Abstract
Background
Since the outbreak of coronavirus disease 2019 (COVID-19) in China in December 2019, considerable attention has been focused on its elucidation. However, it is also important for clinicians and epidemiologists to differentiate COVID-19 from other respiratory infectious diseases such as influenza viruses.Research question
The aim of this study was to explore the different clinical presentations between COVID-19 and influenza A (H1N1) pneumonia in patients with ARDS.Study design and methods
This analysis was a retrospective case-control study. Two independent cohorts of patients with ARDS infected with either COVID-19 (n = 73) or H1N1 (n = 75) were compared. Their clinical manifestations, imaging characteristics, treatments, and prognosis were analyzed and compared.Results
The median age of patients with COVID-19 was higher than that of patients with H1N1, and there was a higher proportion of male subjects among the H1N1 cohort (P < .05). Patients with COVID-19 exhibited higher proportions of nonproductive coughs, fatigue, and GI symptoms than those of patients with H1N1 (P < .05). Patients with H1N1 had higher Sequential Organ Failure Assessment (SOFA) scores than patients with COVID-19 (P < .05). The Pao2/Fio2 of 198.5 mm Hg in the COVID-19 cohort was significantly higher than the Pao2/Fio2 of 107.0 mm Hg in the H1N1 cohort (P < .001). Ground-glass opacities was more common in patients with COVID-19 than in patients with H1N1 (P < .001). There was a greater variety of antiviral therapies administered to COVID-19 patients than to H1N1 patients. The in-hospital mortality of patients with COVID-19 was 28.8%, whereas that of patients with H1N1 was 34.7% (P = .483). SOFA score-adjusted mortality of H1N1 patients was significantly higher than that of COVID-19 patients, with a rate ratio of 2.009 (95% CI, 1.563-2.583; P < .001).Interpretation
There were many differences in clinical presentations between patients with ARDS infected with either COVID-19 or H1N1. Compared with H1N1 patients, patients with COVID-19-induced ARDS had lower severity of illness scores at presentation and lower SOFA score-adjusted mortality.Free full text

Comparison of Hospitalized Patients With ARDS Caused by COVID-19 and H1N1
Abstract
Background
Since the outbreak of coronavirus disease 2019 (COVID-19) in China in December 2019, considerable attention has been focused on its elucidation. However, it is also important for clinicians and epidemiologists to differentiate COVID-19 from other respiratory infectious diseases such as influenza viruses.
Research question
The aim of this study was to explore the different clinical presentations between COVID-19 and influenza A (H1N1) pneumonia in patients with ARDS.
Study Design and Methods
This analysis was a retrospective case-control study. Two independent cohorts of patients with ARDS infected with either COVID-19 (n = 73) or H1N1 (n = 75) were compared. Their clinical manifestations, imaging characteristics, treatments, and prognosis were analyzed and compared.
Results
The median age of patients with COVID-19 was higher than that of patients with H1N1, and there was a higher proportion of male subjects among the H1N1 cohort (P < .05). Patients with COVID-19 exhibited higher proportions of nonproductive coughs, fatigue, and GI symptoms than those of patients with H1N1 (P < .05). Patients with H1N1 had higher Sequential Organ Failure Assessment (SOFA) scores than patients with COVID-19 (P < .05). The Pao2/Fio2 of 198.5 mm Hg in the COVID-19 cohort was significantly higher than the Pao2/Fio2 of 107.0 mm Hg in the H1N1 cohort (P < .001). Ground-glass opacities was more common in patients with COVID-19 than in patients with H1N1 (P < .001). There was a greater variety of antiviral therapies administered to COVID-19 patients than to H1N1 patients. The in-hospital mortality of patients with COVID-19 was 28.8%, whereas that of patients with H1N1 was 34.7% (P = .483). SOFA score-adjusted mortality of H1N1 patients was significantly higher than that of COVID-19 patients, with a rate ratio of 2.009 (95% CI, 1.563-2.583; P < .001).
Interpretation
There were many differences in clinical presentations between patients with ARDS infected with either COVID-19 or H1N1. Compared with H1N1 patients, patients with COVID-19-induced ARDS had lower severity of illness scores at presentation and lower SOFA score-adjusted mortality.
Since December 2019, there has been a cluster of patients with pneumonia of previously unknown cause in Wuhan, China. Research by the Chinese Center for Disease Control and Prevention assessed the lower respiratory tracts of these patients and discovered a novel coronavirus, which has since been named the 2019 novel coronavirus.1 On February 11, 2020, the World Health Organization officially named this novel coronavirus pneumonia as coronavirus disease 2019 (COVID-19), whereas the International Committee on Taxonomy of Viruses has named it severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Huang et al2 reported that the first 41 patients with COVID-19 exhibited fever, cough, myalgia, and/or fatigue as common symptoms, 29% of whom had ARDS and six of whom died (15%). The typical findings from chest CT scans were bilateral ground-glass opacity and subsegmental areas of consolidation. At earlier times during the COVID-19 outbreak, patients with COVID-19 were more likely to report exposure to food from the Huanan Seafood Wholesale Market. With the epidemic gradually growing, it is now clear that human-to-human transmission has been prevalent.3 As of March 10, 2020, there have been a total of 113,702 confirmed cases and 4,012 related deaths, among which 80,924 cases have occurred in China.4
Importantly, when assessing COVID-19, it is noteworthy that influenza viruses share common etiologies and occur in the same season. Recently, global influenza associated with respiratory mortality is occurring at a higher frequency than what has been previously reported.5 From September 2019 through present-day, there have been > 170,000 patients with influenza in the United States, more than one-half of whom have been infected with the influenza A (H1N1) virus. The percentage of deaths attributed to pneumonia induced by influenza is 6.8%.6 During the H1N1 global epidemic in 2009, Jain et al7 found that 5% of patients with H1N1 influenza were admitted to ICUs and 7% died. Another study from Canada showed that the overall mortality among patients critically ill with H1N1 at 28 days was 14.3%.8 The common symptoms of H1N1 infection include fever and productive cough, whereas GI symptoms (eg, nausea, vomiting, diarrhea) are less common. Furthermore, ground-glass opacities are not commonly found on chest CT scans from patients with H1N1.9
Although SARS-CoV-2 and H1N1 virus have loomed as epidemics in different regions at present, such epidemics can easily propagate to further regions over time due to climate change and global travel by individuals. Because of their distinct treatments and prognoses, it is important for clinicians and epidemiologists to accurately identify these two respiroviral infections via their differential clinical manifestations. The aim of the current study therefore was to compare the different clinical presentations between ARDS patients infected with COVID-19 vs those infected with H1N1 to provide some guidance for their differential diagnoses.
