Abstract
Free full text

Chloroquine and Hydroxychloroquine Retinal Toxicity Consideration in the Treatment of COVID-19
Abstract
The proposed doses of chloroquine (CQ) and hydroxychloroquine (HCQ) for treatment of COVID-19 (1000 mg/day for 10 days, CQ; 800
mg/day for 10 days, CQ; 800 mg first day then 400
mg first day then 400 mg/day for 5 days, HCQ) in many guidelines worldwide, are considerably higher than the maximum recommended daily safe doses of both agents (≤2.3
mg/day for 5 days, HCQ) in many guidelines worldwide, are considerably higher than the maximum recommended daily safe doses of both agents (≤2.3 mg/kg/day, CQ; ≤5.0
mg/kg/day, CQ; ≤5.0 mg/kg/day, HCQ) for development of retinal toxicity. Irreversible retinal damage can occur if the exposure to the safe doses is >5 years. It is not known whether exposure to high doses over a short period of time can also cause the damage. We recommend that before prescribing CQ or HCQ, history of ocular disease should be obtained to avoid the prescription if appropriate. If either agent is to be used, routine baseline ocular examination is not absolutely necessary. Patients who do not have ocular disease should also be informed about the potential risk of retinal toxicity. Both agents, however, have not yet been proven to be beneficial to COVID-19.
mg/kg/day, HCQ) for development of retinal toxicity. Irreversible retinal damage can occur if the exposure to the safe doses is >5 years. It is not known whether exposure to high doses over a short period of time can also cause the damage. We recommend that before prescribing CQ or HCQ, history of ocular disease should be obtained to avoid the prescription if appropriate. If either agent is to be used, routine baseline ocular examination is not absolutely necessary. Patients who do not have ocular disease should also be informed about the potential risk of retinal toxicity. Both agents, however, have not yet been proven to be beneficial to COVID-19.
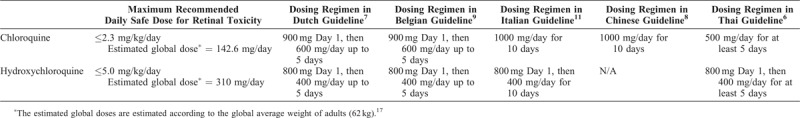
With the occurrence of pandemic of the coronavirus disease 2019 (COVID-19) announced by the World Health Organization in early March 2020 and the number of cases still on the rise in all continents in late March, many therapeutic options have been proposed for this novel and potentially fatal disease. Apart from antiviral agents, chloroquine (CQ) and hydroxychloroquine (HCQ) have been examined for their roles in treatment of COVID-19.1 This may be because both CQ and HCQ have been postulated to reduce viral replication in other coronavirus infections.2,3 According to a recent systematic review, there are almost 20 ongoing randomized controlled clinical trials on both medications for treatment of COVID-19 and all of them are in China. The details including dosing regimens of CQ and HCQ in these trials have been summarized in the review.4 Although there has not yet been a completed clinical trial and the world is waiting eagerly for the results of these trials and other trials on other treatment options, many authorities have chosen to adopt CQ and HCQ in the guidelines for treatment of COVID-19 based on in vitro studies,2,3 nonrandomized trial,5 and anecdotal evidence.6–11 As the therapeutic doses of CQ and HCQ recommended in the trials and guidelines are relatively high compared with the maximum daily safe dose that is related to CQ and HCQ retinal toxicity, this issue of retinal toxicity should be taken into consideration when employing these 2 medications for treatment of COVID-19 worldwide.
According to the recommendation by the American Academy of Ophthalmology, the most significant major risk factors for CQ and HCQ retinal toxicity are high dose and long duration of use.12 Other risk factors include concomitant renal disease and use of tamoxifen.12 The maximum daily dose from this recommendation is ≤5.0 mg/kg real body weight for HCQ, and ≤2.3
mg/kg real body weight for HCQ, and ≤2.3 mg/kg real body weight for CQ. As shown in Table Table1,1, the doses of CQ or HCQ for treatment of COVID-19 in various treatment guidelines worldwide are well beyond these recommended dosing regimens.
mg/kg real body weight for CQ. As shown in Table Table1,1, the doses of CQ or HCQ for treatment of COVID-19 in various treatment guidelines worldwide are well beyond these recommended dosing regimens.
