Abstract
Background
Cardiac fibroblasts (CFs) are principal extracellular matrix-producing cells. In response to injury, CFs transdifferentiate into myofibroblasts. Intracellular calcium (Ca2+) signaling, involved in fibroblast proliferation and differentiation, is activated in fibroblasts through transient receptor potential (TRP) channels, but the function of these channels has not been investigated in human ventricular CFs. Under evaluation in this study, was the role of TRP channels in the differentiation of human ventricular CFs induced by transforming the growth factor beta (TGF-β), a pro-fibrotic cytokine.Methods
Human ventricular CFs were used in this study. The differentiation of CFs into myofibroblast was induced with TGF-β and was identified by the expression of smooth muscle actin.Results
Results indicate that Ca2+ signaling was an essential component of ventricular CF dif-ferentiation. CFs treated with TGF-β demonstrated increased expression of a TRP channel, TRPV4, both at the mRNA and protein levels, which corresponded with CF-myofibroblast trans-differentiation, as evidenced by the upregulation of α-smooth muscle actin, a myofibroblast marker, and plasminogen activator inhibitor-1, which are fibrogenesis markers. An agonist of TRPV4 induced the conversion of CFs into myofibroblasts, whereas it's antagonist as well a Ca2+ chelating agent reduced it, indicating that the Ca2+ influx throughTRPV4 is required for CF trans-differentiation. Overall, these results dem-onstrate that TRPV4-mediated Ca2+ influx participates in regulating the differentiation of human ventricular CFs into myofibroblasts through the MAPK/ERK pathway.Conclusions
Overall, these results demonstrate that TRPV4-mediated Ca2+ influx participates in regulating the differentiation of human ventricular CFs into myofibroblasts through the MAPK/ERK pathway.Free full text

Transient receptor potential channel TRPV4 mediates TGF-β1-induced differentiation of human ventricular fibroblasts
Abstract
Background
Cardiac fibroblasts (CFs) are principal extracellular matrix-producing cells. In response to injury, CFs transdifferentiate into myofibroblasts. Intracellular calcium (Ca2+) signaling, involved in fibroblast proliferation and differentiation, is activated in fibroblasts through transient receptor potential (TRP) channels, but the function of these channels has not been investigated in human ventricular CFs. Under evaluation in this study, was the role of TRP channels in the differentiation of human ventricular CFs induced by transforming the growth factor beta (TGF-β), a pro-fibrotic cytokine.
Methods
Human ventricular CFs were used in this study. The differentiation of CFs into myofibroblast was induced with TGF-β and was identified by the expression of smooth muscle actin.
Results
Results indicate that Ca2+ signaling was an essential component of ventricular CF differentiation. CFs treated with TGF-β demonstrated increased expression of a TRP channel, TRPV4, both at the mRNA and protein levels, which corresponded with CF-myofibroblast trans-differentiation, as evidenced by the upregulation of α-smooth muscle actin, a myofibroblast marker, and plasminogen activator inhibitor-1, which are fibrogenesis markers. An agonist of TRPV4 induced the conversion of CFs into myofibroblasts, whereas it’s antagonist as well a Ca2+ chelating agent reduced it, indicating that the Ca2+ influx through TRPV4 is required for CF trans-differentiation. Overall, these results demonstrate that TRPV4-mediated Ca2+ influx participates in regulating the differentiation of human ventricular CFs into myofibroblasts through the MAPK/ERK pathway.
Conclusions
Overall, these results demonstrate that TRPV4-mediated Ca2+ influx participates in regulating the differentiation of human ventricular CFs into myofibroblasts through the MAPK/ERK pathway.
Introduction
Cardiac fibroblasts (CFs) residing in the interstitial space comprise the largest cell population in the myocardium and provide structural support for cardiomyocytes by producing an extracellular matrix (ECM) which maintains heart architecture under physiological conditions [1]. CFs also play a key role in response to heart injury. Upon pathological stimuli, CFs differentiate into myofibroblasts characterized by contractility and overproduction of ECM, which causes wound retraction and healing by replacing necrotized cardiomyocytes. However, excessive activity of myofibroblasts can cause scarring and cardiac fibrosis followed by heart hypertrophy, enlargement of cardiac chambers, cell apoptosis, and ultimately heart failure [2].
