Abstract
Background
The duration of viral shedding is central to the guidance of decisions about isolation precautions and antiviral treatment. However, studies regarding the risk factors associated with prolonged shedding of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the impact of lopinavir/ritonavir (LPV/r) treatment on viral shedding remain scarce.Methods
Data were collected from all SARS-CoV-2 infected patients who were admitted to isolation wards and had reverse transcription PCR conversion at the No. 3 People's Hospital of Hubei province, China, between 31 January and 9 March 2020. We compared clinical characteristics and SARS-CoV-2 RNA shedding between patients initiated with LPV/r treatment and those without. Logistic regression analysis was employed to evaluate the risk factors associated with prolonged viral shedding.Results
Of 120 patients, the median age was 52 years, 54 (45%) were male and 78 (65%) received LPV/r treatment. The median duration of SARS-CoV-2 RNA detection from symptom onset was 23 days (interquartile range 18-32 days). Older age (OR 1.03, 95% CI 1.00-1.05; p=0.03) and the lack of LPV/r treatment (OR 2.42, 95% CI 1.10-5.36; p=0.029) were independent risk factors for prolonged SARS-CoV-2 RNA shedding. Patients who initiated LPV/r treatment within 10 days from symptom onset, but not initiated from day 11 onwards, had significantly shorter viral shedding duration compared with those without LPV/r treatment (median 19 days versus 28.5 days; log-rank p<0.001).Conclusion
Older age and the lack of LPV/r treatment were independently associated with prolonged SARS-CoV-2 RNA shedding in patients with coronavirus disease 2019 (COVID-19). Earlier administration of LPV/r treatment could shorten viral shedding duration.Free full text

Factors associated with prolonged viral shedding and impact of lopinavir/ritonavir treatment in hospitalised non-critically ill patients with SARS-CoV-2 infection
Associated Data
Abstract
Background
The duration of viral shedding is central to the guidance of decisions about isolation precautions and antiviral treatment. However, studies regarding the risk factors associated with prolonged shedding of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the impact of lopinavir/ritonavir (LPV/r) treatment on viral shedding remain scarce.
Methods
Data were collected from all SARS-CoV-2 infected patients who were admitted to isolation wards and had reverse transcription PCR conversion at the No. 3 People's Hospital of Hubei province, China, between 31 January and 9 March 2020. We compared clinical characteristics and SARS-CoV-2 RNA shedding between patients initiated with LPV/r treatment and those without. Logistic regression analysis was employed to evaluate the risk factors associated with prolonged viral shedding.
Results
Of 120 patients, the median age was 52 years, 54 (45%) were male and 78 (65%) received LPV/r treatment. The median duration of SARS-CoV-2 RNA detection from symptom onset was 23 days (interquartile range 18–32 days). Older age (OR 1.03, 95% CI 1.00–1.05; p=0.03) and the lack of LPV/r treatment (OR 2.42, 95% CI 1.10–5.36; p=0.029) were independent risk factors for prolonged SARS-CoV-2 RNA shedding. Patients who initiated LPV/r treatment within 10 days from symptom onset, but not initiated from day 11 onwards, had significantly shorter viral shedding duration compared with those without LPV/r treatment (median 19 days versus 28.5 days; log-rank p<0.001).
Conclusion
Older age and the lack of LPV/r treatment were independently associated with prolonged SARS-CoV-2 RNA shedding in patients with coronavirus disease 2019 (COVID-19). Earlier administration of LPV/r treatment could shorten viral shedding duration.
Short abstract
Risk factors for prolonged SARS-CoV-2 shedding include older age and the lack of lopinavir/ritonavir treatment. Earlier administration of lopinavir/ritonavir treatment could shorten the duration of SARS-CoV-2 RNA shedding. https://bit.ly/2LxskI9
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused substantial morbidity and mortality worldwide [1–5]. The evidence pertaining to the epidemiological and clinical characteristics of coronavirus disease 2019 (COVID-19) has been emerging rapidly [2–7]. However, few studies have evaluated the duration of viral shedding, which has important implications for guiding the clinical decisions regarding isolation precautions and antiviral treatment in patients with COVID-19 [7, 8]. Factors associated with prolonged duration of viral shedding remain elusive. A recent randomised controlled trial showed that lopinavir/ritonavir (LPV/r) treatment could not provide additional benefits beyond standard-of-care (including in relation to viral shedding) in hospitalised severely ill patients with COVID-19 [9]. However, a post hoc subgroup analysis found that earlier administration of LPV/r treatment accelerated the clinical recovery and reduced mortality [9]. Therefore, it remains crucial to determine whether adding LPV/r treatment could influence the duration of SARS-CoV-2 RNA shedding in non-critically ill patients and whether an earlier administration of LPV/r could shorten the duration of viral shedding.