Patients and Methods
Study Design
This analysis was a retrospective case-control study. All of the COVID-19 subjects were confirmed by using results of laboratory tests and were hospitalized at Wuhan Pulmonary Hospital (Hubei Province of China) between December 24, 2019, and February 7, 2020. The H1N1 pneumonia cases were from a single-center prospective cohort study10 of patients with H1N1-induced ARDS at Beijing Chao-Yang Hospital (China). All of the H1N1 cases were confirmed by using laboratory test results, and corresponding patients were hospitalized from March 2016 to December 2019. All of the patients met the criteria of the Berlin definition11 for diagnosis of ARDS. Following fulfillment of these criteria, all of the patients with COVID-19-induced or H1N1-induced ARDS were included in this study.
The Ethics Committee of Beijing Chao-Yang Hospital (2017-KE-61) and Wuhan Pulmonary Hospital (wufeilunli-2020-02) approved the collection of clinical data from the included patients with H1N1 or COVID-19 infections, respectively. For the H1N1 cohort, written informed consent was obtained from all of the patients or their legal guardians. For the COVID-19 cohort, informed consent from each patient was waived because we prospectively collected and analyzed all of the data from each patient according to the policy for public health outbreak investigation of emerging infectious diseases issued by the National Health Commission of the People’s Republic of China.
Data Collection
Demographic and clinical data of the patients were entered into an electronic case report form. The data included the following: demographic characteristics (age and sex), underlying diseases, comorbidities, clinical symptoms (fever, cough, sputum, dyspnea, chest pain, rash, nausea, vomiting, abdominal pain, diarrhea, and headache), signs (body temperature, heart rate, respiratory frequency, and BP), laboratory tests (blood routine test, arterial blood gas analysis, and blood chemistry), and microbiologic findings/images of the lung (chest CT scan). Antimicrobiologic therapy, respiratory support, complications, and outcomes were also recorded.
Diagnoses of patients infected with COVID-19 or H1N1 were based on clinical presentations, imaging characteristics, and the presence of either SARS-CoV-2 or H1N1 detected in samples from either the respiratory tract or blood.
Statistical Analysis
Data analysis was performed by using SPSS 23.0 (IBM SPSS Statistics, IBM Corporation) software. Categorical variables were summarized by using frequencies and percentages, and continuous data are presented as the medians (interquartile ranges). The Mann-Whitney U test was used for continuous variables, and the χ2 test or the Fisher exact test was used for categorical variables. Variables with a P value < .05 in the univariate analysis were entered into multivariate logistic regression analysis to identify independent risk factors associated with COVID-19 or H1N1. All P values < .05 are considered statistically significant.
Results
From December 24, 2019, to February 7, 2020, there were a total of 179 patients infected with COVID-19 admitted to the Department of Pulmonary and Critical Care at Wuhan Pulmonary Hospital in Hubei Province of China, among which 73 cases included ARDS. There were 345 patients with ARDS induced by pneumonia of various etiologies admitted to the respiratory ICU at Beijing Chao-Yang Hospital from March 2016 to December 2019, among whom 75 patients were infected with H1N1.
COVID-19 and H1N1 Patient Characteristics
The median age of patients with COVID-19 was 67 years, which was significantly higher than that of patients with H1N1 (52 years; P < .001). The proportion of male subjects in the COVID-19 group was 61.5%, which was significantly lower than that of the H1N1 group (80.0%; P = .011). In terms of underlying diseases, 31.5% of COVID-19 patients has a history of cardiovascular disease, whereas that of H1N1 patients was significantly lower (10.7%; P = .002). There was no significant difference in the history of hypertension, diabetes, or chronic airway diseases between the two groups. At the time of admission, septic shock had occurred in 31.5% of patients with COVID-19, which was greater than that reported in patients with H1N1 (13.3%; P < .001). However, the median Sequential Organ Failure Assessment (SOFA) score and the Acute Physiology and Chronic Health Evaluation II (APACHE II) score of COVID-19 patients were 2 and 11, respectively, which were lower than the scores of 5 (P < .001) and 14 (P = .019) for H1N1 patients. There was no significant difference in the duration of onset to ARDS or duration of onset to diagnosis (Table 1 ).
