Abstract
Background
Reports on neurologic manifestations of coronavirus disease 2019 (COVID-19) have attracted broad attention. We present an unusual case of COVID-19-associated encephalitis mimicking a glial tumor.Case description
A 35-year-old woman presented with headache and seizures. T2 fluid-attenuated inverse recovery imaging showed hyperintensities in the left temporal lobe. Magnetic resonance spectroscopy showed an elevated choline peak. Imaging findings were suggestive of high-grade glioma. Antiepileptic medication failed to achieve seizure control. A left anterior temporal lobectomy was performed. The patient had no postoperative deficits, and her symptoms completely improved. Histologic examination revealed encephalitis. Postoperatively, our patient tested positive for COVID-19.Conclusions
Our case raises awareness of neurologic manifestations of the disease and their potential to mimic glial tumors. For prompt diagnosis and prevention of transmission, clinicians should consider COVID-19 in patients with similar presentation.Free full text

COVID-19−Associated Encephalitis Mimicking Glial Tumor
Abstract
Background
Reports on neurologic manifestations of coronavirus disease 2019 (COVID-19) have attracted broad attention. We present an unusual case of COVID-19−associated encephalitis mimicking a glial tumor.
Case Description
A 35-year-old woman presented with headache and seizures. T2 fluid-attenuated inverse recovery imaging showed hyperintensities in the left temporal lobe. Magnetic resonance spectroscopy showed an elevated choline peak. Imaging findings were suggestive of high-grade glioma. Antiepileptic medication failed to achieve seizure control. A left anterior temporal lobectomy was performed. The patient had no postoperative deficits, and her symptoms completely improved. Histologic examination revealed encephalitis. Postoperatively, our patient tested positive for COVID-19.
Conclusions
Our case raises awareness of neurologic manifestations of the disease and their potential to mimic glial tumors. For prompt diagnosis and prevention of transmission, clinicians should consider COVID-19 in patients with similar presentation.
Introduction
In December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in Wuhan, China, causing an outbreak of severe pneumonia. The resulting illness was named coronavirus disease 2019 (COVID-19) and was recognized as pandemic in March 2020. Aside from typical symptoms such as cough, fever and difficulty in breathing, recent reports on neurologic manifestations have attracted broad attention. These included a case of acute necrotizing encephalopathy and a case of encephalitis, which were likely caused by SARS-CoV-2.1 , 2 Central nervous system (CNS) involvement had been seen in the 2002 outbreak of the severe acute respiratory syndrome (SARS) coronavirus before.3 Here, we present a patient with COVID-19−associated encephalitis mimicking a glial tumor. To the best of our knowledge, this is the first case of surgical management and histopathologic confirmation of encephalitis linked to COVID-19.
Case Report
A 35-year-old woman was admitted to our neurosurgical department with headache, nausea, dizziness, and drug-refractory seizures. She was alert and oriented with no motor or sensory deficits. Magnetic resonance imaging (MRI) showed hyperintense signal in the left temporal lobe in T2 and T2 fluid-attenuated inversion recovery (FLAIR) imaging. The patient was hospitalized for further evaluation and magnetic resonance spectroscopy (MRS) was performed. Long echo time MRS showed marked elevation of the choline peak along with a decrease of the N-acetylaspartate peak, suggestive of high-grade glioma rather than a nonneoplastic disease (Figure 1 ). Despite attempts of combined antiepileptic medication, seizure control was not satisfactory. On the basis of clinical and radiologic findings, surgical intervention was inevitable. A left anterior temporal lobectomy was performed. Intraoperative frozen-section biopsy was nondiagnostic. Surgery was uneventful. The patient had no postoperative neurologic deficits, and her symptoms improved completely. Postoperative MRI showed total removal of the anterior portion of the temporal lobe (Figure 2A ).
