Abstract
Free full text

Production of colony-stimulating factors (CSFs) during infection: separate determinations of macrophage-, granulocyte-, granulocyte-macrophage-, and multi-CSFs.
Abstract
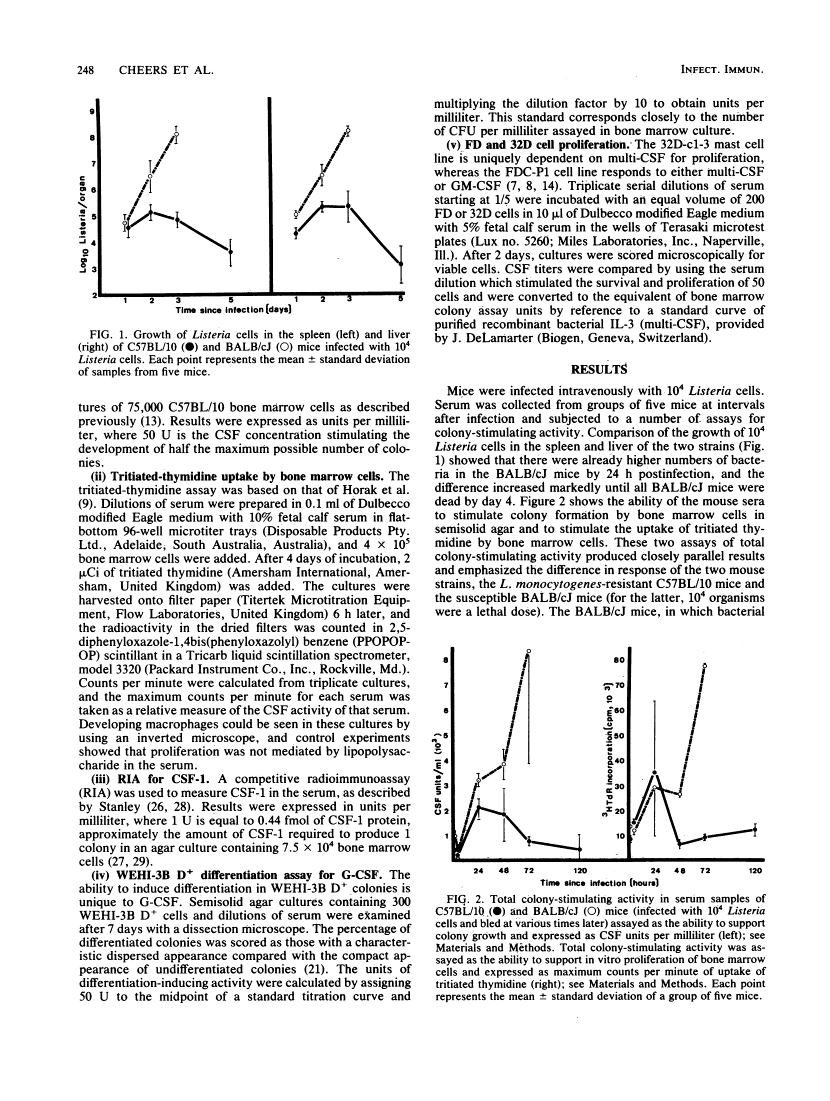
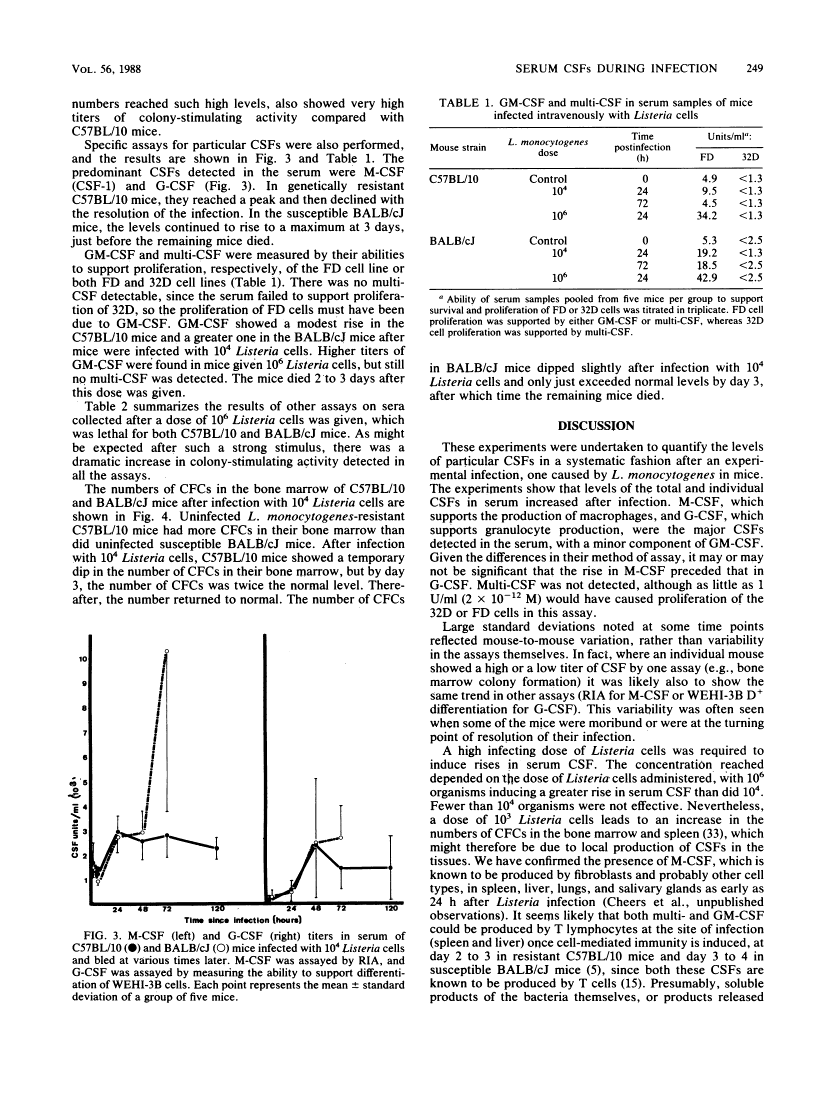
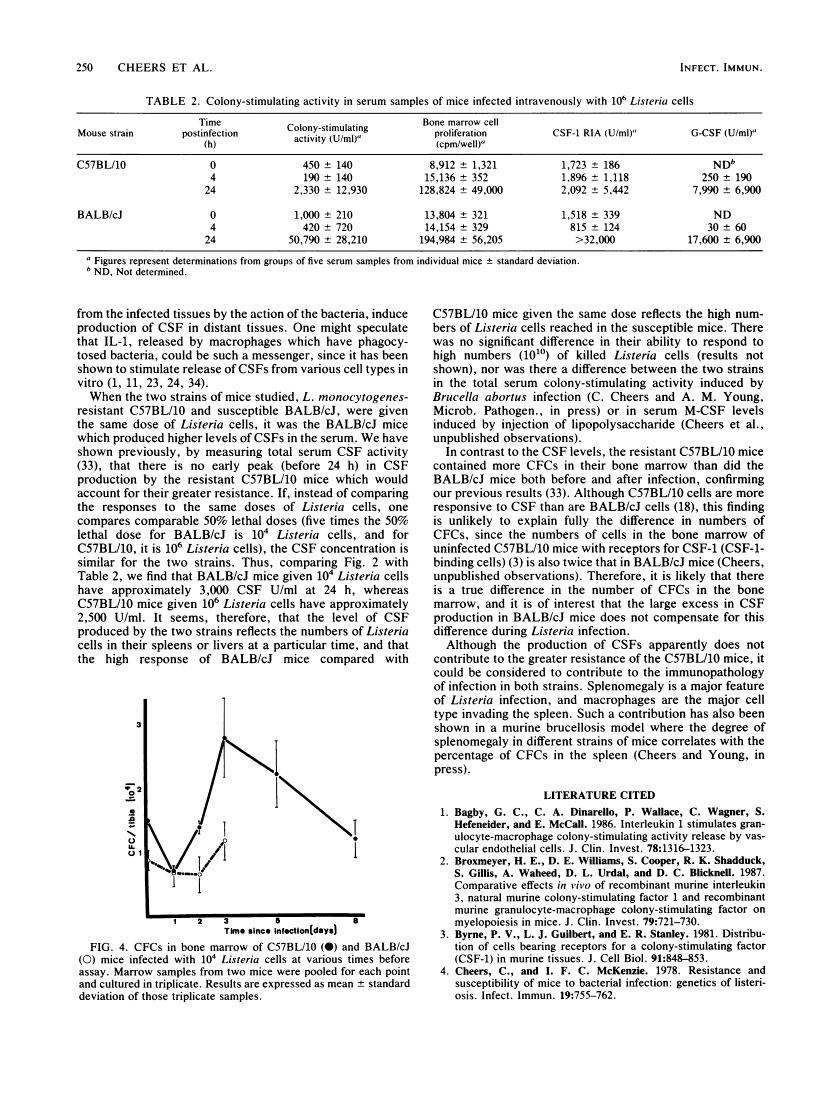
After infection of mice with Listeria monocytogenes, elevated levels of colony-stimulating factors (CSFs) in the serum were quantitated by six different assays: ability to stimulate colony formation, the proliferation of 2 suspension of bone marrow cells (both measuring total colony-stimulating activity), a radioimmunoassay for macrophage-CSF (CSF-1), the WEHI-3B differentiation assay for granulocyte-CSF, and proliferation of 32D-c1-3 and FDC-P1 cell lines (specific for multi-CSF and either multi- or granulocyte-macrophage-CSFs, respectively). The great bulk of serum colony-stimulating activity represented macrophage- and granulocyte-CSFs, with small but measurable amounts of granulocyte-macrophage-CSF. The degree of elevation of serum CSF depended on the infecting dose used and the numbers of bacteria growing in the spleens and livers of the two mouse strains compared, i.e., L. monocytogenes-resistant C57BL/10 and susceptible BALB/cJ. The increase in serum CSFs occurred before the peak in bone marrow granulocyte-macrophage progenitors and before the reduction in bacterial numbers which follows the onset of specific cell-mediated immunity.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (953K), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagby GC, Jr, Dinarello CA, Wallace P, Wagner C, Hefeneider S, McCall E. Interleukin 1 stimulates granulocyte macrophage colony-stimulating activity release by vascular endothelial cells. J Clin Invest. 1986 Nov;78(5):1316–1323. [Europe PMC free article] [Abstract] [Google Scholar]
- Broxmeyer HE, Williams DE, Cooper S, Shadduck RK, Gillis S, Waheed A, Urdal DL, Bicknell DC. Comparative effects in vivo of recombinant murine interleukin 3, natural murine colony-stimulating factor-1, and recombinant murine granulocyte-macrophage colony-stimulating factor on myelopoiesis in mice. J Clin Invest. 1987 Mar;79(3):721–730. [Europe PMC free article] [Abstract] [Google Scholar]