Thus, this study sought to assess the risk factors associated with prolonged viral shedding and the potential impact of earlier administration of LPV/r treatment on the duration of viral shedding in hospitalised non-critically ill patients with SARS-CoV-2 infection between 31 January and 9 March 2020.
Methods
Participants and data collection
This retrospective study included all patients who were admitted to the No. 3 People's Hospital of Hubei province (one of the designated hospitals during the COVID-19 outbreak in Wuhan, China) between 31 January 2020 and 9 March 2020. Eligible patients had laboratory-confirmed SARS-CoV-2 infection and had available RNA virological data to estimate the duration of viral shedding. Demographic, clinical, laboratory, treatment and successive virological data were extracted from electronic medical records using a standardised data collection sheet that was modified based on the World Health Organization/International Severe Acute Respiratory and Emerging Infection Consortium case record form. We assessed the severity of illness according to the Chinese management guideline for COVID-19 (sixth version) [10], and used four classes of severity, as follows. 1) “Mild” represented patients with mild clinical symptoms and no pneumonia on chest imaging. 2) “General” represented patients with clinical symptoms (i.e. fever and respiratory tract symptoms) and pneumonia on chest imaging. 3) “Severe” represented adults who met any of the following criteria: respiratory rate ≥30 breaths·min−1, resting oxygen saturation ≤93% while breathing room air, arterial oxygen tension (PaO2)/inspiratory oxygen fraction (FIO2) ≤300 mmHg, and/or >50% lesion progression within 24–48 h on chest imaging. 4) “Critical” represented adults who met any of the following criteria: development of respiratory failure that required mechanical ventilation, occurrence of shock, and/or other organ failure requiring admission to the intensive care unit. The duration of temperature recovery was defined as the duration between the date of symptom onset and the date when the patient's axillary temperature returned to 37.3°C or below without an increase thereafter. The duration of radiological recovery was defined as the duration between the date of symptom onset and the date when the patient's radiological abnormalities were improved without appearance of new radiological lesions at other sites. We also collected the dose and duration of LPV/r treatment. All data were double-checked by two physicians (D. Yan and X-Y. Liu), with any discrepancy being resolved by consensus discussion (D. Yan, X-Y. Liu and Y-H. Gao). The institutional review board of the No. 3 People's Hospital of Hubei province approved the study, and patient-level informed consent was waived.
Virological investigations
Laboratory identification of SARS-CoV-2 infection was made at Wuhan Jinyintan Hospital and No. 3 People's Hospital of Hubei province using a real-time reverse transcription PCR (RT-PCR) assay. The same methods as previously described were adopted, in which the detection reagents were provided by the local Center for Disease Control [2, 3]. After admission, throat-swab specimens were collected and sent for the re-detection of SARS-CoV-2 RNA using RT-PCR assays every 2 days after symptom remission (including fever, cough and dyspnoea). Quantitative virological data were not available. The duration of viral shedding was defined as the interval from the date of symptom onset to the date when SARS-CoV-2 was undetectable from two consecutive throat-swab specimens (≥24 h apart), without converting positive thereafter. Corticosteroid treatment referred to the administration of methylprednisolone at a dose equivalent to ≥25 mg·day−1 during hospitalisation.
Lopinavir/ritonavir treatment
LPV/r (400 mg and 100 mg, orally, twice daily) was administered to the laboratory-confirmed cases at the discretion of the attending physicians at every isolation ward. The treatment duration was ≥10 days, according to the recommendations from the Chinese management guideline for COVID-19 (sixth version) [10]. Exposure to LPV/r was defined as having received at least one dosage of LPV/r.
Statistical analysis
Data are presented as median (interquartile range (IQR)) for continuous variables and number (percentage) for categorical variables. We employed the Mann–Whitney U-test or Kruskal–Wallis test for analysis of continuous variables, and the Chi-squared test or Fisher exact test for analysis of categorical variables. Univariate and adjusted multivariate logistic regression analyses were used to identify the risk factors associated with prolonged duration of SARS-CoV-2 RNA shedding. Prolonged viral shedding was defined as the duration of SARS-CoV-2 RNA shedding being >23 days. The cut-off was determined a priori based on the median duration of viral shedding. Outcomes were defined as the initial time-point from symptom onset to SARS-CoV-2 RNA assay negativity, which required an undetectable RNA from two consecutive throat-swab specimens (≥24 h apart) and without conversion to a positive test thereafter. Variables used for the analysis of prolonged viral shedding included age, sex, smoking status, comorbidities (including diabetes, hypertension and cardiac disease), corticosteroid treatment and LPV/r treatment. Analysis was also performed using a Cox proportional hazard model to assess the risk factors for prolonged SARS-CoV-2 RNA shedding. We employed Kaplan–Meier survival analysis to estimate the cumulative SARS-CoV-2 RNA-negativity rate and the stratified log-rank statistic to compare the difference in SARS-CoV-2 RNA clearance. All statistical analysis was performed using SPSS version 22.0 software (IBM, Armonk, NY, USA). A two-tailed p-value of <0.05 was regarded as statistically significant.