Myofibroblasts are absent in normal cardiac tissue and only appear following injury [3]. The differentiation of CFs into myofibroblasts is a crucial process in heart remodeling, and it is established that the transforming growth factor (TGF)-β1 secreted by inflammatory cells directly induces a switch from the quiescent phenotype of CFs to active synthetic and contractile phenotype of myofibroblasts [4, 5]. The latter can be distinguished from CFs by the expression of several smooth muscle cell markers, including smooth muscle actin (α-SMA), which provide myofibroblast contractility [6, 7].
Although a number of signaling pathways are known to mediate fibroblast-to-myofibroblast conversion and fibrogenesis in the heart, there are few effective therapies to treat cardiac fibrosis, emphasizing the need to identify new molecular targets. Ca2+ signals regulate diverse cellular functions, and are known to be involved in fibroblast proliferation and ECM synthesis [8]. Fibroblasts have a relatively depolarized resting membrane potential, which is between −31 and −16 mV in atrial fibroblasts [9], and lack functional voltage-gated Ca2+ channels [10]. Instead, they express transient receptor potential (TRP) channels which provide nonselective intracellular Ca2+ flux and are widely present in many cell types [11]. Recently four TRP channels; TRPM7, TRPC3, TRPC6, and TRPV4, were found to be essential for myofibroblast trans differentiation [12–15]. However, their function appears to be species- and tissue-specific and it is not known whether TRP-mediated Ca2+ signaling is specifically required for the conversion of human ventricular CFs into myofibroblasts [16]. Therefore, the aim of this study was to investigate the role of TRP channels in the TGF-β-mediated transformation of human ventricular CFs.
Methods
Materials
Normal human ventricular cardiac fibroblasts (NHCF-V) (Product code; CC-2904) were purchased from Lonza (Walkersville Inc., MD, USA). An intracellular calcium chelator BAPTA-AM, a selective antagonist (RN-9893), agonist (GSK1016790A) of TRPV4 and selective inhibitor of MAPK/ERK kinase (U0126) were purchased from Sigma-Aldrich (St. Louis, MO, USA) [17]. Polyclonal antibodies against TRPV4 were obtained from Alomone Labs (Jerusalem, Israel), plasminogen activator inhibitor-1 (PAI-1)-specific antibody was from Santa Cruz Biotechnology (Santa Cruz, CA, USA), α-SMA antibody was from Abcam (Cambridge, MA, USA), and monoclonal antibodies against phosphorylated (p)-ERK and total ERK were obtained from Cell Signaling Technology (Danvers, MA, USA). TGF-β was purchased from PeproTech (Rocky Hill, NJ, USA).
Cell culture and TGF-β treatment
Cardiac fibroblasts were cultured in fibroblast growth medium (FGM, Lonza, Basel, Switzerland) supplemented with fetal calf serum (FCS), streptomycin (50 μg/mL), and penicillin (100 U/mL) at 37°C in a humidified atmosphere of 5% CO2. After 24 h starvation in serum-free FGM, the medium was replaced by FGM containing 2 ng/mL recombinant human TGF-β. CFs were cultured for different times (0, 6, 12, 24, and 48 h).
Polymerase chain reaction
Gene-specific primers were used to detect the expression of human TRP channels as described previously [14]. Ventricular CFs were seeded into 10-cm dishes, cultured in serum-free FGM for 24 h, and then stimulated with TGF-β (2 ng/mL) for 3, 6, 12, 24, and 48 h. Cells were collected, and total RNA was extracted by Trizol (Invitrogen, Paisley, UK), and cDNA was synthesized, which was used as a template for the polymerase chain reaction (PCR). The amplified products were resolved by electrophoresis on 2% agarose gels and photographed using the FluorChem FC2 system (Cell Biosciences, Santa Clara, CA, USA).
Western blotting
Cardiac fibroblast differentiation was induced with 2 ng/mL TGF-β in the absence or presence of 1 μM/mL Ca2+ chelator BAPTA-AM for 48 h, or different concentrations of TRPV4 agonist GSK1016790A (10, 20 and 50 nM) or antagonist RN-9893 (10, 20, and 50 μM) for 24 h. DMSO was used as the vehicle control. For inhibition of ERK signaling, CFs were treated with U0126 (10 μM), an inhibitor of MAPK/ERK kinase for 48 h. CFs were homogenized and lysed in RIPA buffer containing 50 mM Tris pH 7.4, 150 mM NaCl, 1% NP40, 0.5% sodium deoxycholate, 0.1% SDS, and protease inhibitor cocktail (Sigma, St. Louis, MO, USA). Protein concentration in cell lysates was determined by the Bradford assay (Bio-Rad, Richmond, CA, USA). Proteins were separated in 10% SDS-PAGE gels under reducing conditions, electrophoretically transferred onto equilibrated polyvinylidene difluoride membranes (Immobilon-P, Millipore, Billerica, MA, USA), and probed with primary antibodies against TRPV4, α-SMA, p-ERK (T202/Y204), total ERK, PAI-1, and β-actin was used as an internal control. Reactivity to the anti-PAI-1 antibody was also determined in the cell culture supernatant.