- Byrne PV, Guilbert LJ, Stanley ER. Distribution of cells bearing receptors for a colony-stimulating factor (CSF-1) in murine tissues. J Cell Biol. 1981 Dec;91(3 Pt 1):848–853. [Europe PMC free article] [Abstract] [Google Scholar]
- Cheers C, McKenzie IF. Resistance and susceptibility of mice to bacterial infection: genetics of listeriosis. Infect Immun. 1978 Mar;19(3):755–762. [Europe PMC free article] [Abstract] [Google Scholar]
- Cheers C, McKenzie IF, Pavlov H, Waid C, York J. Resistance and susceptibility of mice to bacterial infection: course of listeriosis in resistant or susceptible mice. Infect Immun. 1978 Mar;19(3):763–770. [Europe PMC free article] [Abstract] [Google Scholar]
- Czuprynski CJ, Henson PM, Campbell PA. Killing of Listeria monocytogenes by inflammatory neutrophils and mononuclear phagocytes from immune and nonimmune mice. J Leukoc Biol. 1984 Feb;35(2):193–208. [Abstract] [Google Scholar]
- Dexter TM, Garland J, Scott D, Scolnick E, Metcalf D. Growth of factor-dependent hemopoietic precursor cell lines. J Exp Med. 1980 Oct 1;152(4):1036–1047. [Europe PMC free article] [Abstract] [Google Scholar]
- Greenberger JS, Sakakeeny MA, Humphries RK, Eaves CJ, Eckner RJ. Demonstration of permanent factor-dependent multipotential (erythroid/neutrophil/basophil) hematopoietic progenitor cell lines. Proc Natl Acad Sci U S A. 1983 May;80(10):2931–2935. [Europe PMC free article] [Abstract] [Google Scholar]
- Horak H, Turner AR, Shaw AR, Yau OW. Stimulation of [3H]thymidine uptake in mouse marrow by granulocyte-macrophage colony stimulating factor from mouse lung conditioned medium. J Immunol Methods. 1983 Jan 28;56(2):253–260. [Abstract] [Google Scholar]
- Kindler V, Thorens B, de Kossodo S, Allet B, Eliason JF, Thatcher D, Farber N, Vassalli P. Stimulation of hematopoiesis in vivo by recombinant bacterial murine interleukin 3. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1001–1005. [Europe PMC free article] [Abstract] [Google Scholar]
