Abstract
Free full text

Time Course of Lung Changes On Chest CT During Recovery From 2019 Novel Coronavirus (COVID-19) Pneumonia
Abstract
Background
Chest CT is used to assess the severity of lung involvement in COVID-19 pneumonia.
Purpose
To determine the change in chest CT findings associated with COVID-19 pneumonia from initial diagnosis until patient recovery.
Materials and Methods
This retrospective review included patients with RT-PCR confirmed COVID-19 infection presenting between 12 January 2020 to 6 February 2020. Patients with severe respiratory distress and/ or oxygen requirement at any time during the disease course were excluded. Repeat Chest CT was obtained at approximately 4 day intervals. The total CT score was the sum of lung involvement (5 lobes, score 1-5 for each lobe, range, 0 none, 25 maximum) was determined.
Results
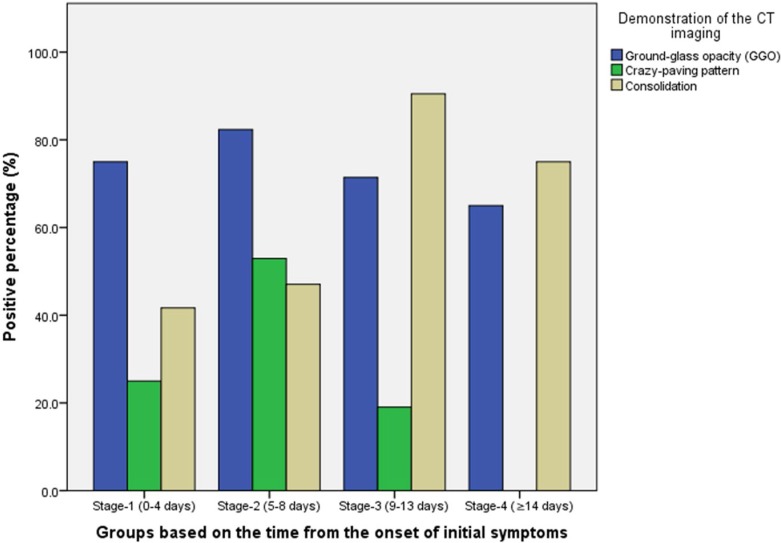
Twenty one patients (6 males and 15 females, age 25-63 years) with confirmed COVID-19 pneumonia were evaluated. These patients under went a total of 82 pulmonary CT scans with a mean interval of 4±1 days (range: 1-8 days). All patients were discharged after a mean hospitalized period of 17±4 days (range: 11-26 days). Maximum lung involved peaked at approximately 10 days (with the calculated total CT score of 6) from the onset of initial symptoms (R2=0.25), p<0.001). Based on quartiles of patients from day 0 to day 26 involvement, 4 stages of lung CT were defined: Stage 1 (0-4 days): ground glass opacities (GGO) in 18/24 (75%) patients with the total CT score of 2±2; (2)Stage-2 (5-8d days): increased crazy-paving pattern 9/17 patients (53%) with a increase in total CT score (6±4, p=0.002); (3) Stage-3 (9-13days): consolidation 19/21 (91%) patients with the peak of total CT score (7±4); (4) Stage-4 (≥14 days): gradual resolution of consolidation 15/20 (75%) patients with a decreased total CT score (6±4) without crazy-paving pattern.
Conclusion
In patients recovering from COVID-19 pneumonia (without severe respiratory distress during the disease course), lung abnormalities on chest CT showed greatest severity approximately 10 days after initial onset of symptoms.
Introduction
Since December 2019, many gathered cases of “unknown viral pneumonia” have been reported, initially linked to exposure at the Huanan Seafood Market, Wuhan, China (1). A novel coronavirus was detected, capable of infecting humans, on 6 January 2020 (2, 3) and termed COVID-19 (2). By 7 February 2020, there were 43103 confirmed cases with 2019-nCoV (COVID-19) pneumonia in 25 countries (4). Similar to other coronaviral pneumonia such as severe acute respiratory syndrome caused by coronavirus and Middle East respiratory syndrome coronavirus, COVID-19 can also lead to acute respiratory distress syndrome (ARDS) (1, 5). With the gradual recognition of COVID-19 pneumonia, professional consensus, guidelines, and criteria were steadily established with the aim of preventing transmission and facilitating diagnosis and treatment (6-8).