Results
Patient characteristics
From 31 January 2020 to 9 March 2020, 168 confirmed cases were admitted to our hospital. As of 9 March 2020, a total of 48 patients (28.6%) still tested positive for SARS-CoV-2 RNA and eight patients (4.8%) had died. The main characteristics of the patients who were included in and excluded from the final analyses are provided in supplementary table E1. There was no notable selection bias between the two groups. Thus, 120 patients (71.4%) who had a conversion of SARS-CoV-2 RNA shedding were included in the final analysis. All the respiratory specimens tested were derived from throat swabs.
Table 1 shows the main characteristics of the 120 patients who were included in the final analysis. Of these, the median age was 52 years. 54 patients (45%) were male, and 12 (10%) were current smokers. The common comorbidities included hypertension (32 (26.7%)), diabetes (10 (8.3%)) and cardiac disease (7 (5.8%)). 89 patients (74.2%), 30 patients (25%) and one patient (0.8%) were categorised as having general, severe and critical COVID-19, respectively. 54 patients (45%) received systemic corticosteroid treatment and 78 (65%) received LPV/r treatment. The median duration of recovery of the body temperature and radiological manifestations from illness onset were 10 days and 17 days, respectively. The median length of stay in hospital was 21 days.
TABLE 1
Characteristics of 120 hospitalised patients with SARS-CoV-2 infection in Wuhan
| Characteristic | Total patients | Days to negativity | p-value# | |
| ≤23 | >23 | |||
| Patients | 120 | 61 | 59 | |
| Age years | 52 (35–63) | 48 (33–60) | 56 (42–65) | 0.04 |
| Male sex | 54 (45) | 30 (49.2) | 24 (40.7) | 0.35 |
| Current smoker | 12 (10) | 5 (8.2) | 7 (11.9) | 0.50 |
| Comorbidity | ||||
 Hypertension Hypertension | 32 (26.7) | 19 (31.1) | 13 (22.0) | 0.26 |
 Diabetes Diabetes | 10 (8.3) | 3 (4.9) | 7 (11.9) | 0.20 |
 Cardiac disease¶ Cardiac disease¶ | 7 (5.8) | 3 (4.9) | 4 (6.8) | 0.72 |
 Stroke Stroke | 3 (2.5) | 1 (1.6) | 2 (3.4) | 0.62 |
 COPD or asthma COPD or asthma | 2 (1.6) | 2 (3.2) | 0 (0) | |
 Chronic renal insufficiency Chronic renal insufficiency | 1 (0.8) | 0 | 1 (1.7) | |
 Malignancy Malignancy | 7 (5.8) | 4 (6.6) | 3 (5.1) | 0.73 |
| Disease severity | 0.36 | |||
 General General | 89 (74.2) | 48 (78.7) | 41 (69.5) | |
 Severe Severe | 30 (25.0) | 13 (21.3) | 17 (28.8) | |
 Critical Critical | 1 (0.8) | 0 (0) | 1 (1.7) | |
| Laboratory findings on admission+ | ||||
 White blood cell count ×109 cells·L−1 White blood cell count ×109 cells·L−1 | 5.65 (4.14–7.33) | 0.57 | ||
  <4 <4 | 27 (22.7) | 16 (26.7) | 11 (18.6) | |
  4–10 4–10 | 82 (68.9) | 39 (65) | 43 (72.9) | |
  >10 >10 | 10 (8.4) | 5 (8.3) | 5 (8.5) | |
 Lymphocyte count ×109 lymphocytes·L−1 Lymphocyte count ×109 lymphocytes·L−1 | 1.14 (0.81–1.55) | |||
  <0.8 <0.8 | 29 (24.3) | 18 (62.1) | 11 (37.9) | 0.15 |
 Platelet count ×109 platelets·L−1 Platelet count ×109 platelets·L−1 | 195 (144–269) | |||
  <100 <100 | 7 (5.9) | 5 (4.2) | 2 (1.0) | 0.44 |
 Creatinine level μmol·L−1 Creatinine level μmol·L−1 | 61 (52–74) | |||
  >133 >133 | 4 (3.4) | 0 (0) | 4 (3.4) | |
 AST level U·L−1 AST level U·L−1 | 31 (24–42.4) | |||
  >40 >40 | 33 (27.7) | 14 (11.8) | 19 (16.0) | 0.18 |
| Treatment | ||||
 Corticosteroid therapy Corticosteroid therapy | 54 (45.0) | 28 (45.9) | 26 (44.1) | 0.78 |