- Løvhaug D, Pelus LM, Nordlie EM, Bøyum A, Moore MA. Monocyte-conditioned medium and interleukin 1 induce granulocyte-macrophage colony-stimulating factor production in the adherent cell layer of murine bone marrow cultures. Exp Hematol. 1986 Dec;14(11):1037–1042. [Abstract] [Google Scholar]
- Metcalf D. Acute antigen-induced elevation of serum colony stimulating factor (CFS) levels. Immunology. 1971 Sep;21(3):427–436. [Abstract] [Google Scholar]
- Metcalf D. Molecular control of granulocyte and macrophage production. Prog Clin Biol Res. 1985;191:323–337. [Abstract] [Google Scholar]
- Metcalf D. The molecular biology and functions of the granulocyte-macrophage colony-stimulating factors. Blood. 1986 Feb;67(2):257–267. [Abstract] [Google Scholar]
- Metcalf D, Begley CG, Johnson GR, Nicola NA, Lopez AF, Williamson DJ. Effects of purified bacterially synthesized murine multi-CSF (IL-3) on hematopoiesis in normal adult mice. Blood. 1986 Jul;68(1):46–57. [Abstract] [Google Scholar]
- Metcalf D, Johnson GR, Mandel TE. Colony formation in agar by multipotential hemopoietic cells. J Cell Physiol. 1979 Feb;98(2):401–420. [Abstract] [Google Scholar]
- Metcalf D, Russell S. Inhibition by mouse serum of hemopoietic colony formation in vitro. Exp Hematol. 1976 Nov;4(6):339–353. [Abstract] [Google Scholar]
- Newborg MF, North RJ. On the mechanism of T cell-independent anti-Listeria resistance in nude mice. J Immunol. 1980 Feb;124(2):571–576. [Abstract] [Google Scholar]
- Nicola NA, Metcalf D, Matsumoto M, Johnson GR. Purification of a factor inducing differentiation in murine myelomonocytic leukemia cells. Identification as granulocyte colony-stimulating factor. J Biol Chem. 1983 Jul 25;258(14):9017–9023. [Abstract] [Google Scholar]
- Rennick D, Yang G, Gemmell L, Lee F. Control of hemopoiesis by a bone marrow stromal cell clone: lipopolysaccharide- and interleukin-1-inducible production of colony-stimulating factors. Blood. 1987 Feb;69(2):682–691. [Abstract] [Google Scholar]