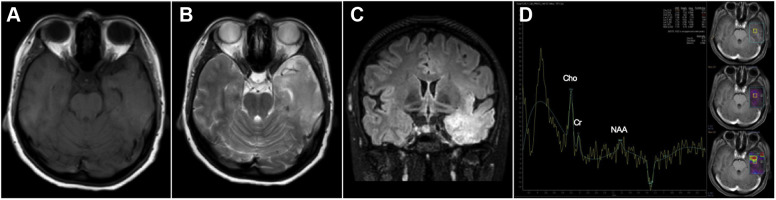
T1-weighted axial magnetic resonance imaging (MRI) (A) showed an isointense lesion in the left temporal lobe. Lesion appeared hyperintense in T2-weighted axial MRI (B) and fluid-attenuated inversion recovery (C). In long echo time magnetic resonance spectroscopy (D), marked elevation of the choline peak was seen along with a decrease of the N-acetylaspartate peak. Findings were suggestive of high-grade glioma.
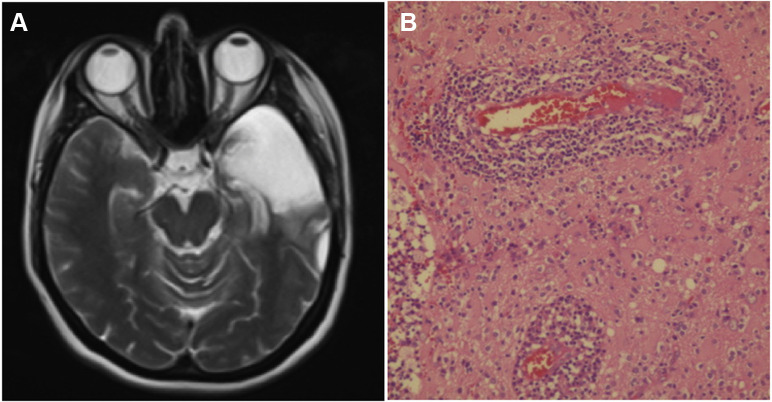
Postoperative T2-weighted axial magnetic resonance imaging (A) showed total removal of the left anterior temporal lobe. Histopathologic examination (B) showed concentric lymphocytic infiltration into perivascular spaces causing neuronal damage and diffuse hypoxic changes in surrounding brain parenchyma (hematoxylin-eosin, ×400).
On the day of surgery, the patient's husband presented to the emergency department with signs of respiratory tract infection and tested positive for COVID-19. Postoperatively, our patient tested positive for COVID-19 in reverse-transcriptase-polymerase-chain-reaction and antibody tests. She was referred to a designated infectious diseases clinic and monitored until testing negative for SARS-CoV-2. She retrospectively declared that she had mild flulike complaints 2 weeks before onset of neurologic symptoms. On postoperative day 5, the diagnosis of encephalitis was confirmed on histopathologic examination (see Figure 2B).
Discussion
Coronaviruses interact with target cells through membrane-bound spike proteins. The angiotensin-converting enzyme 2 was identified as an entry receptor for SARS-CoV-2. Due to its broad expression pattern, COVID-19 can affect multiple organs including the nervous system, where the receptor is predominantly expressed by neurons. SARS-CoV-2 is believed to reach the CNS via 2 major routes. After infecting the nasal mucosa, coronaviruses can invade the brain through the cribriform plate, advancing along the olfactory nerve. Alternatively, coronaviruses can reach the capillaries via the bloodstream and interact with angiotensin-converting enzyme 2 to invade and replicate within the endothelium. Viral budding causes damage to the endothelial lining, allowing for greater viral access into the neural milieu.4
Neuroinvasion has been observed previously in the SARS coronavirus (SARS-CoV) and MERS coronavirus (MERS-CoV). Among 70 patients with MERS-CoV, 26% had an altered mental status and 9% suffered from seizures.5 SARS-CoV responsible for the 2002–2004 outbreak was reported to induce polyneuropathy, ischemic stroke, and encephalitis.3 Autopsy results of patients with SARS showed ischemic neuronal damage and demyelination. Viral RNA was detected in brain tissue, particularly accumulating in and around the hippocampus.6 In SARS, neurologic symptoms were reported to develop around 2–4 weeks after onset of respiratory symptoms.3 Similarly, our patient had, although mild, flulike symptoms 2 weeks before onset of neurologic symptoms, possibly suggesting a parallel course of the disease. However, it remains unproven whether her flulike symptoms were caused by her SARS-CoV-2 infection.