 Lopinavir/ritonavir treatment Lopinavir/ritonavir treatment | 78 (65) | 46 (75.4) | 33 (55.9) | 0.02 |
 Antibiotics Antibiotics | 102 (85.0) | 52 (85.2) | 50 (84.7) | 0.77 |
 High-flow nasal canula oxygen therapy High-flow nasal canula oxygen therapy | 21 (17.5) | 10 (16.4) | 11 (18.6) | 0.88 |
 Noninvasive mechanical ventilation Noninvasive mechanical ventilation | 2 (1.7) | 0 (0) | 2 (3.4) | |
 Invasive mechanical ventilation Invasive mechanical ventilation | 1 (0.8) | 0 (0) | 1 (1.7) | |
| Outcome | ||||
 Viral shedding days Viral shedding days | 23 (18–32) | 18 (15–20.5) | 32 (28–38) | <0.001 |
 Symptom onset to temperature recovery days§ Symptom onset to temperature recovery days§ | 10 (6–15.5) | 7.5 (5–11) | 12 (8–20) | <0.001 |
 Symptom onset to radiological recovery days Symptom onset to radiological recovery days | 17 (12–21) | 13.5 (11–18) | 20 (12–24) | <0.001 |
 Hospital length of stay days Hospital length of stay days | 21 (17–26) | 20 (15–24) | 22 (18–28) | 0.02 |
Data are presented as n, median (interquartile range) or n (%), unless otherwise stated. AST: aspartate aminotransferase. #: p-value represents comparisons between patients with prolonged viral shedding and those without; ¶: includes congestive heart disease and coronary atherosclerotic heart disease; +: data were available for 119 patients; §: data were from 99 patients.
Duration of viral shedding and risk factors
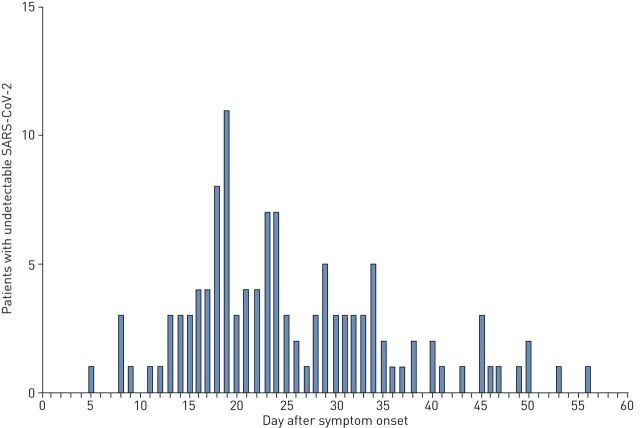
The median duration of SARS-CoV-2 RNA shedding was 23 days (IQR 18–32 days). Only five patients (4.2%) had undetectable SARS-CoV-2 RNA within 10 days, 46 (38.3%) tested negative within 20 days, and 85 (70.8%) tested negative within 30 days from symptom onset. Most patients (86.7%) had undetectable SARS-CoV-2 RNA within 37 days after symptom onset, but a subgroup of 12 patients had detectable SARS-CoV-2 RNA up to 40 days after symptom onset (figure 1). The median duration of SARS-CoV-2 shedding did not differ significantly among the three groups with different disease severity (general 23 days versus severe 26 days versus critical 28 days; p=0.51).
In our study, a duration of SARS-CoV-2 RNA shedding of >23 days (above the median) was defined as prolonged viral shedding. The main characteristics for patients with prolonged viral shedding and those without are shown in table 1. Prolonged RNA shedding was associated with a delayed recovery of radiological manifestations (median 20 days versus 13.5 days; p<0.001) and a delayed recovery of the body temperature (median 12 days versus 7.5 days; p<0.001). In the final multivariate logistic model that included all 120 patients, older age (OR 1.03, 95% CI 1.00–1.05; p=0.03) and the lack of LPV/r treatment (OR 2.42, 95% CI 1.10–5.36; p=0.029) were independent risk factors associated with prolonged SARS-CoV-2 shedding (table 2 and supplementary table E2). However, comorbidities (including current smoking, hypertension, diabetes and cardiac disease) and the administration of systemic corticosteroids were not associated with prolonged viral shedding (table 2 and supplementary table E2). Similar risk factors for prolonged SARS-CoV-2 RNA clearance were observed when the data were analysed using a Cox proportional hazards model (supplementary table E3, and supplementary figures E1 and E2).