From the recently published literature, thetypical radiological imaging of COVID-19 pneumonia demonstrated clear destruction of the pulmonary parenchyma including interstitial inflammation and extensive consolidation, similar to the previously reported coronavirus infection (1, 9-11). However, some patients with COVID-19 pneumonia consistently demonstrated no hypoxemia or respiratory distress during the course of hospitalization. The purpose ofthis study was determine the change in chest CT findings associated with COVID-19 pneumonia from initial diagnosis until patient recovery.
Materials and Methods
This study was approved by the Ethics of Committees of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, and in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent for this retrospective study was waived. The anonymous data was collected and analyzed to facilitate better clinical decisions and treatment.
Diagnostic and Cure Criteria of COVID-19 Pneumonia
Based on the preliminary diagnosis and treatment protocols from the National Health Commission of the People’s Republic of China, the diagnostic criteria of COVID-19 pneumonia were: 1. epidemiological history - travel/residence history in Wuhan or exposure history to fevered patients with respiratory symptoms from Wuhan within 14 days before the onset of illness; 2. clinical manifestations - fever, imaging characteristics of pneumonia, and/or normal or decreased white blood cells countor decreased lymphocyte count and 3. laboratory diagnosis-real-time fluorescence polymerase chain reaction revealed positive detection of COVID-19 in throat swabs or lower respiratory tract (7, 8). The patients with confirmed COVID-19 pneumonia were hospitalized and isolated for treatment. The discharge criteria were: 1. afebrile for greater than 3 days; 2. respiratory symptoms significantly improved and 3. improvement in the radiological abnormalities on chest radiograph or CT; and 4. two consecutive negative COVID-19 nucleic acid detection at least 24 h apart (7).
Patients
In this study, records for patients diagnosed with COVID-19 pneumonia were reviewed retrospectively for the period from 12 January 2020 to 6 February 2020 in this single center study. Patients with severe pneumonia during the disease course were excluded, defined as: severe respiratory distress (respiratory rate >30 breaths/min); 2. Requirement for oxygen treatment or mechanical ventilation and 3. SpO2<90% on room air (6).
CT Protocol
Chest CT scans were performed using a single inspiratory phase in two commercial multi-detector CT scanners (Philips Ingenuity Core 128, Philips Medical Systems, Best, the Netherlands; SOMATOM Definition AS, Siemens Healthineers, Germany). To minimize motion artifacts, patients were instructed on breath-holding; CT images were then acquired during a single breath-hold. For CT acquisition, the tube voltage was 120kVp with automatic tube current modulation. From the raw data, CT images were reconstructed with a matrix size of 512 ×512 as axial images (thickness of 1.5mm and increment of 1.5mm) in transverse slice orientation with either hybrid iterative reconstruction (iDose level 5, Philips Medical Systems, Netherlands) or a pulmonary B70F kernel and a mediastinal B30f kernel (Siemens Healthineers, Germany). The mean CTDIvol was 8.4 ±2.0 mGy (range: 5.2-12.6 mGy).
×512 as axial images (thickness of 1.5mm and increment of 1.5mm) in transverse slice orientation with either hybrid iterative reconstruction (iDose level 5, Philips Medical Systems, Netherlands) or a pulmonary B70F kernel and a mediastinal B30f kernel (Siemens Healthineers, Germany). The mean CTDIvol was 8.4 ±2.0 mGy (range: 5.2-12.6 mGy).
Chest CT Evaluation
The major CT demonstrations were described using internationally standard nomenclature defined by the Fleischner Society glossary and peer-reviewed literature on viral pneumonia, using terms including ground glass opacity (GGO), crazy-paving pattern, and consolidation (12-14). A semi-quantitative scoring system was used to quantitatively estimate the pulmonary involvement of all these abnormalities on the basis of the area involved (15). Each of the 5 lung lobes was visually scored from 0 to 5 as: 0, no involvement; 1, <5% involvement; 2, 25% involvement; 3, 26%-49% involvement; 4, 50%-75% involvement; 5, >75% involvement. The total CT score was the sum of the individual lobar scores and ranged from 0 (no involvement) to 25 (maximum involvement).
The distribution of lung abnormalities was recorded as predominantly subpleural (involving mainly the peripheral one-third of the lung), random (without predilection for subpleural or central regions), or diffuse (continuous involvement without respect to lung segments) (16).