Measurement of cytosolic Ca2+
Ca2+ concentration was measured using a ratiometric indicator Fura2 as described previously [18]. CFs were seeded onto black-walled 96-well plates (5 × 104 cells/well) and treated with 2 mM Ca2+ with or without TGF-β for 24 h to induce differentiation into myofibroblasts. Cells were loaded with 5 μM Fura2/AM (Invitrogen, Carlsbad, CA, USA) in Krebs-Ringer bicarbonate buffer (126 mM NaCl, 4 mM KCl, 10 mM HEPES, 1 mM MgCl2, 5.5 mM glucose, pH 7.4) for 30 min at 37°C, and fluorescence was measured at 510 nm in response to alternate excitation at 340 nm and 380 nm using a Flexstation fluorescence reader (Molecular Devices, Sunnyvale, CA, USA). The changes in the Fura2 fluorescence ratio (F340/F380) caused by 2 mM Ca2+ were estimated by the area under the curve (AUC) and normalized to that caused by 10 μM ionomycin-elicited Ca2+ signal. To assess the effects of TRPV4 activation, CFs were treated with 100 nM GSK1016790A.
Statistical analysis
Statistical analysis was performed with one-way ANOVA followed by the Tukey post hoc analysis or Student t-test; changes at p < 0.05 were considered statistically significant. All analyses were performed using SPSS v18 (SPSS, Chicago, IL, USA).
Results
TGF-β induces Ca2+-dependent differentiation of ventricular fibroblasts and expression of Ca2+-permeable channel TRPV4
To determine whether Ca2+ was required for TGF-β-mediated CF differentiation into myofibroblasts, TGF-β-stimulated cells were treated with the intracellular Ca2+ chelator BAPTA-AM and the expression of myofibroblast differentiation marker α-SMA (Fig. 1) was analyzed. The expression of α-SMA was suppressed with intracellular Ca2+ chelator. The results showed that chelation of intracellular Ca2+ inhibited CF differentiation, indicating that Ca2+ signaling was essential for CF conversion into myofibroblasts.
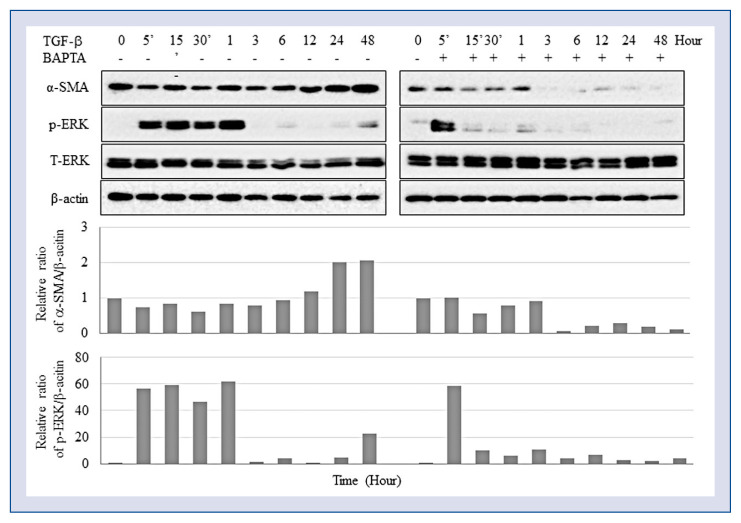
Fibroblast differentiation depends on Ca2+. Cardiac fibroblasts induced with 2 ng/mL transforming growth factor beta (TGF-β) were treated or not with 1 μg/mL BAPTA-AM and analyzed for protein expression by Western blotting.