TABLE 2
Multivariable logistic regression analysis of factors associated with duration of SARS-CoV-2 RNA detection in 120 hospitalised patients in Wuhan
| Variable | Unadjusted OR (95% CI) | p-value | Adjusted OR (95% CI) | p-value# |
| Demographic characteristic | ||||
 Age Age | 1.02 (1.00–1.04) | 0.04 | 1.03 (1.00–1.05) | 0.03 |
 Age ≥50 years Age ≥50 years | 2.13 (1.02–4.44) | 0.04 | 2.26 (1.07–4.78) | 0.03 |
 Male sex Male sex | 0.71 (0.34–1.46) | 0.35 | 0.60 (0.28–1.28) | 0.19 |
| Comorbidity | ||||
 Current smoking Current smoking | 0.86 (0.23–3.18) | 0.82 | ||
 Hypertension Hypertension | 0.63 (0.28–1.42) | 0.26 | ||
 Cardiac disease Cardiac disease | 1.41 (0.30–6.57) | 0.67 | ||
 Diabetes Diabetes | 2.60 (0.64–10.59) | 0.18 | ||
| Drug treatment | ||||
 Corticosteroid Corticosteroid | 0.90 (0.44–1.85) | 0.78 | 0.80 (0.38–1.70) | 0.57 |
 Lack of lopinavir/ritonavir Lack of lopinavir/ritonavir | 2.59 (1.19–5.62) | 0.02 | 2.42 (1.10–5.36) | 0.03 |
OR >1 indicates that the variable increases the duration of SARS-CoV-2 RNA shedding. ORs in multivariable analysis were adjusted for age and sex. #: by use of the logistic regression model, with the cut-off determined according to the median duration of SARS-CoV-2 RNA shedding (23 days).
The effect of lopinavir/ritonavir treatment
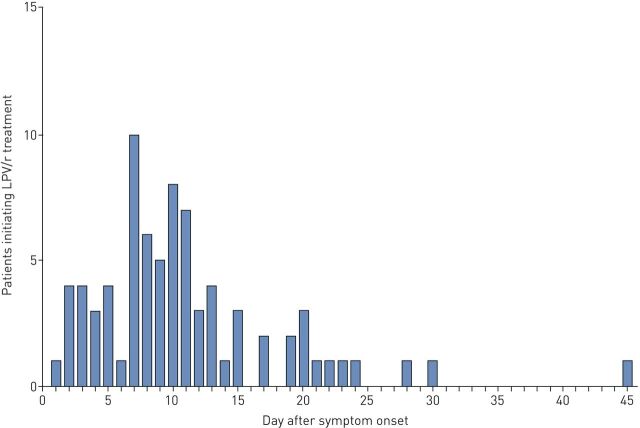
Of the 120 patients, 78 patients (65%) were administered LPV/r treatment. Patients receiving LPV/r treatment and those not receiving LPV/r treatment had similar baseline characteristics (table 3). However, patients receiving LPV/r treatment were more likely to have severe COVID-19 and a larger proportion of these patients had a lymphocyte count <0.8×109 cells·L−1, compared those without LPV/r treatment. LPV/r therapy was initiated at a median of 10 days (IQR 7–13 days) from symptom onset. Figure 2 shows the distribution of time to the initiation of LPV/r treatment from symptom onset. The median duration of LPV/r treatment was 10 days (IQR 9–10 days). 61 patients (78.2%) received ≥10 days of LRV/r treatment. The median duration of SARS-CoV-2 shedding in the LPV/r treatment group was 22 days (IQR 18–29 days), which was significantly shorter than that in the group with no LPV/r treatment (28.5 days, IQR 19.5–38 days; log-rank p=0.009) (supplementary figure E3). Patients who initiated LPV/r treatment within 10 days of symptom onset had a shorter duration of SARS-CoV-2 RNA shedding compared with those without LPV/r treatment (median 19 days versus 28.5 days; log-rank p<0.0001) (figure 3a). In contrast, the median duration of viral shedding did not differ between patients who initiated LPV/r treatment >10 days from symptom onset and patients who did not receive LPV/r treatment (median 27.5 days versus 28.5 days; log-rank p=0.51) (figure 3b).