Image analysis was performed using the institutional digital database system (Vue PACS, version 11.3.5.8902, Carestream Health, Canada) by three radiologists (C.Z, B.L, and L.Y, who had 26, 25 and 22 years of experience in thoracic radiology, respectively) and final scores were determined by consensus.
Statistical Analysis
Statistical analyses were performed using IBM SPSS Statistics Software (version 24; IBM, New York, USA). Quantitative data were presented as mean ± standard deviation (minimum-maximum) and the counting data were presented as the percentage of the total unless otherwise specified. The total CT score of pulmonary as a function of time was quantitively assessed using the SPSS curve estimation module. The comparisons of non-paired and paired quantitative data were evaluated using the Mann-Whitney U test and Wilcoxon test, according to the normal distribution assessed by the Shapiro–Wilk test. A p-value of <0.05 was defined as statistical significance.
Results
1. Patient characteristics:
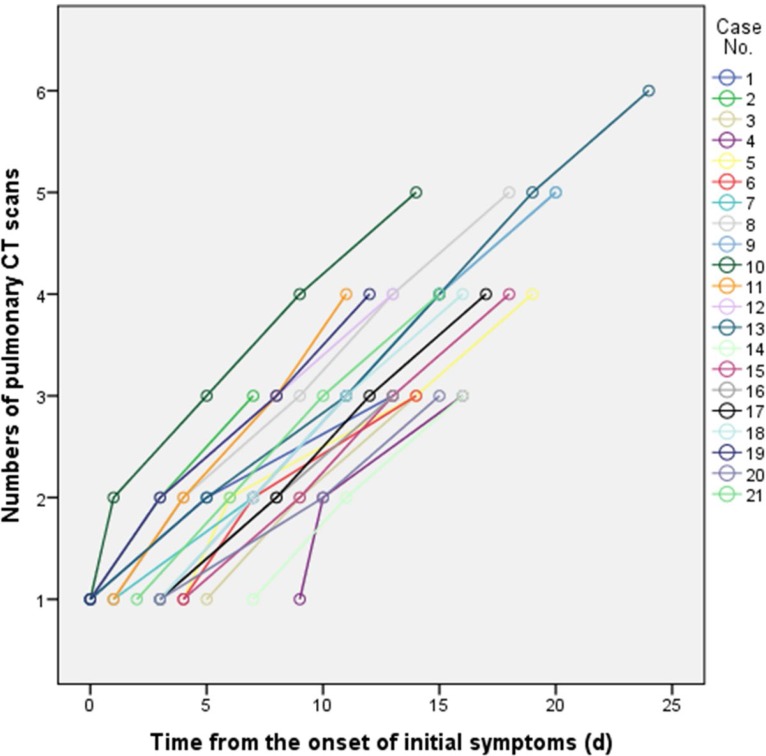
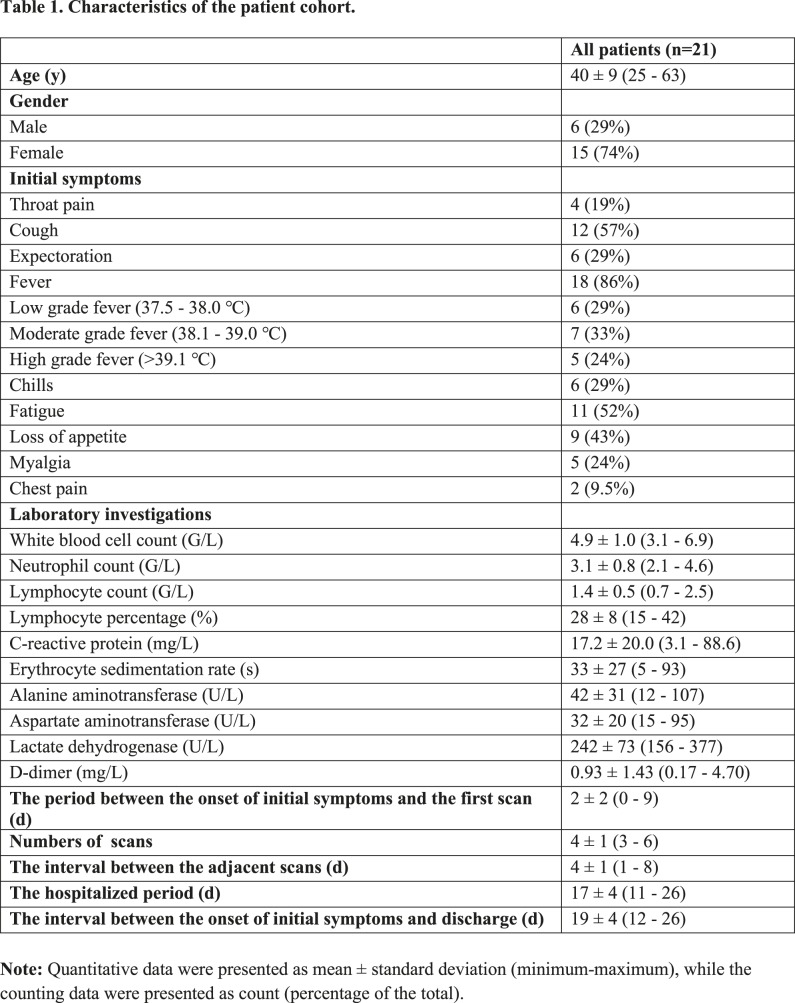
A total of 21 patients(6 males and 15 females) were included in the study. The average age was 40 ± 9 years (Table 1). The most prevalent presenting symptoms were fever (86%)and cough (57%). Four patients had no chest CT abnormalities at base line evaluation. Laboratory investigations were often normal, with the most frequent abnormalities being a mildly elevated C-reactive protein (17.2±20.0mg/L), erythrocyte sedimentation rate (33±27s), lactate dehydrogenase (242±73 U/L), and D-dimer (0.93±1.43) (Table 1). The first pulmonary CT scan was obtained 2±2 days (range: 0–9) after the onset of