpoint inhibitor–treated patients. Moreover, it is unclear based on our analyses whether BMI could be considered as a treatment effect modifier owing to lack of adequate power. Future research on the effect of BMI subgroups across all immune checkpoint inhibitor therapy trials may provide adequate power to evaluate this question.
It is well recognized that men and women have different body composition and adiposity with varying prevalence of obesity. However, the interaction between sex and immune checkpoint inhibitor therapy outcomes is inconsistent. A recent report identified that sex may be a predictor of response to ipilimumab, a CTLA4 antibody, but not to PD-1/PD-L1 inhibitors, with men having better OS compared with women owing to sexual dimorphism in immune response.26 An updated meta-analysis reported that both men and women had similar OS benefit with immune checkpoint inhibitor therapies.27 However, women with NSCLC have better overall outcomes than men, even after adjusting for smoking, cancer histologic characteristics, and oncogene mutations.28,29 Contrary to a previous report in which men with obesity had a better outcome with immunotherapy in melanoma,12 our data suggested that sex had no significant effect on the improved survival seen with men and women with obesity.
The association between BMI and TRAEs and irAEs associated with immune checkpoint inhibitors has been variably reported. In our data set, we did not find that patients with obesity had an increased incidence of any grade of TRAEs compared with those whose BMI was normal; these results are similar to those of McQuade et al.12 However, a retrospective series by Cortellini et al13 that included various cancer types, such as lung, kidney, melanoma, and others, as well as those with poor performance status, reported a higher incidence of any grade of irAEs in patients who were overweight or obese. Given the expected improved accuracy of data collected through prospective clinical trials in our analysis, it is unlikely that obesity is associated with increased TRAEs. Among the irAEs, those affecting the skin were the only ones consistently associated with high BMI. Future research using large data sets from all immune checkpoint inhibitor trials could robustly evaluate the association between obesity and the incidence of irAEs.
In the present post hoc exploratory analysis, pooling of prospectively collected clinical trial data provided, to our knowledge, one of the largest cohorts of patients who received uniform treatment with atezolizumab. The data were of high quality and allowed analysis with adjustments for key clinical confounders. Furthermore, the data contained only a small amount of missing information, improving the accuracy of our analyses. The ITT analysis that compared atezolizumab and docetaxel arms for BMI subgroups is unique in our study.
Limitations and Strengths
There are several limitations in our study. The results from this analysis should be considered as exploratory—not preplanned—and need to be confirmed in subsequent clinical trials. Moreover, BMI alone as a measure of obesity is problematic because of its inability to differentiate fat and lean muscle mass and to diagnose sarcopenia; in addition, BMI is a poor reflection of body fat distribution. It is likely that a combination of clinical and biochemical markers may be required to more accurately characterize obesity.
Another known prognostic factor associated with survival in patients with NSCLC is pretreatment weight loss, which is a measure of cachexia.4,30,31 Weight loss, either before or during treatment, may have variable consequences in treatment response. In the present analysis, we used 1-time recorded height and weight at screening or day 1 of trial treatment for the calculation of BMI. Because pretreatment weight loss was variably recorded in the data set provided, the role of this prognostic factor could not be assessed. It would be relevant to analyze data from other trials that have prospectively collected information on pretreatment weight loss. Despite these limitations, the association between BMI and OS from atezolizumab, especially in patients with PD-L1–positive cancer, appears to be strong.
Conclusions
Baseline high BMI may be independently associated with improved survival with atezolizumab in patients with advanced NSCLC. Baseline BMI should therefore be considered as a stratification factor in future immune checkpoint inhibitor therapy trials.
Notes
Supplement.
eTable 1. Baseline Characteristics of Atezolizumab Treated Patients
eTable 2. Cox Proportional Hazards Regression Analysis for Overall Survival/Progression Free Survival for Atezolizumab Treated Patients Across Trials
eTable 3. Sex-Specific Association Between BMI and Overall Survival for Patients With Advanced NSCLC Treated With Atezolizumab
eTable 4. Cause of Death by Trials
eTable 5. Cause of Death by BMI Groups
eTable 6. Pooled Adverse Events Related to Atezolizumab Across All Trials
eTable 7. Baseline Characteristics of Docetaxel Treated Patients
eTable 8. Baseline Characteristics of the Pooled Intention-to-Treat Populations of the OAK and POPLAR Trials Excluding Participants With Missing or Underweight BMI
eFigure 1. Forest Plots for Adverse Events From Atezolizumab
eFigure 2. OS as per BMI Categories for Pooled OAK and POPLAR Trials
References
Full text links
Read article at publisher's site: https://doi.org/10.1001/jamaoncol.2019.5241
Read article for free, from open access legal sources, via Unpaywall:
https://jamanetwork.com/journals/jamaoncology/articlepdf/2757521/jamaoncology_kichenadasse_2019_oi_190098.pdf
Citations & impact
Impact metrics
Article citations
Obesity-dependent selection of driver mutations in cancer.
Nat Genet, 56(11):2318-2321, 28 Oct 2024
Cited by: 0 articles | PMID: 39468367 | PMCID: PMC11549034
Prediction of 90-day mortality risk after unplanned emergency department visits of advanced stage cancer patients.
Support Care Cancer, 32(11):732, 16 Oct 2024
Cited by: 0 articles | PMID: 39414641 | PMCID: PMC11485181
A multidimensional analysis of the impact of obesity on immune checkpoint inhibitor therapy efficacy.
Cancer Cell Int, 24(1):358, 29 Oct 2024
Cited by: 0 articles | PMID: 39472922 | PMCID: PMC11523605
Obesity and survival in advanced non-small cell lung cancer patients treated with chemotherapy, immunotherapy, or chemoimmunotherapy: a multicenter cohort study.
BMC Med, 22(1):463, 14 Oct 2024
Cited by: 0 articles | PMID: 39402614 | PMCID: PMC11475647
Impact of baseline body mass index on the long-term prognosis of advanced hepatocellular carcinoma treated with immunotherapy.
World J Gastroenterol, 30(37):4132-4148, 01 Oct 2024
Cited by: 0 articles | PMID: 39474397 | PMCID: PMC11514531
Go to all (164) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials (4)
- (1 citation) ClinicalTrials.gov - NCT02008227
- (1 citation) ClinicalTrials.gov - NCT01903993
- (1 citation) ClinicalTrials.gov - NCT02031458
- (1 citation) ClinicalTrials.gov - NCT01846416
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial.
Lancet, 387(10030):1837-1846, 10 Mar 2016
Cited by: 1599 articles | PMID: 26970723
CONTACT-01: A Randomized Phase III Trial of Atezolizumab + Cabozantinib Versus Docetaxel for Metastatic Non-Small Cell Lung Cancer After a Checkpoint Inhibitor and Chemotherapy.
J Clin Oncol, 42(20):2393-2403, 29 Mar 2024
Cited by: 1 article | PMID: 38552197 | PMCID: PMC11227305
Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial.
Lancet, 389(10066):255-265, 13 Dec 2016
Cited by: 2499 articles | PMID: 27979383 | PMCID: PMC6886121
Atezolizumab: A Review in Previously Treated Advanced Non-Small Cell Lung Cancer.
Target Oncol, 13(3):399-407, 01 Jun 2018
Cited by: 8 articles | PMID: 29785577
Review
 1
,
2
1
,
2