To determine which Ca2+ channel is responsible for the TGF-β-induced differentiation of human ventricular CFs, the expression of TRP channels in CFs were examined. TRPV4 was the only TRP channel upregulated by TGF-β1 both at the mRNA (Fig. 2) and protein (Fig. 3) levels, which corresponded to the induction of CF differentiation into myofibroblasts manifested by an increased expression of α-SMA and PAI-1 (Fig. 3). PAI-1 is the main inhibitor of serine proteases urinary and tissue plasminogen activators, which are responsible for plasmin formation, and of plasmin-dependent matrix metalloproteinases. Since plasmin and matrix metalloproteases degrade the ECM, their downregulation promotes ECM deposition; therefore, PAI-1 is considered a biomarker of excessive fibrogenesis and is implicated in the pathophysiology of fibrosis [19].
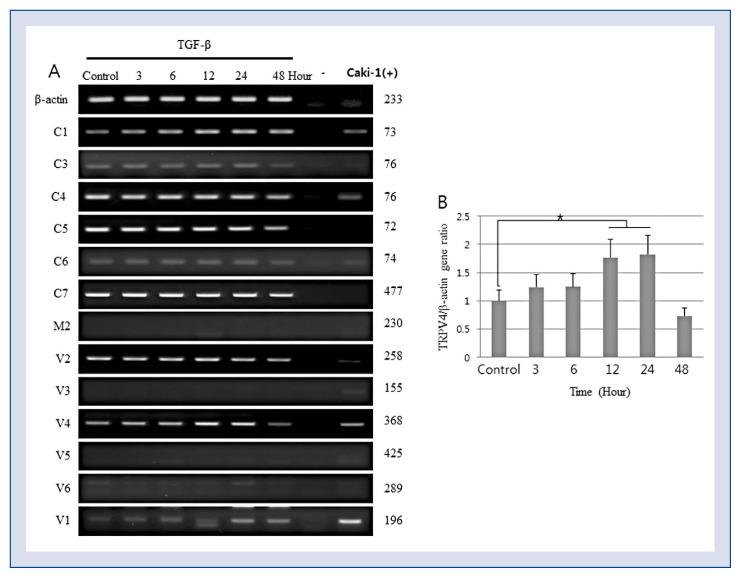
Transforming growth factor beta (TGF-β) induces TRPV4 mRNA expression. Cardiac fibroblasts were treated with 2 ng/mL TGF-β and analyzed for the expression of transient receptor potential (TRP) channels by a polymerase chain reaction (PCR); A. Representative image of an agarose gel showing PCR amplification products; B. Semi-quantitative analysis of TRPV4 mRNA expression relative to α-actin (*p < 0.05 vs. untreated cells).
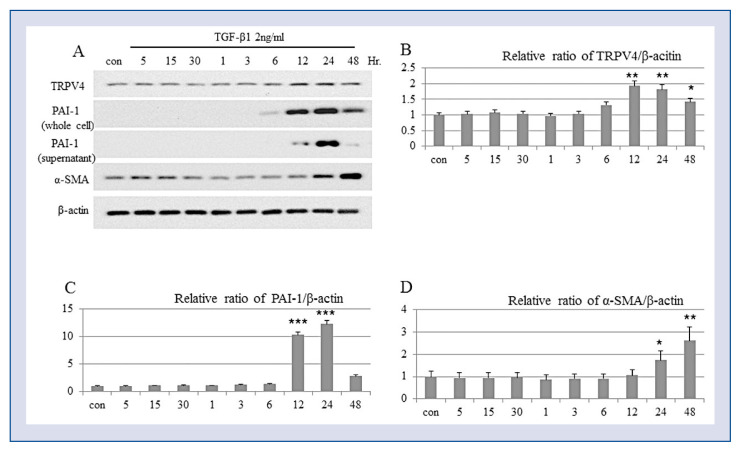
Transforming growth factor beta (TGF-β) induces TRPV4 protein expression and differentiation of ventricular fibroblasts. Cardiac fibroblasts were treated with 2 ng/mL TGF-β1 and analyzed for protein expression of TRPV4, smooth muscle actin alpha (α-SMA), and plasminogen activator inhibitor-1 (PAI-1) by Western blotting; A. Representative Western blotting image; B–D. Relative protein expression of TRPV (B), plasminogen activator inhibitor- 1 (PAI-1) (C), and smooth muscle actin alpha (α-SMA) (D) after TGF-β treatment (*p < 0.05, **p < 0.01, and ***p < 0.001, respectively, vs. untreated cells); con — control.
TRPV4 is required for the differentiation of ventricular fibroblasts
To determine the involvement of TRPV4 in myofibroblast trans-differentiation, CFs were cultured with TRPV4 agonist GSK1016790A or antagonist RN-9893 and were then stimulated with TGF-β for 24 h. The results indicate that the TRPV4 agonist significantly promoted and TRPV4 antagonist suppressed the conversion of CFs into myofibroblasts, as evidenced by dose-dependent increase and decrease, respectively, in α-SMA expression (Fig. 4).