The high genetic similarity between SARS-CoV and the novel SARS-CoV-2 raises attention for similar and potentially life-threatening CNS manifestations in COVID-19. As in our case, recent reports on encephalopathies associated with COVID-19 showed a predominant involvement of the temporal lobe. Poyiadji et al1 described a presumptive case of acute necrotizing encephalopathy in a female patient with COVID-19. Hyperintense lesions in the thalamus and medial temporal lobes were detected in T2 FLAIR. Another male patient showed T2 FLAIR hyperintensity in the right temporal lobe and hippocampus, suggesting encephalitis.2 Similar to our patient, he presented with nausea and generalized seizures.
It has been reported that encephalitis may mimic other CNS pathologies in MRS. MRS findings of encephalitis and glioma may in some cases be indistinguishable.7 As our patient had medical treatment−resistant seizures, our final diagnosis of encephalitis would not have changed our decision for surgery. Anterior temporal lobectomy has previously been proposed for herpes simplex virus encephalitis with focal epilepsy if refractory to maximal medical therapy.8
Conclusion
We report, to the best of our knowledge, the first case of surgical management and histologic confirmation of encephalitis in a patient with COVID-19. In view of the rising number of infections worldwide, our case aims to raise awareness of severe neurologic manifestations of COVID-19. These cases may mimic glial neoplasm. Radiologic findings, especially MRI and MRS, can thus be misleading. For timely diagnosis and prevention of transmission, physicians should consider SARS-CoV-2 infection in patients with similar presentation.
Footnotes
Conflict of interest statement: The authors declare that the article content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Citations & impact
Impact metrics
Article citations
Autoimmune encephalitis: what the radiologist needs to know.
Neuroradiology, 66(5):653-675, 20 Mar 2024
Cited by: 2 articles | PMID: 38507081 | PMCID: PMC11031487
Review Free full text in Europe PMC
SARS-CoV-2 and Brain Health: New Challenges in the Era of the Pandemic.
Microorganisms, 11(10):2511, 08 Oct 2023
Cited by: 0 articles | PMID: 37894169 | PMCID: PMC10609574
Review Free full text in Europe PMC
COVID-19 associated psychosis.
Ind Psychiatry J, 32(2):215-221, 12 Jul 2023
Cited by: 1 article | PMID: 38161482 | PMCID: PMC10756597
Review Free full text in Europe PMC
Neurological Complications of COVID-19 in the Elderly.
Neurosci Behav Physiol, 52(5):625-634, 13 Sep 2022
Cited by: 0 articles | PMID: 36119647 | PMCID: PMC9468529
Encephalitis in Patients with COVID-19: A Systematic Evidence-Based Analysis.
Cells, 11(16):2575, 18 Aug 2022
Cited by: 18 articles | PMID: 36010650 | PMCID: PMC9406394
Review Free full text in Europe PMC
Go to all (46) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Neurological manifestations of COVID-19: available evidences and a new paradigm.
J Neurovirol, 26(5):619-630, 24 Aug 2020
Cited by: 57 articles | PMID: 32839951 | PMCID: PMC7444681
Review Free full text in Europe PMC
[Encephalitis associated with COVID-19 in a 13-year-old girl: A case report].
Medwave, 20(7):e7984, 03 Aug 2020
Cited by: 8 articles | PMID: 32804920
SARS-CoV-2-Associated Acute Hemorrhagic, Necrotizing Encephalitis (AHNE) Presenting with Cognitive Impairment in a 44-Year-Old Woman without Comorbidities: A Case Report.
Am J Case Rep, 21:e925641, 16 Aug 2020
Cited by: 44 articles | PMID: 32799213 | PMCID: PMC7447297
First motor seizure as presenting symptom of SARS-CoV-2 infection.
Neurol Sci, 41(7):1651-1653, 16 May 2020
Cited by: 21 articles | PMID: 32417987 | PMCID: PMC7229435