symptoms. A total of 82 pulmonary CT scans were performed and each patient underwent an average of 4±1 CT scans (range: 3-6) with a mean interval of 4±1 days (range: 1-8 days) (Figure 1). All patients were discharged after a mean hospitalized period of 17±4 days (range: 11-26 days).
Table 1:
Characteristics of the patient cohort.
2. Pulmonary CT Evaluation
Ground glass opacities (GGO), crazy-paving pattern (GGO with superimposed inter- and intralobular septal thickening) and consolidation were the most frequent CT findings in mild COVID-19 pneumonia (Figure 2). No mediastinal lymphadenopathy was observed. In most patients (18 out of 21) the total CT score increased to approximately ten days after the onset of symptoms, and then gradually decreased (Figure 3a). The total CT score peaked at ten days after the onset of initial symptoms (Figure 3, calculated total CT Score, 6 at 10 days).
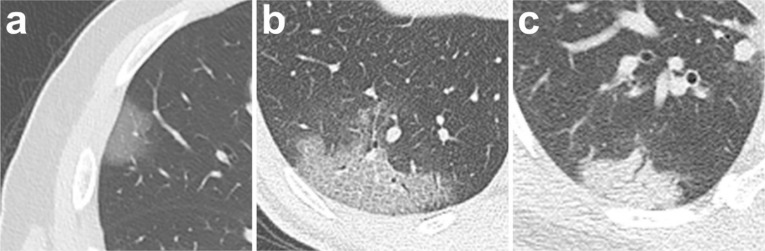
Chest CT findings of COVID-19 pneumonia on transaxial images. (a) GGO; (b) crazy-paving pattern (GGO with superimposed inter- and intralobular septal thickening); (c) Consolidation. All images have the same window level of -600 and window width of 1600.
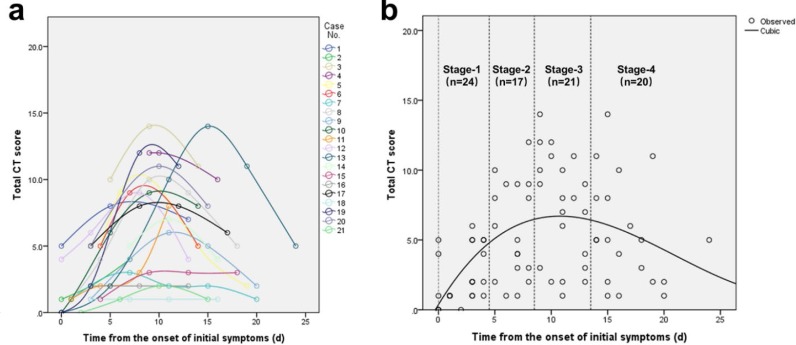
Change in lung involvement on chest CT from time of onset of initial symptoms (in days). (a) The dynamic changes in total CT score for each patient; (b) Peak total CT lung involvement occurred at day 10 (curve fitting equation: y=0.001*x3-0.083*x2+1.329x+0.373, in which x = time from the onset of the initial symptoms, y = total CT score of the pulmonary involvement; R2=0.25, p<0.001). Quartiles of patients between 0 and 26 days are shown as stages 1 to 4.