Table 1
Characteristics of Patients With COVID-19 or H1N1
| Characteristic | Total (N = 148) | COVID-19 (n = 73) | H1N1 (n = 75) | P Value |
|---|---|---|---|---|
| Age, y | 62 (47, 69) | 67 (57, 72) | 52 (41, 64) | < .001 |
| Male sex | 105 (70.9) | 45 (61.6) | 60 (80.0) | .011 |
| Onset to ARDS, d | 8 (6, 11) | 8 (6, 10) | 8 (6, 12) | .755 |
| Onset to confirm diagnosis, d | 10 (7, 14) | 11 (8, 14) | 9 (7, 13) | .079 |
| CURB-65 score | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) | .255 |
| SOFA score | 4 (2, 6) | 2 (2, 4) | 5 (4, 8) | < .001 |
| APACHE II score | 12 (8, 15) | 11 (8, 13) | 14 (9, 19) | .019 |
| Highest temperature, °C | 38.5 (36.8, 39.3) | 36.8 (36.5, 38.2) | 39 (38.7, 39.8) | < .001 |
| Systolic BP, mm Hg | 127 (110, 140) | 123 (118, 128) | 128 (108, 143) | .626 |
| Diastolic BP, mm Hg | 70 (62, 82) | 76 (70, 84) | 70 (60, 82) | .554 |
| Respiratory rate, breaths/min | 22 (20, 31) | 21 (20, 30) | 26 (21, 33) | .021 |
| Heart rate, beats/min | 90 (80, 104) | 86 (78, 101) | 96 (81, 112) | .006 |
| Underlying diseases | ||||
| Smoke | 43 (29.3) | 8 (11.0) | 35 (47.3) | < .001 |
| Hypertension | 70 (47.3) | 38 (52.1) | 32 (42.7) | .323 |
| Diabetes | 35 (23.6) | 20 (27.4) | 15 (20.0) | .336 |
| Cardiovascular disease | 31 (20.9) | 23 (31.5) | 8 (10.7) | .002 |
| Chronic kidney failure | 9 (6.1) | 3 (4.1) | 6 (8.0) | .494 |
| Chronic respiratory disease | 2 (1.4) | 1 (1.4) | 1 (1.3) | .745 |
| Complications | ||||
| Leukocytopenia | 125 (84.5) | 60 (82.2) | 65 (86.7) | .502 |
| Septic shock | 33 (22.3) | 23 (31.5) | 10 (13.3) | .010 |
| Acute kidney injury | 21 (14.2) | 13 (17.8) | 8 (10.7) | .245 |
| Liver disfunction | 67 (45.3) | 33 (45.2) | 34 (45.3) | .999 |
Data are presented as medians (interquartile ranges) or No. (%). APACHE = Acute Physiology and Chronic Health Evaluation; COVID-19 = coronavirus disease 2019; CURB-65 = confusion, urea nitrogen, respiratory rate, blood pressure, 65 years of age and older; H1N1 = influenza A (H1N1); SOFA = Sequential Organ Failure Assessment.
Clinical Symptoms and Laboratory Examinations
Both COVID-19 and H1N1 groups presented with fever, cough, and dyspnea, whereas hemoptysis was less common. Furthermore, 53.4% of patients with COVID-19 had productive cough, which was significantly less than that of patients with H1N1 (78.7%; P = .002). The proportions of fatigue (63.0%), GI symptoms (37.0%), and myalgia (34.2%) in patients with COVID-19 were higher than those of patients with H1N1 (18.7%, P < .001; 6.7%, P < .001; and 14.7%, P = .007, respectively) (Table 2 ).
Table 2
Clinical Symptoms of Patients With COVID-19 or H1N1
| Symptom | Total (N = 148) | COVID-19 (n = 73) | H1N1 (n = 75) | P Value |
|---|---|---|---|---|
| Fever | 141 (95.3) | 72 (98.6) | 69 (92.0) | .116 |
| Cough | 125 (84.5) | 58 (79.5) | 67 (89.3) | .115 |
| Sputum | 98 (66.2) | 39 (53.4) | 59 (78.7) | .002 |
| Dyspnea | 108 (73.0) | 52 (71.2) | 56 (74.7) | .712 |
| Fatigue | 60 (63.0) | 46 (63.0) | 14 (18.7) | < .001 |
| GI symptoms | 32 (21.6) | 27 (37.0) | 5 (6.7) | < .001 |
| Myalgia | 36 (24.3) | 25 (34.2) | 11 (14.7) | .007 |
| Hemoptysis | 9 (6.1) | 4 (5.5) | 5 (6.7) | .517 |
Data are presented as No. (%). See Table 1 legend for expansion of abbreviations.
The median Pao 2/Fio 2 in patients with COVID-19 was 198.5 mm Hg, which was significantly higher than the 107.0 mm Hg of patients with H1N1 (P < .001). Following biochemical testing, aspartate transaminase, lactate dehydrogenase, and troponin I levels in patients with COVID-19 were all significantly lower than those in patients with H1N1 (25.5 vs 70.0 U/L, 483 vs 767 U/L, and 0.03 vs 0.14 ng/mL, respectively; P < .001 for each). Both COVID-19 and H1N1 cohorts exhibited impairments in cellular immune function. However, the median CD3+ T lymphocyte concentration in patients with COVID-19 was 193 cells/μL, and the median CD4+CD3+ T lymphocyte concentration was 97 cells/μL, which were significantly lower than those in patients with H1N1 (303 cells/μL, P = .007; and 185 cells/μL, P < .001) (Table 3 ).