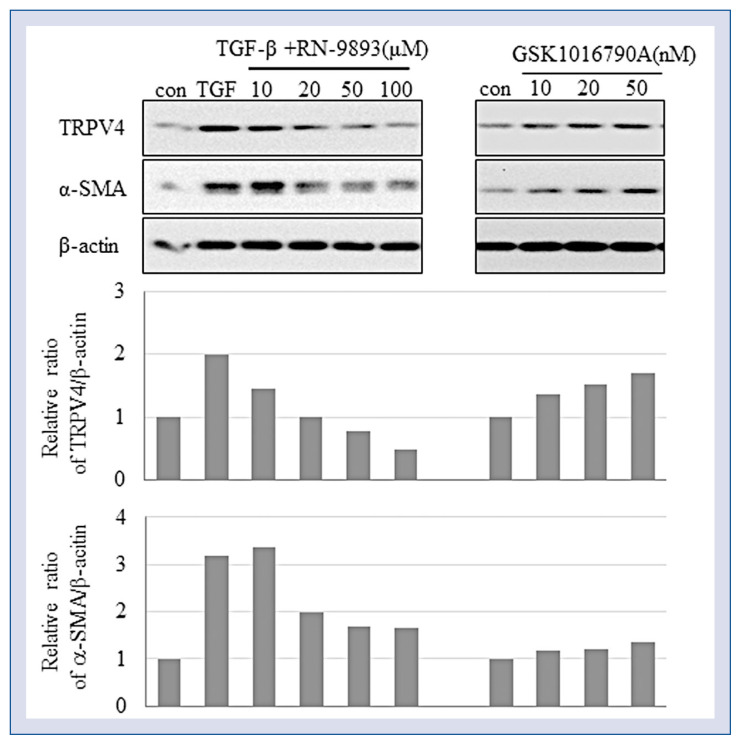
Transient receptor potential V4 (TRPV4) is involved in fibroblast differentiation. Cardiac fibroblasts (CFs) were pre-treated with the indicated concentrations of TRPV4 agonist GSK1016790A or antagonist RN-9893 and then treated with 2 ng/mL Transforming growth factor beta (TGF-β) for 24 h. CF conversion into myofibroblasts was assessed based on smooth muscle actin alpha (α-SMA) expression analyzed by Western blotting.
TRPV4 downstream signaling is mediated by ERK
The data obtained indicates that TRPV4 is involved in TGF-β-stimulated CF differentiation. Therefore, potential downstream signaling mechanisms mediating TRPV4 activity were subsequently addressed. Since it is known that fibroblast differentiation is induced by reactive oxygen species (ROS) and inhibited through mitogen-activated protein kinase (MAPK)/ERK signaling, and thus ERK1/2 phosphorylation in TGF-β-induced CFs treated with 1 μM of Ca2+ chelator BAPTA-AM was analyzed. ERK phosphorylation induced by TGF-β during the first hour of treatment was suppressed in a time-dependent manner by BAPTA-AM, which did not affect ERK activation at the early phase, but inhibited it at the late phase (Fig. 1). Furthermore, ERK inhibitor U0126 caused the same effects as shown by TRPV4 antagonist RN-9893, since both these suppressed ERK phosphorylation as well as CF differentiation (Fig. 5). Overall, these results suggest the involvement of the MAPK/ERK pathway in TGF-β-induced CF differentiation into myofibroblasts. However, N-acetyl-L-cysteine (NAC), a well-known scavenger of ROS did not influence either ERK activation or CF differentiation (Fig. 5).