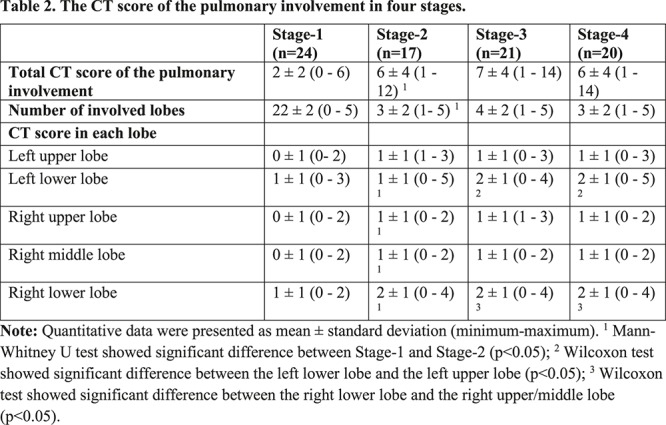
Based on quartiles of patients and degree of lung involvement from day 0 to day 26 after disease onset, four stages were identified from the onset of initial symptoms (Figure 3b): Stage-1 (0-4 days, n=24); Stage-2 (5-8 days, n=17); Stage-3 (9-13 days, n=21); and Stage-4 (≥14 days, n=20). In each group, the lower lobes were more inclined to be involved with higher CT scores. The CT scores of bilateral lower lobes were especially reaching significant differences than the corresponding upper/middle lobes in Stage-3 and Stage-4 (In Stage-3 and Stage-4, left lower lobe versus left upper lobe: 2 ± 1 vs. 1 ± 1, p=0.002, p=0.004, respectively; and right lower lobe versus right upper/middle lobe: 2 ± 1 versus 1 ± 1/1 ± 1, p<0.001/=0.002, p=0.001/0.003, respectively). In Stage-2, the total CT score was greater than that of Stage-1 (6±4 versus 2±2, p=0.002) (Table 2).
Table 2:
The CT score of the pulmonary involvement in four stages.
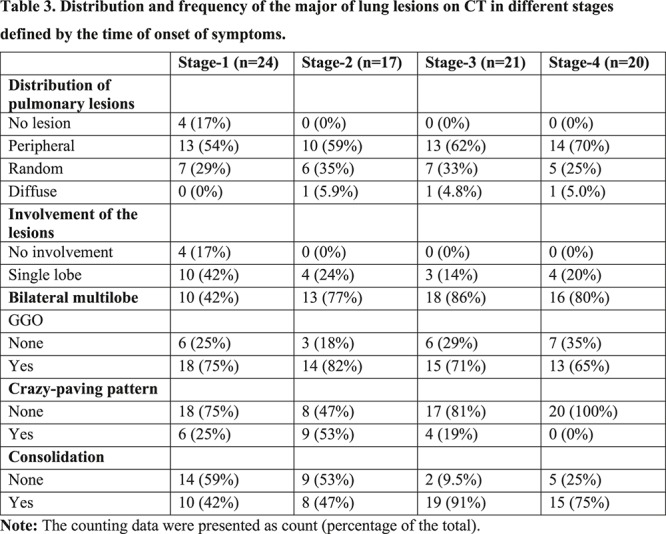
The distribution of lesions and major CT findings were compared at the four stages of recovery (Table 3). Overal, subpleural lesions were more common than central lung lesions. The majority of patients had bilateral lung involvement during the course of the diease. In Stage-1, GGO [18/24 (75%) of patients] with partial crazy-paving pattern [6/24 (25%) of patients] and consolidation [10/24 (42%) of patients] could be mostly found (Figures 4, ,5).5). In Stage-2, the GGO [14/17 (82%) of patients] extended to more pulmonary lobes with more crazy-paving pattern [9/17 (53%) of patients] and consolidation [8/17 (47%) of patients]. In Stage-3, consolidation [19/21 (91%) of patients] was the main demonstration with the obviously deceased ratio of GGO [15/21 (71%) of patients] and crazy-paving pattern [4/21 (19%) of patients]. In Stage-4, the consolidation [15/20 (75%) of patients] was partially absorbed without any crazy-paving pattern [0/20 (0%) of patients].