TABLE 3
Comparison of clinical features of patients with SARS-CoV-2 infection who were treated with lopinavir/ritonavir (LPV/r) and without LPV/r, in Wuhan
| Variable | LPV/r | No LPV/r | p-value |
| Patients | 78 | 42 | |
| Age years | 50 (34–61) | 57 (36.5–66) | 0.11 |
| Male sex | 35 (44.9) | 19 (45.2) | 0.83 |
| Current smoker | 8 (10.3) | 4 (9.5) | 0.74 |
| Comorbidity | |||
 Hypertension Hypertension | 19 (24.4) | 13 (31.0) | 0.39 |
 Diabetes Diabetes | 8 (10.3) | 2 (4.8) | 0.49 |
 Cardiac disease# Cardiac disease# | 5 (6.4) | 2 (4.8) | 1.00 |
 Stroke Stroke | 2 (2.6) | 1 (2.4) | 1.00 |
 COPD or asthma COPD or asthma | 1 (1.3) | 1 (2.4) | 0.34 |
 Chronic renal insufficiency Chronic renal insufficiency | 1 (1.3) | 0 (0) | |
 Malignancy Malignancy | 4 (5.1) | 3 (7.1) | 0.69 |
| Disease severity | 0.02 | ||
 General General | 53 (67.9) | 36 (85.7) | |
 Severe Severe | 25 (32.1) | 5 (11.9) | |
 Critical Critical | 0 (0) | 1 (2.4) | |
| Laboratory findings on admission | |||
 White blood cell count ×109 cells·L−1 White blood cell count ×109 cells·L−1 | 0.32 | ||
  <4 <4 | 22 (28.2) | 5 (11.9) | |
  4–10 4–10 | 49 (62.8) | 33 (78.6) | |
  >10 >10 | 7 (9.0) | 3 (7.1) | |
 Lymphocyte count ×109 lymphocytes·L−1 Lymphocyte count ×109 lymphocytes·L−1 | |||
  <0.8 <0.8 | 25 (32.6) | 4 (9.5) | 0.01 |
 Platelet count ×109 platelets·L−1 Platelet count ×109 platelets·L−1 | |||
  <100 <100 | 6 (7.7) | 1 (2.4) | 0.42 |
 Creatinine level μmol·L−1 Creatinine level μmol·L−1 | |||
  >133 >133 | 1 (1.3) | 3 (7.1) | 0.11 |
 AST level U·L−1 AST level U·L−1 | |||
  >40 >40 | 21 (26.9) | 12 (28.6) | 0.87 |
| Treatment | |||
 Corticosteroid therapy Corticosteroid therapy | 44 (56.4) | 10 (23.8) | 0.001 |
 Antibiotics Antibiotics | 73 (93.6) | 30 (71.4) | 0.001 |
 High-flow nasal canula oxygen therapy High-flow nasal canula oxygen therapy | 17 (21.8) | 4 (9.5) | 0.13 |
 Noninvasive mechanical ventilation Noninvasive mechanical ventilation | 1 (1.3) | 1 (2.4) | 1.00 |
 Invasive mechanical ventilation Invasive mechanical ventilation | 1 (1.3) | 0 (0) | 1.00 |
| Outcome | |||
 Viral shedding days Viral shedding days | 22 (18–29) | 28.5 (19.5–38) | 0.02 |
 Hospital length of stay days Hospital length of stay days | 23 (19–27) | 18.5 (13–22.5) | <0.01 |
Data are presented as n, median (interquartile range) or n (%), unless otherwise stated. AST: aspartate aminotransferase. #: includes congestive heart disease and coronary atherosclerotic heart disease.
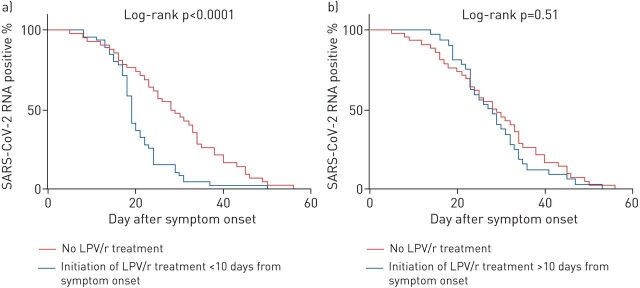
The cumulative proportions of patients with detectable SARS-CoV-2 RNA over time, by day after symptom onset, comparing patients without lopinavir/ritonavir (LPV/r) treatment with a) patients initiating LPV/r treatment <10 days from symptom onset, and b) patients initiating LPV/r treatment >10 days from symptom onset.
Discussion
Viral shedding has commonly been used as a proxy measure for infectivity. Therefore, identification of the duration of viral shedding would be central to inform control policies and antiviral treatment in patients with COVID-19. In the current study of 120 hospitalised non-critically ill patients with COVID-19, we have for the first time identified that older age and the lack of LPV/r treatment were independent risk factors for prolonged viral shedding. We have also found that an initial administration of LPV/r treatment within 10 days from symptom onset, but not afterwards, could shorten the duration of SARS-CoV-2 RNA shedding. However, we did not observe an impact of comorbidities and corticosteroid use on the duration of viral shedding.