Table 3
Laboratory Examinations and Imaging Characteristics at Admission in Patients With COVID-19 or H1N1
| Variable | Total (N = 148) | COVID-19 (n = 73) | H1N1 (n = 75) | P Value |
|---|---|---|---|---|
| Blood routine test | ||||
| WBC (×109/L) | 6.9 (4.6, 10.0) | 7.2 (4.8, 10.0) | 6.6 (4.3, 10.1) | .511 |
| Neutrophil granulocyte (×109/L) | 6.0 (3.3, 9.1) | 6.3 (3.2, 9.2) | 5.5 (3.4, 9.0) | .511 |
| Neutrophil granulocyte, % | 86.0 (77.9, 91.2) | 85.4 (75.4, 90.2) | 86.6 (80.0, 92.0) | .439 |
| Lymphocyte (×109/L) | 0.6 (0.4, 0.8) | 0.7 (0.5, 0.9) | 0.5 (0.4, 0.8) | .251 |
| Lymphocyte, % | 9.2 (5.0, 13.8) | 9.2 (6.1, 16.0) | 9.2 (4.8, 12.3) | .930 |
| Hemoglobin, g/L | 126.0 (105.5, 138.5) | 136.0 (127.5, 147.0) | 124 (104.5, 138.0) | .094 |
| Platelet (×109/L) | 129.0 (99, 176.5) | 166.5 (145.5, 192.5) | 123.0 (96.5, 173.0) | .117 |
| Coagulation function | ||||
| Prothrombin time, s | 13.0 (12.0, 14.8) | 14.2 (12.6, 15.6) | 12.1 (11.5, 13.8) | < .001 |
| Activated partial thromboplastin time, s | 33.8 (28.8, 39.9) | 36.2 (30.4, 40.8) | 31.6 (26.2, 37.8) | .020 |
| D-dimer, mg/L | 2.4 (0.6, 6.6) | 0.6 (0.4, 3.4) | 4.2 (1.8, 9.2) | < .001 |
| Biochemical test | ||||
| Albumin, g/L | 30.7 (26.8, 33.4) | 33.2 (30.8, 36.2) | 27.3 (24.8, 30.8) | < .001 |
| AST, U/L | 29.5 (21.0, 51.0) | 25.5 (20.0, 42.5) | 70.0 (49.0, 123.0) | < .001 |
| ALT, U/L | 52.0 (31.0, 88.0) | 34.5 (24.0, 61.0) | 35.0 (23.0, 55.0) | .742 |
| Total bilirubin, μmol/L | 11.1 (8.2, 16.8) | 9.8 (8.0, 14.5) | 12.1 (9.1, 18.5) | .208 |
| Direct bilirubin, μmol /L | 4.6 (2.7, 7.2) | 3.1 (2.2, 5.4) | 6.2 (3.4, 10.3) | < .001 |
| Urea nitrogen, mmol/L | 5.3 (7.4, 10.8) | 7.5 (6.1, 8.6) | 8.1 (5.6, 12.5) | .247 |
| Creatinine, μmol /L | 81.0 (59.0, 107.0) | 81.0 (62.0, 95.0) | 84.3 (57.7, 116.4) | .320 |
| Lactate dehydrogenase, U/L | 577.0 (440.0, 826.0) | 483.0 (351.0, 602.0) | 767.0 (504.0, 1026.0) | < .001 |
| Troponin I, ng/mL | 0.04 (0.02, 0.20) | 0.03 (0.03, 0.05) | 0.14 (0.02, 0.37) | .014 |
| Type B natriuretic peptide, pg/mL | 217.0 (60.0, 1072.0) | 619.0 (264.0, 2159.0) | 169 (46.5, 649) | .009 |
| Infection and immunity | ||||
| Procalcitonin, ng/mL | 0.4 (0.1, 2.6) | 0.1 (0.0, 0.24) | 1.0 (0.5, 5.9) | < .001 |
| C-reactive protein, mg/dL | 22.8 (10.0, 88.9) | 87.2 (32.6, 104.5) | 11.7 (7.9, 19.8) | < .001 |
| CD3+ T lymphocyte (/μL) | 243 (141, 363) | 193 (98, 295) | 303 (198, 495) | .007 |
| CD4+CD3+ T lymphocyte (/μL) | 150 (75, 240) | 97 (57, 194) | 185 (119, 299) | < .001 |
| CD8+CD3+ T lymphocyte (/μL) | 82 (46, 136) | 70 (36, 116) | 89 (58, 150) | .073 |
| CD4+/CD8+ T lymphocyte | 1.8 (1.3, 2.6) | 1.6 (1.0, 2.3) | 2.2 (1.5, 2.8) | .125 |
| Arterial blood gas analysis | ||||
| pH | 7.42 (7.36, 7.45) | 7.48 (7.45, 7.52) | 7.42 (7.36, 7.45) | .099 |
| Pao2, mm Hg | 74.6 (64.0, 89.0) | 58.0 (49.0, 67.0) | 74.6 (64.0, 89.0) | .018 |
| Paco2, mm Hg | 38.0 (32.0, 44.0) | 35.0 (31.5, 39.5) | 38.0 (32.0, 43.9) | .253 |
| Pao2/Fio2, mm Hg | 138.0 (92.0, 207.3) | 198.5 (147.6, 255.2) | 107.0 (76.0, 148.0) | < .001 |
| Lung CT scan | ||||
| Ground-glass opacity | 103 (69.6) | 69 (94.5) | 34 (45.3) | < .001 |
| Consolidation | 55 (37.2) | 21 (28.8) | 34 (45.3) | .042 |
| Mixed manifestationa | 37 (25.0) | 21 (28.8) | 16 (21.3) | .345 |
Data are presented as medians (interquartile ranges) or No. (%). ALT = alanine aminotransferase; AST = aspartate transaminase. See Table 1 legend for expansion of other abbreviations.
In terms of imaging characteristics, ground-glass opacity on chest CT scans was more common in patients with COVID-19 (94.5%) than in patients with H1N1 (45.3%; P < .001). In contrast, consolidation was more common in patients with H1N1 than in those with COVID-19 (P = .042) (Fig 1 , Table 3).