Table 1
Comparison Between the Maximum Daily Safe Doses of Chloroquine and Hydroxychloroquine for Development of Retinal Toxicity and the Recommended Doses in COVID-19 Treatment Guidelines From Different Countries
The Royal College of Ophthalmologists (RCO) in the UK also addressed the importance of safe dose and duration of prescription of CQ and HCQ for the development of retinal toxicity. Although no absolute safe dose was identified, the RCO recommends the daily dose of HCQ to be <5 mg/kg/day for <5 years as relatively safe for retinal toxicity. However, no safe dose of CQ was recommended and the RCO identified those who receive CQ for >1 year as having risk of retinal toxicity.13
mg/kg/day for <5 years as relatively safe for retinal toxicity. However, no safe dose of CQ was recommended and the RCO identified those who receive CQ for >1 year as having risk of retinal toxicity.13
Despite the fact that the daily doses of both CQ and HCQ for the treatment of COVID-19 exceed the daily safe doses of both agents, the treatment may still be considered relatively safe for retinal toxicity. The toxicity, which causes irreversible retinal damage and visual loss despite ceasing prescription, requires exposure to the safe dose for a long period of time, generally in excess of 5 years. In general, the recommendation for screening for retinal toxicity from CQ and HCQ is within the first year of use as baseline and then annual screening after a year of use for CQ13 and 5 years of use for HCQ.12 Both American Academy of Ophthalmology and RCO recommended the screening should be conducted sooner if the major risk factors are present.12,13 In the case of treatment of COVID-19 using CQ and HCQ, the major risk factor is the use of higher than generally recommended dosage, although over a relatively short period of time, that is for about a week. There has not yet been a report on retinal toxicity associated with this kind of treatment. Nonetheless, it has been reported that retinal toxicity can develop even after <1 year of high dose of HCQ use (1000 mg daily) in an oncology trial.14
mg daily) in an oncology trial.14
In this report, 2 of 7 patients who received the high dose of HCQ showed abnormalities of the macula on retinal imaging modalities and multifocal electroretinogram without visual symptoms. These patients did not have any known risk factors, such as renal disease, concomitant retinotoxic agents, or co-existing retinal disease. It is not known whether the retinal toxicity from high-dose CQ and HCQ is underreported in the literature due to suboptimal and nonuniform ocular screening methods.14
Both CQ and HCQ are known for their binding affinity with melanin in retinal pigment epithelium which can be a mechanism of the toxic effects. Both agents have also been shown to cause damage to the photoreceptor layer and outer nuclear layer of the retina, whereas CQ can cause damage to the inner retina as well. Light absorption and metabolism of cone cells may also play roles for the damages. These mechanisms may lead to clinically characteristic “bull's eye” maculopathy after chronic exposure to both agents even in the safe dose.15 It is not known whether exposure to the high dose over a short period may also cause similar cellular damages as with the chronic exposure. Given that patients with COVID-19 who may require treatment are commonly older patients, it is possible that some may already have coexistent age-related macular degeneration. It is still not known whether the diseased macula would be more vulnerable to damage with exposure to the high dose of either CQ or HCQ even over a short duration.