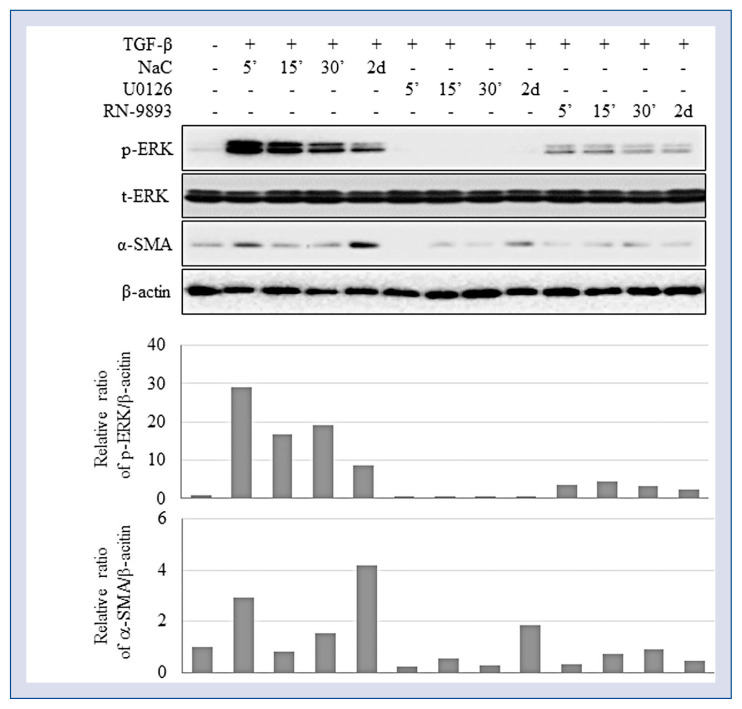
ERK activation is involved in TRPV4-mediated differentiation of human ventricular fibroblasts. Cardiac fibroblasts (CFs) were treated with 2 ng/mL of transforming growth factor beta (TGF-β) in the presence or absence of selective ERK inhibitor U0126 (10 μM), reactive oxygen species scavenger N-acetyl-L-cysteine (NAC 0.5 mM), or TRPV4 antagonist RN-9893 (50 μM) and analyzed for the expression of p-ERK (T202/Y204) and smooth muscle actin alpha (α-SMA).
TRPV4 is essential for Ca2+ entry into human ventricular fibroblasts
To analyze Ca2+ influx into differentiating CFs, cytosolic Ca2+ levels were measured using a ratiometric fluorescent dye Fura2. Intracellular Ca2+ concentration increased in CFs after treatment with the TRPV4 agonist as well as with TGF-β. Given that both TRPV4 and TGF-β induced CF differentiation, these results suggest that TRPV4 as a Ca2+-permeable channel has a role in the differentiation of human CFs into myofibroblasts (Fig. 6).
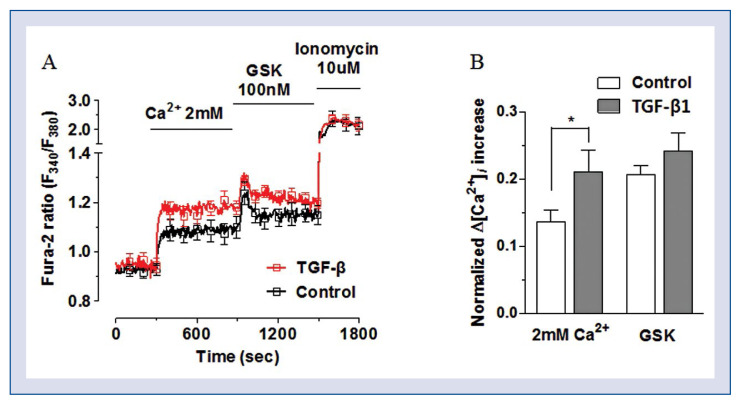
TRPV4 mediates Ca2+ influx required for cardiac fibroblasts (CFs) differentiation; A. Ca2+ influx was elicited by 2 mM Ca2+ and further augmented by the treatment with TRPV4 agonist GSK1016790A; B. Ca2+ influx was compared between control CFs (clear bar) and transforming growth factor beta (TGF-β)-treated CFs (gray bar); the treatment with TRPV4 agonist GSK1016790A further increased Ca2+ influx (*p < 0.05 vs. control).
Discussion
In the current study, the pathophysiological function of the Ca2+-permeable channel TRPV4 in human ventricular CFs were under investigation. Results confirm that Ca2+ is essential for CF differentiation into myofibroblasts; most importantly, they also revealed that TGF-β upregulates TRPV4 expression and that TRPV4 mediates TGF-β-induced differentiation of human ventricular CFs into myofibroblasts via Ca2+-dependent ERK phosphorylation.
Ca2+ signaling is a ubiquitous intracellular mechanism responsible for diverse cellular functions [8]. Several studies suggest that Ca2+ is essential for CF proliferation and differentiation. The mitogenic effect of substance P in rat CFs was abolished by chelation of extracellular Ca2+ with EGTA, which inhibited cell proliferation [20]. Furthermore, a Ca2+ channel blocker prevented angiotensin II-mediated myocardial fibrosis and accumulation of myofibroblasts in vivo [21]. Consistent with these findings, the present results indicate that TGF-β-induced human ventricular CF differentiation into myofibroblasts was inhibited by intracellular Ca2+ chelation. Overall, these data prove that Ca2+ entry through Ca2+-permeable ion channels are important for fibroblast gene expression and differentiation.