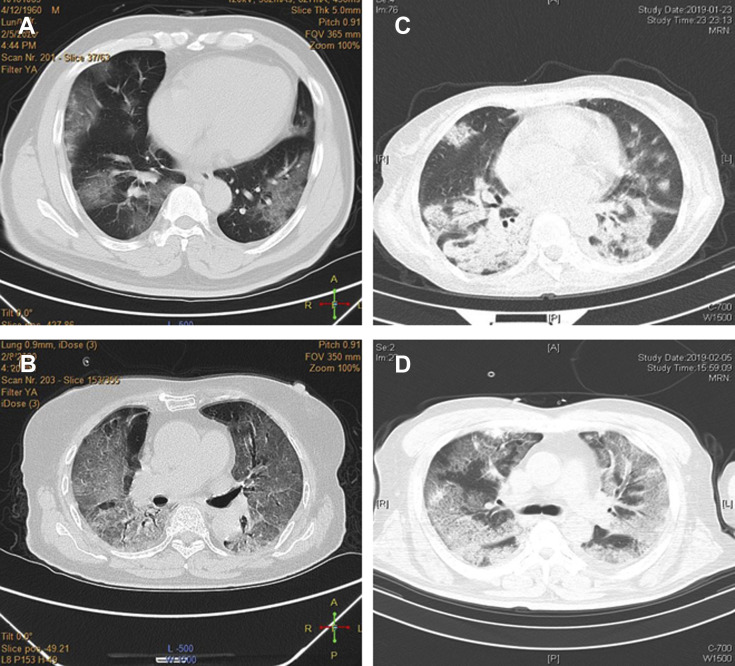
A-D, Imaging characteristics of chest CT scans from patients with coronavirus disease 2019 (COVID-19) and influenza A (H1N1) . A, A 60-year-old man with COVID-19 exhibited multiple ground-glass opacities in both lungs. B, A 75-year-old man with COVID-19 exhibited diffuse ground-glass opacities in both lungs. C, A 46-year-old woman with H1N1 exhibited exudation and consolidation distributed with bronchus in multiple lobes and segments. D, A 66-year-old man with H1N1 exhibited ground-glass opacities with little exudation and consolidation distributed diffusely in both lungs.
Treatment Process and Prognosis
All of the patients received antiviral therapies. Oseltamivir was administered in all of the patients with H1N1. However, patients with COVID-19 were administered a variety of antiviral treatments, including 83.6% with lopinavir/ritonavir, 62.7% with interferon-α2b, 46.6% with oseltamivir, 32.9% with ganciclovir, and 27.4% with traditional Chinese medicines. In addition to antiviral treatments, 79.5% of patients with COVID-19 received glucocorticoids, which was significantly higher than the proportion of 49.3% in patients with H1N1 (P < .001). In contrast, there were no differences in the dosage or course of glucocorticoid treatments between the two groups. Immunoglobulin was administered in 58.9% of patients with COVID-19, which was higher than that administered to patients with H1N1 (29.3%; P < .001) (Table 4 ).
Table 4
Treatments and Prognosis of the Patients With COVID-19 or H1N1
| Variable | Total (N = 148) | COVID-19 (n = 73) | H1N1 (n = 75) | P Value |
|---|---|---|---|---|
| Oxygenation stratification | < .001 | |||
| Pao2/Fio2 > 200 mm Hg | 41 (27.7) | 32 (43.8) | 9 (12.0) | |
| 100 mm Hg < Pao2/Fio2 ≤ 200 mm Hg | 66 (44.6) | 36 (49.3) | 30 (40.0) | |
| Pao2/Fio2 ≤ 100 mm Hg | 41 (27.7) | 5 (6.8) | 36 (48.0) | |
| Initial respiratory support | < .001 | |||
| COT | 54 (38.3) | 49 (67.1) | 5 (7.4) | |
| HFNC | 16 (11.3) | 14 (19.2) | 2 (2.9) | |
| NIV | 29 (20.6) | 5 (6.8) | 24 (35.3) | |
| IMV | 42 (29.8) | 5 (6.8) | 37 (54.4) | |
| Initial respiratory support failure | ||||
| COT failure | 20/54 (37.0) | 20/49 (40.8) | 0/5 (0.0) | .145 |
| HFNC failure | 3/16 (18.8) | 3/14 (21.4) | 0/2 (0.0) | .650 |
| NIV failure | 11/29 (37.9) | 5/5 (100.0) | 6/24 (25.0) | .004 |
| Respiratory support during hospitalization | ||||
| COT | 61 (47.3) | 29 (39.7) | 32 (57.1) | .053 |
| HFNC | 54 (40.6) | 22 (30.1) | 32 (53.3) | .008 |
| NIV | 42 (31.3) | 8 (11.0) | 34 (55.7) | < .001 |
| IMV | 73 (51.4) | 14 (19.2) | 59 (85.5) | < .001 |
| ECMO | 35 (25.2) | 10 (13.7) | 25 (25.2) | .002 |
| Antiviral therapy | ||||
| Interferon-α2b | 42 (29.8) | 42 (62.7) | … | … |
| Ganciclovir | 24 (16.2) | 24 (32.9) | … | … |
| Lopinavir/ritonavir | 61 (47.3) | 61 (83.6) | … | … |
| Oseltamivir | 102 (68.9) | 34 (46.6) | 68 (90.7) | < .001 |
| Chinese traditional medicine | 20 (13.5) | 20 (27.4) | … | … |
| Glucocorticoid | 94 (64.4) | 58 (79.5) | 36 (49.3) | < .001 |
| Initial dosage, mg/d | 80 (40, 80) | 80 (40, 80) | 80 (40, 80) | .770 |
| Duration, d | 8 (5, 11) | 8 (5, 11) | 6 (5, 13) | .502 |
| Immunoglobulin | 65 (43.9) | 43 (58.9) | 22 (29.3) | < .001 |
| Outcome | ||||
| Discharge | 75 (50.7) | 26 (35.6) | 49 (65.3) | .001 |
| Death | 47 (31.8) | 21 (28.8) | 26 (34.7) | .483 |
| In-hospital | 26 (17.6) | 26 (35.6) | … | … |
| Hospital stay, d | 14 (9, 21) | 13 (10, 18) | 16 (9, 30) | .247 |
Data are presented as medians (interquartile ranges) or No. (%). COT = conventional oxygen therapy; ECMO = extracorporeal membrane oxygenation; HFNC = high-flow nasal cannula oxygen therapy; IMV = invasive mechanical ventilation; NIV = noninvasive mechanical ventilation. See Table 1 legend for expansion of other abbreviations.