Routine baseline ocular examination is not absolutely necessary for patients with COVID-19 who are undergoing treatment with CQ and HCQ but should be considered if manpower and expertise are available and extreme precautions should be taken during the examination. It is relevant, however, to take a history of ocular disease, particularly macular disease, in patients with COVID-19 who are older than 50 years before prescribing CQ or HCQ as treatment, to rule out age-related macular degeneration or other macular abnormalities. Coexistent retinal pathology is listed as a contraindication of using CQ and HCQ in patients with COVID-19 in the treatment guideline of Belgium.9 As treatment with CQ or HCQ is not yet proven to be beneficial, but instead can be harmful,16 in COVID-19, choosing other options of treatment in this group of patients with the preexisting disease may be more appropriate. For patients in whom CQ or HCQ is still considered as a treatment option, the potential benefits and risks of retinal toxicity and other systemic complications shall be thoroughly discussed with patients and well documented on written consent form before the treatment or trial of CQ or HCQ. Following recovery from COVID-19 with the treatment using CQ or HCQ, the patients should also be informed to visit ophthalmologists if they encounter any abnormal visual symptoms.
In summary, the bottom line at the present time is that neither CQ nor HCQ has been proven to be effective in the treatment of COVID-19, although there is certainly a vast interest in its possible benefit. Further controlled clinical trial data will be necessary to help better address this issue. Despite the current situation of COVID-19 pandemic, many adverse effects of either CQ or HCQ should still be weighed against its potential benefit. For retinal toxicity, the risk of having irreversible retinal damage and visual loss may outweigh the unproven benefit of both agents in some patients. Detecting the risk is easy. It can be done by simply taking a history of previous or co-existing ocular disease from the patients, then other options of treatment should be considered if appropriate.
ACKNOWLEDGEMENT
The authors acknowledge Varis Ruamviboonsuk, MD, in assisting manuscript preparation.
REFERENCES
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1097/apo.0000000000000289
Article citations
Update on overview of ocular manifestations of COVID-19.
Front Med (Lausanne), 9:877023, 13 Sep 2022
Cited by: 10 articles | PMID: 36177323 | PMCID: PMC9513125
Review Free full text in Europe PMC
The marine natural product, dicitrinone B, induces apoptosis through autophagy blockade in breast cancer.
Int J Mol Med, 50(4):130, 02 Sep 2022
Cited by: 1 article | PMID: 36052845 | PMCID: PMC9448296
Retinal microvasculature alteration in patients with systemic sclerosis and chloroquine treatment.
Quant Imaging Med Surg, 12(10):4885-4899, 01 Oct 2022
Cited by: 0 articles | PMID: 36185048 | PMCID: PMC9511431
Use of Tox21 Screening Data to Evaluate the COVID-19 Drug Candidates for Their Potential Toxic Effects and Related Pathways.
Front Pharmacol, 13:935399, 14 Jul 2022
Cited by: 5 articles | PMID: 35910344 | PMCID: PMC9333127
Review Free full text in Europe PMC
Peripheral Neuropathies Derived from COVID-19: New Perspectives for Treatment.
Biomedicines, 10(5):1051, 02 May 2022
Cited by: 5 articles | PMID: 35625788 | PMCID: PMC9138404
Review Free full text in Europe PMC
Go to all (15) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
COVID-19 and Chloroquine/Hydroxychloroquine: Is There Ophthalmological Concern?
Am J Ophthalmol, 216:A1-A2, 08 May 2020
Cited by: 3 articles | PMID: 32439074 | PMCID: PMC7205730
COVID-19 Pandemic - A Narrative Review of the Potential Roles of Chloroquine and Hydroxychloroquine.
Pain Physician, 23(4s):S351-S366, 01 Aug 2020
Cited by: 9 articles | PMID: 32942793
Review
Chloroquine and Hydroxychloroquine in COVID-19: Challenges and the Need for Caution in Low-Resource Settings.
J Coll Physicians Surg Pak, 30(6):78, 01 Jun 2020
Cited by: 0 articles | PMID: 32723461
Does zinc supplementation enhance the clinical efficacy of chloroquine/hydroxychloroquine to win today's battle against COVID-19?
Med Hypotheses, 142:109815, 06 May 2020
Cited by: 91 articles | PMID: 32408070 | PMCID: PMC7202847