As an emerging novel coronavirus, data on the duration of SARS-CoV-2 RNA shedding have been limited. Our previous study reported a median duration of SARS-CoV-2 RNA shedding of 19.5 days [7]. A recent study including 191 confirmed cases showed that SARS-CoV-2 RNA was detectable for a median of 20 days in survivors and until death in non-survivors [8]. Our corresponding estimate in the current study was 23 days using throat-swab specimens for viral detection. In addition, the median duration of SARS-CoV-2 RNA shedding did not differ between severe and general patients in the current study, which was in contrast to a study that recruited patients infected with Middle East respiratory syndrome coronavirus (MERS-CoV), in which more severely ill patients typically had higher RNA levels and more prolonged shedding [11]. Previous studies have reported that detectable SARS-CoV-2 RNA could persist until death in non-survivors [8, 9]. Whether critically ill patients have longer viral shedding compared with the generally or severely ill patients with COVID-19 needs further investigation. Nevertheless, given a long duration of SARS-CoV-2 RNA shedding as detected by RT-PCR, a more stringent approach to infection control, such as quarantine for an extended period of time and strict droplet precautions, may be pertinent and applicable to avoid wide-spread transmission.
Our results demonstrated that older age was independently associated with prolonged SARS-CoV-2 RNA shedding. Previous studies have shown that older age is a risk factor for SARS-CoV-2 infection and has been associated with a greater risk of development of acute respiratory distress syndrome and mortality [8, 12]. Our findings further contribute to the literature by addressing the association between age and SARS-CoV-2 RNA shedding. One possible explanation would be that older patients typically generate less robust innate and adaptive immune responses, limiting the viral clearance, which predisposes to prolonged duration of viral shedding [13]. Of interest, a previous study of SARS-CoV has shown that the polymorphism of genes involved in innate immunity (i.e. interleukin-18, interleukin-1A and fibrinogen-like protein 2), which could be regulated in an age- and sex-dependent manner, was associated with viral load during the initial stage of the disease [14]. This highlights the importance of host genetic factors in the process of viral infections and merits further investigations in COVID-19.
Lopinavir, an HIV-1 protease inhibitor, was administered in combination with ritonavir to increase drug bioavailability of lopinavir [15]. LPV/r has been shown to be effective in patients infected with SARS-CoV [16]. LPV/r also improved clinical parameters in MERS-CoV-infected marmosets and mice [17, 18]. Our study has, for the first time, shown that the lack of LPV/r treatment was independently associated with prolonged SARS-CoV-2 RNA shedding in non-critically ill patients with COVID-19. Administration of LPV/r treatment could shorten the duration of viral shedding compared with no LPV/r treatment. Interestingly, subgroup analysis revealed that the early administration of LPV/r treatment within 10 days from symptom onset, but not late administration (initiated after 10 days), could shorten the duration of SARS-CoV-2 RNA shedding compared with no LPV/r treatment. A recent randomised controlled trial showed that LPV/r treatment could not provide additional benefit, including in relation to viral shedding, beyond standard-of-care in hospitalised severely ill adult patients with COVID-19 who required a range of ventilatory support modes [9]. However, a post hoc subgroup analysis of the LPV/r treatment in the randomised controlled trial found that earlier administration of antiviral treatment could accelerate the clinical recovery and reduce the mortality, which was consistent with our findings. Admittedly, patients recruited in the trial were in an advanced phase of infection (as evidenced by the 25% mortality in the control group). In contrast, most patients (99.2%) in our cohort were non-critically ill patients with COVID-19, due to triage strategies. Because LPV/r was not particularly designed to act against SARS-CoV-2, it might reasonably be anticipated that LPV/r action on SARS-CoV-2 was not as effective as neuraminidase inhibitor action on influenza [19]. The median duration of viral shedding, even in patients who received LPV/r treatment within 10 days from symptom onset, was 19 days in the current study, demonstrating that LPV/r treatment could not completely inhibit viral replication. In this regard, earlier administration of LPV/r therapy after symptom onset, to patients who were not in the late phase of infection, may be crucial to determine the efficacy. Nevertheless, randomised clinical trials remain crucial to test whether earlier administration of LPV/r treatment could shorten the duration of viral shedding, accelerate the clinical recovery and improve survival in patients with COVID-19, especially in non-critically ill patients.
Our investigation has some limitations. First, the presence of SARS-CoV-2 RNA does not necessarily indicate the production of infectious virus. Secondly, owing to triage strategies, almost all patients in our hospital were categorised as having general and severe COVID-19; therefore, extrapolating these findings to critically ill patients needs caution. Thirdly, the estimation of the duration of SARS-CoV-2 RNA shedding was limited by the type of respiratory specimen, the frequency of respiratory specimen collection and the lack of quantitative viral RNA detection. Fourthly, we excluded fatal cases from the final analysis because all of them had detectable SARS-CoV-2 RNA until death, and the time to death could not accurately reflect the duration of viral shedding; therefore, the association between viral shedding and mortality could be not assessed. Finally, interpretation of our findings was limited by the sample size and the lack of genetic analysis of the host. Further large-scale cohort studies are still needed to better define the risk factors for prolonged viral shedding, including testing the effect of corticosteroid use, in patients with COVID-19.