In terms of respiratory support, 67.1% of patients with COVID-19 received conventional oxygen therapy as initial support, whereas 89.7% of patients with H1N1 received mechanical ventilation (P < .001). However, the failure rates of conventional oxygen therapy, high-flow nasal cannula oxygen therapy, and noninvasive mechanical ventilation were higher in patients with COVID-19. During the entire process of treatment, the proportions of patients with H1N1 who received high-flow nasal cannula oxygen therapy, noninvasive mechanical ventilation, invasive mechanical ventilation, and extracorporeal membrane oxygenation (ECMO) were significantly higher than those of patients with COVID-19 (P < .05) (Table 4).
In terms of prognosis, 26 patients (35.6%) with COVID-19 were not discharged by the time that the current study was published. The in-hospital mortality of patients with COVID-19-induced ARDS was 28.8%, whereas that of patients with H1N1-induced ARDS was 34.7% (P = .483). The SOFA score was then used to adjust the mortality of these patients. SOFA score-adjusted mortality of patients with H1N1 was significantly higher than that of patients with COVID-19; the rate ratio was 2.009 (95% CI, 1.563-2.583; P < .001). There was no difference in the duration of hospitalization between patients with COVID-19 (13 days) and patients with H1N1 (16 days) (Table 4).
Multivariate Analysis
Variables with a P value< .05 in the univariate analysis were entered into multivariate logistic regression analysis. Compared with parameters in patients with COVID-19, patients with H1N1 were more inclined to have productive cough (OR, 9.576; 95% CI, 1.729-64.711; P = .011), consolidation manifested on chest CT imaging (OR, 4.956; 95% CI, 1.518-16.176; P = .008), and higher SOFA scores (OR, 2.263; 95% CI, 1.124-3.574; P = .006). Furthermore, compared with additional parameters in patients with H1N1, patients with COVID-19 had a greater disposition to be older (OR, 0.908; 95% CI, 0.843-0.978; P = .011), exhibit symptoms of fatigue (OR, 0.117; 95% CI, 0.021-0.941]; P = .013), exhibit GI symptoms (OR, 0.100; 95% CI, 0.009-0.984; P = .044), and present with ground-glass opacities on chest CT scans (OR, 0.086; 95% CI, 0.015-0.490; P = .006) (Fig 2 , Table 5 ).
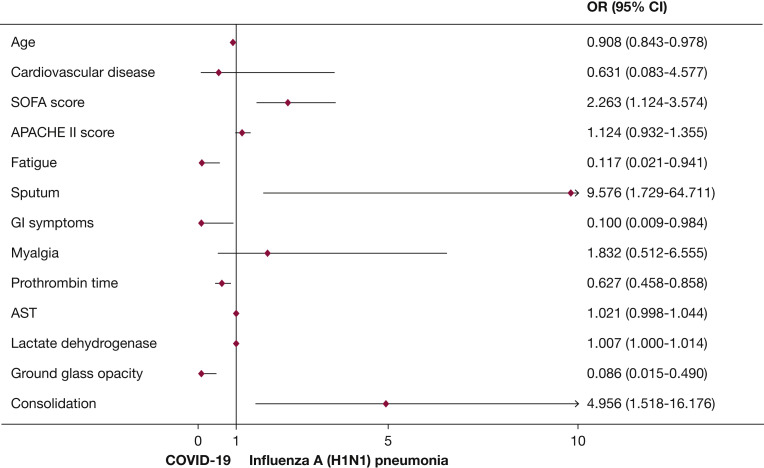
Multivariate model of the specific risk factors for COVID-19 or H1N1. Plots reporting variables independently associated with the risk for COVID-19 or H1N1 in the final model, with their 95% CIs. APACHE = Acute Physiology and Chronic Health Evaluation; AST = aspartate transaminase; SOFA = sequential organ failure assessment. See Figure 1 legend for expansion of other abbreviations.