Fibroblasts are electrically non-excitable cells and have high membrane resistance and a relatively depolarized resting membrane potential of −15.9 ± 2.1 mV [22]. CFs typically do not express voltage-gated Ca2+ channels including those of L-type characteristic for cardiomyocytes [23] and are known to be mechanosensitive [10]. Therefore, CFs need a different Ca2+-signaling pathway to facilitate differentiation into myofibroblasts. The TRP superfamily of ion channels comprises non-selective cation channels permeable for Ca2+ and Mg2+. There are 28 mammalian TRP genes divided into six subfamilies based on amino acid homology: TRPC (Canonical), TRPV (Vanilloid), TRPM (Melastatin), TRPA (Ankyrin), TRPP (Polycystin), and TRPML (Mucolipin). Among these, all TRPC and TRPV channels as well as TRPM1, 2, 3, 6, 7, and 8, TRPA1, TRPP2, 3, and 5, and TRPML1, 2, and 3 are permeable for Ca2+ [24]. Several previous studies indicate that TRP channels are involved in Ca2+-dependent regulation of CF differentiation into myofibroblasts in different mammalian species. Thus, Du et al. [14] suggested that TRPM7 played a major role in Ca2+ entry into human atrial fibroblasts by showing that the expression of TRPM7 mRNA was increased by TGF-β which required a TRPM7-mediated Ca2+ signal for its effect on CF proliferation and differentiation. Other investigators showed that the proliferation and differentiation of rat CFs is regulated by Ca2+ influx through TRPC3 [15], whereas TRPC6 was necessary for the transformation of mouse embryonic CFs into myofibroblasts [13]. Furthermore, it has been demonstrated that a mechanosensitve channel TRPV4 is required for TGF-β-induced differentiation of adult rat CFs into myofibroblasts and that TGF-β upregulated TRPV4 expression and TRPV4-mediated Ca2+ influx [12]. These findings indicate that TRP channels regulate CF differentiation into myofibroblasts, but their activity is species-and tissue-specific. The present study is the first to investigate the role of TRP channels in human ventricular CFs and the obtained results indicate that TRPV4 is the channel involved in TGF-β-induced conversion of human ventricular CFs into myofibroblasts. TRPV4 expression was upregulated by TGF-β both at the mRNA and protein levels, which correlated with the induction of Ca2+ influx as well as myofibroblast transdifferentiation. At the same time, TRPV4 inhibition suppressed, while its activation induced the differentiation of CFs, confirming the regulatory role of TRPV4 in the process.
Transient receptor potential channels belonging to different subfamilies induce distinct downstream signaling mechanisms. TRPC6 was shown to upregulate Ca2+-responsive protein phosphatase calcineurin through its downstream transcriptional effector, nuclear factor of activated T-cells (NFAT), which resulted in myofibroblast trans-differentiation, whereas TRPC6 expression was induced by TGF-β through the MAPK/p38 pathway [13]. It has been suggested that TRPC3 expression mediating CF differentiation could be via a potential downstream mechanism involving the activation of ERK1/2 [15]. Protein kinases ERK1/2 are key intracellular signaling molecules regulated by phosphorylation and implicated in numerous biological processes, including cell growth, survival, and differentiation. In the current study, CF differentiation and ERK1/2 phosphorylation were increased by TGF-β, but were decreased by the intracellular Ca2+ chelator, TRPV4 antagonist, and ERK inhibitor, indicating the involvement of ERK1/2 in TRPV4-dependent conversion of ventricular CFs into myofibroblasts.
Based on the current findings, the following mechanism underlying fibroblast trans-differentiation in the ventricle can be suggested: TGF-β secreted by inflammatory cells after heart injury upregulates the expression of TRPV4 and Ca2+ influx, which activates the MAPK/ERK pathway and downstream transcription of the genes encoding myofibroblast markers such as α-SMA, leading to a phenotypic switch from CFs to myofibroblasts (Fig. 7).