In summary, older age and the lack of LPV/r treatment correlate with prolonged SARS-CoV-2 RNA shedding. Earlier administration of LPV/r treatment may shorten the duration of SARS-CoV-2 RNA shedding. Randomised clinical trials to determine the effectiveness of LPV/r treatment in non-critically ill patients with COVID-19 are needed.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00799-2020.SUPPLEMENT
Shareable PDF
Acknowledgement
We thank Wei-jie Guan (State Key Laboratory of Respiratory Disease and National Clinical Research Center for Respiratory Disease, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China) for his assistance in language editing during revision.
Footnotes
This article has supplementary material available from https://www.erj.ersjournals.com
Author contributions: Y-H. Gao designed the project. D. Yan, X-Y. Liu and B-T. Dan carried out the data collection. Y-H. Gao, Y-N. Zhu and L. Huang analysed the data and prepared the figures. Y-H. Gao, D. Yan, Y-N. Zhu and L. Huang drafted the manuscript. All the authors have revised the manuscript critically, approved the version submitted for publication and have agreed to be accountable for all aspects of the work.
Conflict of interest: D. Yan has nothing to disclose.
Conflict of interest: X-Y. Liu has nothing to disclose.
Conflict of interest: Y-N. Zhu has nothing to disclose.
Conflict of interest: L. Huang has nothing to disclose.
Conflict of interest: B-T. Dan has nothing to disclose.
Conflict of interest: G-J. Zhang has nothing to disclose.
Conflict of interest: Y-H. Gao has nothing to disclose.
References
Full text links
Read article at publisher's site: https://doi.org/10.1183/13993003.00799-2020
Read article for free, from open access legal sources, via Unpaywall:
https://erj.ersjournals.com/content/erj/56/1/2000799.full.pdf
Citations & impact
Impact metrics
Article citations
Therapeutic Management with Repurposing Approaches: A Mystery During COVID-19 Outbreak.
Curr Mol Med, 24(6):712-733, 01 Jan 2024
Cited by: 0 articles | PMID: 37312440
Review
Impact of Tuberculosis on Disease Severity and Viral Shedding Duration in COVID-19 Patients.
Viruses, 16(2):260, 06 Feb 2024
Cited by: 0 articles | PMID: 38400036 | PMCID: PMC10893069
Risk Factors Associated with Prolonged Nasopharyngeal Carriage of SARS-CoV-2 and Length of Stay among Patients Admitted to a COVID-19 Referral Center in Manila, Philippines.
Acta Med Philipp, 57(12):66-72, 18 Dec 2023
Cited by: 0 articles | PMID: 39429759 | PMCID: PMC11484558
Estimation of shedding time in laboratory-confirmed COVID-19 cases in South Africa: a population-based record linkage study, March-December 2020.
Pan Afr Med J, 46:24, 15 Sep 2023
Cited by: 0 articles | PMID: 38107342 | PMCID: PMC10724037
Age-dependent immune responses in COVID-19-mediated liver injury: focus on cytokines.
Front Endocrinol (Lausanne), 14:1139692, 15 Aug 2023
Cited by: 4 articles | PMID: 37654571 | PMCID: PMC10465349
Review Free full text in Europe PMC
Go to all (108) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Lopinavir/ritonavir did not shorten the duration of SARS CoV-2 shedding in patients with mild pneumonia in Taiwan.
J Microbiol Immunol Infect, 53(3):488-492, 03 Apr 2020
Cited by: 46 articles | PMID: 32331982 | PMCID: PMC7194913
Lopinavir/ritonavir and interferon combination therapy may help shorten the duration of viral shedding in patients with COVID-19: A retrospective study in two designated hospitals in Anhui, China.
J Med Virol, 92(11):2666-2674, 29 Jun 2020
Cited by: 29 articles | PMID: 32492211 | PMCID: PMC7300569
Associations of clinical characteristics and treatment regimens with the duration of viral RNA shedding in patients with COVID-19.
Int J Infect Dis, 98:252-260, 30 Jun 2020
Cited by: 59 articles | PMID: 32619760 | PMCID: PMC7326382
Compounds with Therapeutic Potential against Novel Respiratory 2019 Coronavirus.
Antimicrob Agents Chemother, 64(5):e00399-20, 21 Apr 2020
Cited by: 224 articles | PMID: 32152082 | PMCID: PMC7179632
Review Free full text in Europe PMC