Table 5
Multivariate Analysis of Independent Risk Factors for Differentiating COVID-19 From H1N1
| Variable | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Age | 0.928 | 0.092-0.956 | < .001 | 0.908 | 0.843-0.978 | .011 |
| Cardiovascular disease | 0.260 | 0.107-0.628 | .003 | 0.631 | 0.083-4.577 | .649 |
| Septic shock | 0.334 | 0.146-0.766 | .010 | … | … | … |
| Respiratory rate | 1.018 | 0.983-1.054 | .325 | … | … | … |
| Heart rate | 1.021 | 1.004-1.039 | .015 | … | … | … |
| SOFA score | 1.820 | 1.462-2.266 | < .001 | 2.263 | 1.124-3.574 | .006 |
| APACHE II score | 1.136 | 1.062-1.214 | < .001 | 1.124 | 0.932-1.355 | .221 |
| Fatigue | 0.135 | 0.064-0.285 | < .001 | 0.117 | 0.021-0.941 | .013 |
| Sputum | 3.215 | 1.567-6.597 | .001 | 9.576 | 1.729-64.711 | .011 |
| GI symptoms | 0.122 | 0.044-0.339 | < .001 | 0.100 | 0.009-0.984 | .044 |
| Myalgia | 0.330 | 0.148-0.736 | .007 | 1.832 | 0.512-6.555 | .352 |
| Prothrombin time | 0.673 | 0.555-0.817 | < .001 | 0.627 | 0.458-0.858 | .004 |
| APTT | 0.986 | 0.954-1.019 | .409 | … | … | … |
| D-dimer | 1.036 | 0.993-1.080 | .100 | … | … | … |
| AST | 1.035 | 1.021-1.049 | < .001 | 1.021 | 0.998-1.044 | .074 |
| Direct bilirubin | 1.155 | 1.055-1.265 | .002 | … | … | … |
| Lactate dehydrogenase | 1.004 | 1.002-1.005 | < .001 | 1.007 | 1.000-1.014 | .025 |
| Troponin I | 1.517 | 0.883-2.605 | .131 | … | … | … |
| CD3+ T lymphocyte | 1.004 | 1.002-1.006 | .001 | … | … | … |
| CD4+CD3+ T lymphocyte | 1.007 | 1.003-1.010 | < .001 | … | … | … |
| Ground-glass opacity | 0.048 | 0.016-0.145 | < .001 | 0.086 | 0.015-0.490 | .006 |
| Consolidation | 2.053 | 1.039-4.056 | .038 | 4.956 | 1.518-16.176 | .008 |
Discussion
The outbreak of COVID-19 began in December 2019, which also corresponded with the flu season. The current study compares the clinical courses between patients with COVID-19-induced ARDS and those with H1N1-induced ARDS. We found that, compared with features in patients with H1N1, patients with COVID-19 were more likely to exhibit nonproductive cough with obvious constitutional symptoms such as fatigue, GI symptoms, and a prevalence in the elderly. In addition, imaging results more commonly presented as ground-glass opacities in patients with COVID-19. However, although the conditions of patients with H1N1 seemed to be more critical than those of patients with COVID-19, there was no difference in the prognoses between ARDS patients infected with COVID-19 vs those infected with H1N1.
Huang et al2 reported that 93% of the first 41 patients with COVID-19 received oseltamivir as an antiviral therapy, which indicated that it was difficult to differentiate COVID-19 from influenza via only clinical manifestations prior to viral identification. Similar to H1N1, SARS-CoV-2 exhibits prevalent human-to-human transmission through close contact, and its basic reproductive number is estimated to be 2.2.3 However, the basic reproductive number estimated during the H1N1 outbreak in Mexico in 2009 ranged from 1.3 to 1.7.12 Acute respiratory infection is always the initial manifestation of these two respiratory infectious diseases. Because of their different therapies, prognoses, and protective measures, it is important to differentiate these two diseases via early clinical presentations. The current study revealed that COVID-19 manifested as nonproductive cough with nonspecific systemic symptoms, which is consistent with previous studies. Wang et al13 analyzed the clinical characteristics of 138 hospitalized COVID-19 patients and reported that fever, fatigue, and dry cough were the most common symptoms, and that the mean incubation period was 5.2 days. However, in addition to fever and productive cough, rhinorrhea is more common in patients with H1N1, and the median incubation period of this virus is 2 days.9 Therefore, we speculate from previous research and our current findings that COVID-19 infection may present as a slow onset with fewer productive coughs and more obvious systemic symptoms compared with the clinical presentations of H1N1 infection.
The current study found that ground-glass opacity was more common in patients with COVID-19 than in patients with H1N1, whereas consolidation was more frequent in H1N1 patients, which is consistent with previous studies. The radiologic findings of 81 patients with COVID-19 pneumonia from Shi et al14 showed that diffused bilateral ground-glass opacities were the most predominant pattern of abnormalities on chest CT scans within 1 to 3 weeks following disease onset. In addition, studies on H1N1-associated pneumonia have shown that critical cases present as areas of consolidation on CT imaging, with or without ground-glass opacities.15 , 16 In addition to diffuse alveolar damage in pathologic findings of lungs indicating ARDS, COVID-19 is accompanied by cellular fibromyxoid exudates,17 whereas H1N1 is accompanied by necrotizing bronchiolitis and extensive hemorrhage.18 Therefore, these differential pathologic changes may present as distinguishing imaging characteristics during clinical assessments.
We also found that patients with COVID-19 received a wider variety of treatments compared with patients with H1N1. In contrast to definitive treatment measures for H1N1,19 there is no evidence to approve the effectiveness of any therapy for COVID-19. More than one hundred clinical studies have been conducted by Chinese researchers, and the interim research data may provide some help for the current urgent demand for COVID-19 drug treatments.20 The application of glucocorticoids was common in both COVID-19 and H1N1 patients in the current study, but the proportion in COVID-19 patients was greater than that in H1N1 patients. However, there was no difference in the dosage or duration of glucocorticoids between these two groups. The observational data currently available suggest that glucocorticoids for the treatment of respiratory infections increase mortality and secondary infection rates in influenza, impair clearance of SARS-CoV and Middle East respiratory syndrome coronavirus, and complicate corticosteroid therapies in survivors.21 Therefore, indications for glucocorticoids should be carefully evaluated in such patients.
Both COVID-19 and H1N1 infections may be accompanied by ARDS. Respiratory support in such cases should be in accordance with therapeutic strategies for ARDS.22 In the current study, we found that the severity of respiratory failure was not equal between COVID-19 and H1N1 patients. The Pao 2/Fio 2 levels in patients with COVID-19 were higher than those in patients with H1N1, such that respiratory support in COVID-19 patients was initially via noninvasive methods and ultimately yielded higher failure rates. The ECMO to Rescue Lung Injury in Severe ARDS (EOLIA) trial23 provided information about the posterior probability of a mortality benefit for patients with acute respiratory failure,24 especially in terms of reporting the success of the application of ECMO in ARDS patients with influenza.25 We speculate that ECMO may also have potential in treating patients with COVID-19. However, the rapid growth of cases and lack of medical resources and medical staff have limited standardized respiratory support in accordance with related guidelines.