Table 3:
Distribution and frequency of the major of lung lesions on CT in different stages defined by the time of onset of symptoms.
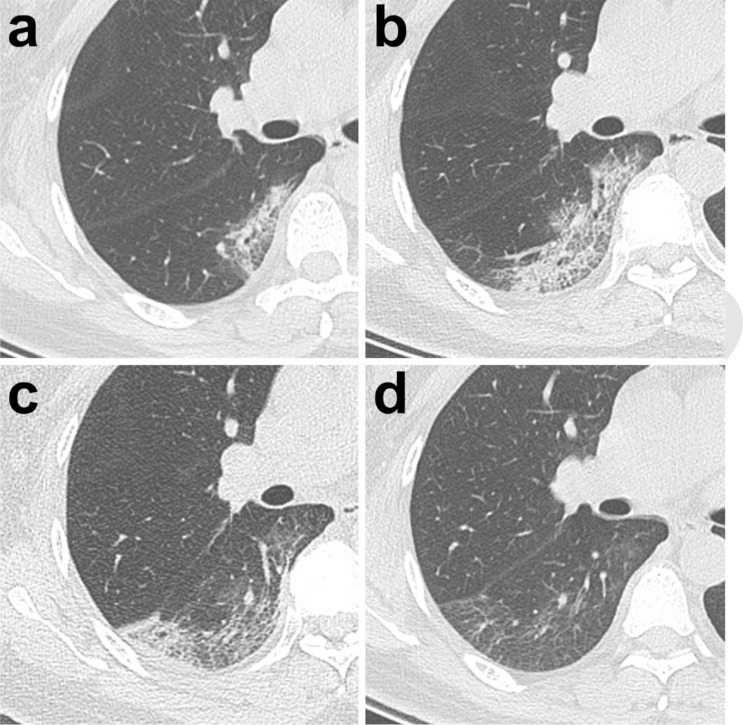
Typical evolution of CT findings in a47-year-old female patient presenting with persistent fever (38.8°C) for three days. (a) At presentation (day 3), a small region of subpleural GGO with partial consolidation was demonstrated in the right lower lobe; (b) day 7, there was an enlarged region of GGO with superimposed inter-and intralobular septal thickening (crazy-paving pattern) with partial consolidation; (c) day 11, partial resolution of the initial GGO, with a new area of subpleural consolidation; (d) day 20, continued resolution with minimal residual GGO and parenchymal bands were observed. All images have the same window level of -600 and window width of 1600.
Discussion
The purpose of this study was determine the change in chest CT findings associated with COVID-19 pneumonia from initial diagnosis until patient recovery. We studied patients with COVID-19 at approximately 4 day intervals from the onset of disease until recovery. Patients with complicated pneumonia including severe respiratory distress (respiratory rate >30 breaths/min), requirement for oxygen treatment or mechanical ventilation or SpO2<90% on room air were excluded. In patients without severe respiratory disease, the major pulmonary CT findings of COVID-19 pneumonia were GGO, crazy-paving pattern and consolidation predominantly in subpleural locations in the lower lobes. Chest CT abnormalities increased in number and severity of lesions in the first 10 weeks (peaking at apprxoimately 10 days). Subseqquently, there was a short plateau phase and a gradual decrease in abnormalities.
In this study, the patients underwent multiple pulmonary CT scans (≥3 times) providing reliable data of the dynamic radiological pattern. The typical mild COVID-19 pneumonia mainly starts as small subpleural, unilateral or bilateral GGO in the lower lobes, which then develops into the crazy-paving pattern and subsequent consolidation. After more than two weeks, the lesions are gradually absorbed with residual GGO and subpleural parenchymal bands. In these patients who recovered from COVID- 19 pneumonia, 4 stages of lung involvement were defined on CT:
1. Early stage (0-4 days after onset of the initial symptom): In this stage, GGO was the main radiological demonstration (Figure 2a) distributed subpleurally in the lower lobes unilaterally or bilaterally. In our ochort, negative findings were revealed in four patients (total CT score=0) (Figure 3a). However, repeat pulmonary CT showed lung abnormalities in these 4 patients.