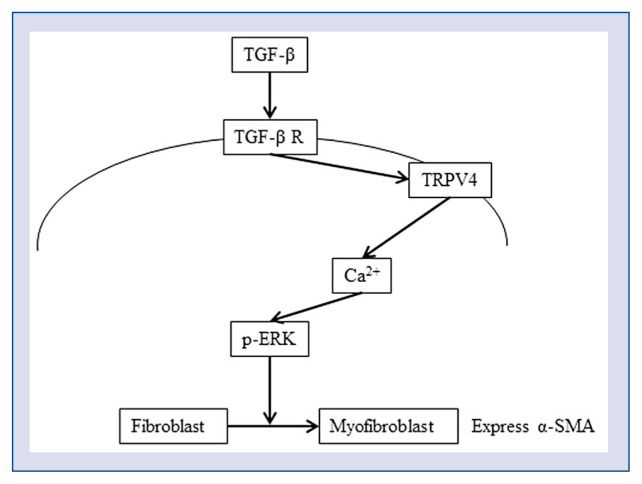
A signaling model for TRPV4-mediated differentiation of human ventricular fibroblasts. Transforming growth factor beta (TGF-β) signaling upregulates the expression of TRPV4, which induces Ca2+ influx and ERK1/2 activation, ultimately resulting in cardiac fibroblasts conversion into myofibroblasts; α-SMA — smooth muscle actin alpha.
Conclusions
In conclusion, it was demonstrated that the Ca2+-permeable cation channel TRPV4 participated in Ca2+-mediated signaling in human ventricular fibroblasts, promoting their differentiation into myofibroblasts through the MAPK/ERK pathway. Findings herein, indicate that TRPV4 plays an important biological role in the regulation of left ventricular function and fibrogenesis in the heart, suggesting TRPV4 as a potential molecular target to attenuate cardiac fibrosis.
References
Articles from Cardiology Journal are provided here courtesy of Via Medica sp. z o.o. sp. k.
Full text links
Read article at publisher's site: https://doi.org/10.5603/cj.a2019.0050
Read article for free, from open access legal sources, via Unpaywall:
https://journals.viamedica.pl/cardiology_journal/article/download/CJ.a2019.0050/50887
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.5603/cj.a2019.0050
Article citations
Mechanosensitive TRPV4 channel guides maturation and organization of the bilayered mammary epithelium.
Sci Rep, 14(1):6774, 21 Mar 2024
Cited by: 0 articles | PMID: 38514727 | PMCID: PMC10957991
The Multifaceted Functions of TRPV4 and Calcium Oscillations in Tissue Repair.
Int J Mol Sci, 25(2):1179, 18 Jan 2024
Cited by: 1 article | PMID: 38256251 | PMCID: PMC10816018
Review Free full text in Europe PMC
TRP (transient receptor potential) ion channel family: structures, biological functions and therapeutic interventions for diseases.
Signal Transduct Target Ther, 8(1):261, 05 Jul 2023
Cited by: 54 articles | PMID: 37402746 | PMCID: PMC10319900
Review Free full text in Europe PMC
Pathophysiological Roles of the TRPV4 Channel in the Heart.
Cells, 12(12):1654, 17 Jun 2023
Cited by: 5 articles | PMID: 37371124 | PMCID: PMC10296986
Review Free full text in Europe PMC
Progress on role of ion channels of cardiac fibroblasts in fibrosis.
Front Physiol, 14:1138306, 09 Mar 2023
Cited by: 5 articles | PMID: 36969589 | PMCID: PMC10033868
Review Free full text in Europe PMC
Go to all (10) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
TRPV4 channels mediate cardiac fibroblast differentiation by integrating mechanical and soluble signals.
J Mol Cell Cardiol, 54:45-52, 08 Nov 2012
Cited by: 132 articles | PMID: 23142541 | PMCID: PMC3935769
Translocation of TRPV4-PI3Kγ complexes to the plasma membrane drives myofibroblast transdifferentiation.
Sci Signal, 12(607):eaau1533, 12 Nov 2019
Cited by: 21 articles | PMID: 31719171 | PMCID: PMC7001959
Featured Article: TGF-β1 dominates extracellular matrix rigidity for inducing differentiation of human cardiac fibroblasts to myofibroblasts.
Exp Biol Med (Maywood), 243(7):601-612, 04 Mar 2018
Cited by: 29 articles | PMID: 29504479 | PMCID: PMC6582399
TRPV4 Mechanotransduction in Fibrosis.
Cells, 10(11):3053, 06 Nov 2021
Cited by: 13 articles | PMID: 34831281 | PMCID: PMC8619244
Review Free full text in Europe PMC
 1
1