In the current study, the mortality of ARDS patients infected with COVID-19 was 28.8%. According to the median Pao 2/Fio 2 of 198.5 mm Hg in patients with COVID-19 in the current study, the corresponding mortality rate was consistent with the definition of ARDS.11 Although patients with H1N1 in this study exhibited significantly lower oxygenation than that of patients with COVID-19, there was no difference in the mortality rate between the two groups. From the adjusted mortality analysis, we found that patients with H1N1 had a significantly worse prognosis than patients with COVID-19. All of the included COVID-19 cases in the current study were at the early stage of this epidemic. The rapidly growing cases of unknown diseases, inadequate responses, insufficient medical staff, and lack of medical supplies have adversely affected the treatments and prognoses of COVID-19 cases. Therefore, as a novel respiratory infectious disease, the relatively higher mortality rate of COVID-19 cases is to be expected. From the experiences gained from treating early COVID-19 patients, subsequent cases may benefit from better and more standard therapies, including specific medical treatments and respiratory support.
The current study had some limitations. First, this was a retrospective study that included data from two independent single-center cohorts, which may have resulted in unavoidable bias. Second, the conditions of patients with H1N1 was more severe than those of the COVID-19 cohort, which may have led to statistical disequilibrium. Third, 35.6% of the patients with COVID-19 were still hospitalized at the time of manuscript submission, meaning that the mortality rate presented in COVID-19 is likely an underestimate of the real overall hospital mortality rate. Finally, the data from the H1N1 cohort originated from a 3-year span, whereas the data from the COVID-19 cohort originated from only a 1-month span, which may also have affected the study’s results.
Interpretation
There were many differences in clinical presentations between patients with ARDS infected with either COVID-19 or H1N1. Compared with H1N1, patients with COVID-19-induced ARDS had lower severity of illness scores at presentation and lower SOFA score-adjusted mortality. Future studies investigating COVID-19 should focus on well-designed, prospective, case-controlled trials with large sample sizes, which could provide more experience and evidence regarding COVID-19 treatment measures.
Acknowledgments
Author contributions: B. S. takes responsibility for the content of the manuscript, including the data and analysis. H. Z. S., P. P., and B. S. conceived the idea, designed and supervised the study, drafted the manuscript, and had full access to all of the data and take responsibility for the integrity of the data. X. T., R. H. D., R. W., T. Z. C., L. L. G., C. Q. Y., Q. Z., M. H., X. Y. L., and Y. L. collected data. L. R. L., and Z. H. T., analyzed data and performed statistical analysis. All of the authors reviewed and approved the final version of the manuscript.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
Drs Tang, Du, and Peng contributed equally to this study.
FUNDING/SUPPORT: This work was supported by the Beijing Municipal Administration of Hospitals’ Mission Plan [SML20150301], the 1351 Talents Program of Beijing Chao-Yang Hospital [WXZXZ-2017-01], and Novel Coronavirus Pneumonia Key Technology Research and Development Funding of the Beijing Hospital Authority [Covid-19-BHA03].
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.chest.2020.03.032
Read article for free, from open access legal sources, via Unpaywall:
http://journal.chestnet.org/article/S0012369220305584/pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.chest.2020.03.032
Article citations
Targeting GPVI with glenzocimab in COVID-19 patients: Results from a randomized clinical trial.
PLoS One, 19(6):e0302897, 17 Jun 2024
Cited by: 0 articles | PMID: 38885234 | PMCID: PMC11182546
Influenza, SARS-CoV-2, and Their Impact on Chronic Lung Diseases and Fibrosis: Exploring Therapeutic Options.
Am J Pathol, 194(10):1807-1822, 18 Jul 2024
Cited by: 0 articles | PMID: 39032604
Review
Differences of respiratory mechanics in mechanical ventilation of acute respiratory distress syndrome between patients with COVID-19 and Influenza A.
Respir Res, 25(1):112, 07 Mar 2024
Cited by: 0 articles | PMID: 38448933 | PMCID: PMC10919012
The value of lung ultrasound score in neonatal respiratory distress syndrome: a prospective diagnostic cohort study.
Front Med (Lausanne), 11:1357944, 08 Feb 2024
Cited by: 0 articles | PMID: 38390571 | PMCID: PMC10881781
Relationship between the Pre-ECMO and ECMO Time and Survival of Severe COVID-19 Patients: A Systematic Review and Meta-Analysis.
J Clin Med, 13(3):868, 01 Feb 2024
Cited by: 0 articles | PMID: 38337562 | PMCID: PMC10856383
Review Free full text in Europe PMC
Go to all (196) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study.
Lancet, 395(10229):1054-1062, 11 Mar 2020
Cited by: 15136 articles | PMID: 32171076 | PMCID: PMC7270627
Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma.
JAMA, 323(16):1582-1589, 01 Apr 2020
Cited by: 1303 articles | PMID: 32219428 | PMCID: PMC7101507
Outcomes of severe H1N1 pneumoniae: A retrospective study at intensive care units.
J Formos Med Assoc, 119(1 pt 1):26-33, 07 Mar 2019
Cited by: 5 articles | PMID: 30852002
A Comparison of Clinical and Chest CT Findings in Patients With Influenza A (H1N1) Virus Infection and Coronavirus Disease (COVID-19).
AJR Am J Roentgenol, 215(5):1065-1071, 26 May 2020
Cited by: 40 articles | PMID: 32452731
Funding
Funders who supported this work.
Beijing Municipal Administration of Hospitals’ Mission Plan (1)
Grant ID: SML20150301
Key Technology Research and Development
Talents Program of Beijing Chao-Yang Hospital (1)
Grant ID: WXZXZ-2017-01