2. Progressive stage (5-8 days after the onset of the initial symptom): In this stage, the infection rapidly aggravated and extended to a bilateral multi-lobe distribution with diffuse GGO, crazy-paving pattern and consolidation (Figure 5b).
3. Peak stage (9-13 days after the onset of the initial symptom): In this stage, the involved area of the lungs slowly increased to the peak invovlment and dense consolidation became more prevalent. Findings included diffuse GGO, crazy-paving pattern, consolidation, and residual parenchymal bands.
4. Absorption stage (≥14 days after the onset of the initial symptom): In this stage, the infection was controlled and the consolidation was gradually absorbed. No crazy-paving pattern was present anymore. However, in this process, extensive GGO could be observed as the demonstration of the consolidation absorption (Figure 5d). Noticeably, in this study, no crazy-paving was observed in this stage, likely as a result of recovering. Based on the total CT score, the absorption stage extended beyond 26 days (our last days of follow-up) from the onset of initial symptoms.
Limitations of this study include the retrospective nature of our analysis and lack of a severe COVID- 19 comparison group. Most of the patients with severe pneumonia and ARDS are still hospitalized (17). Therefore, the analysis was limited to patients with mild COVID-19 pneumonia.
In summary, most patients who recovered from COVID-19 pneumonia showed greatest severity of lung disease on CT at approximately 10 days after initial onset of symptoms. Chest CT signs of improvment began at approximately 14 days after the onset of initial symptoms.
Acknowledgements
The authors would like to express their appreciation for all of the emergency services, nurses, doctors, and other hospital staff for their efforts to combat the COVID-19 outbreak.
References
Full text links
Read article at publisher's site: https://doi.org/10.1148/radiol.2020200370
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc7233367?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1148/radiol.2020200370
Article citations
The severity assessment and nucleic acid turning-negative-time prediction in COVID-19 patients with COPD using a fused deep learning model.
BMC Pulm Med, 24(1):515, 14 Oct 2024
Cited by: 0 articles | PMID: 39402509 | PMCID: PMC11476205
Genetic signatures of <i>AKT1</i> variants associated with worse COVID-19 outcomes - a multicentric observational study.
Front Immunol, 15:1422349, 08 Oct 2024
Cited by: 0 articles | PMID: 39439795 | PMCID: PMC11493623
Predicting the severity of mycoplasma pneumoniae pneumonia in pediatric and adult patients: a multicenter study.
Sci Rep, 14(1):22978, 03 Oct 2024
Cited by: 0 articles | PMID: 39362944 | PMCID: PMC11450145
Prognostic Value of Coronary Artery Calcification in Patients with COVID-19 and Interstitial Pneumonia: A Case-Control Study.
J Cardiovasc Dev Dis, 11(10):319, 11 Oct 2024
Cited by: 0 articles | PMID: 39452289 | PMCID: PMC11508648
U-Net-based computed tomography quantification of viral pneumonia can predict fibrotic interstitial lung abnormalities at 3-month follow-up.
Front Med (Lausanne), 11:1435337, 30 Sep 2024
Cited by: 0 articles | PMID: 39403283 | PMCID: PMC11471527
Go to all (1,518) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Frequency and Distribution of Chest Radiographic Findings in Patients Positive for COVID-19.
Radiology, 296(2):E72-E78, 27 Mar 2020
Cited by: 775 articles | PMID: 32216717 | PMCID: PMC7233401
Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2.
Eur J Nucl Med Mol Imaging, 47(5):1275-1280, 28 Feb 2020
Cited by: 421 articles | PMID: 32107577 | PMCID: PMC7080117
The Performance of Chest CT in Evaluating the Clinical Severity of COVID-19 Pneumonia: Identifying Critical Cases Based on CT Characteristics.
Invest Radiol, 55(7):412-421, 01 Jul 2020
Cited by: 78 articles | PMID: 32304402 | PMCID: PMC7173027
Chest CT features of coronavirus disease 2019 (COVID-19) pneumonia: key points for radiologists.
Radiol Med, 125(7):636-646, 04 Jun 2020
Cited by: 98 articles | PMID: 32500509 | PMCID: PMC7270744
Review Free full text in Europe PMC

 and
and