- Segal GM, McCall E, Stueve T, Bagby GC., Jr Interleukin 1 stimulates endothelial cells to release multilineage human colony-stimulating activity. J Immunol. 1987 Mar 15;138(6):1772–1778. [Abstract] [Google Scholar]
- Shimamura M, Kobayashi Y, Yuo A, Urabe A, Okabe T, Komatsu Y, Itoh S, Takaku F. Effect of human recombinant granulocyte colony-stimulating factor on hematopoietic injury in mice induced by 5-fluorouracil. Blood. 1987 Jan;69(1):353–355. [Abstract] [Google Scholar]
- Stanley ER. Colony-stimulating factor (CSF) radioimmunoassay: detection of a CSF subclass stimulating macrophage production. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2969–2973. [Europe PMC free article] [Abstract] [Google Scholar]
- Stanley ER. The macrophage colony-stimulating factor, CSF-1. Methods Enzymol. 1985;116:564–587. [Abstract] [Google Scholar]
- Stanley ER, Metcalf D, Maritz JS, Yeo GF. Standardized bioassay for bone marrow colony stimulating factor in human urine: levels in normal man. J Lab Clin Med. 1972 Apr;79(4):657–668. [Abstract] [Google Scholar]
- Wing EJ, Barczynski LK, Sherbondy JM. Effect of acute nutritional deprivation on macrophage colony-stimulating factor and macrophage progenitor cells in mice. Infect Immun. 1986 Oct;54(1):245–249. [Europe PMC free article] [Abstract] [Google Scholar]
- Wing EJ, Waheed A, Shadduck RK. Changes in serum colony-stimulating factor and monocytic progenitor cells during Listeria monocytogenes infection in mice. Infect Immun. 1984 Jul;45(1):180–184. [Europe PMC free article] [Abstract] [Google Scholar]
- Wood PR, Spanidis V, Frangos K, Cheers C. The in vitro bactericidal activity of peritoneal and spleen cells from Listeria-resistant and -susceptible mouse strains. Cell Immunol. 1986 Apr 15;99(1):160–169. [Abstract] [Google Scholar]
- Young AM, Cheers C. Colony-forming cells and colony-stimulating activity during listeriosis in genetically resistant or susceptible mice. Cell Immunol. 1986 Feb;97(2):227–237. [Abstract] [Google Scholar]
- Zucali JR, Dinarello CA, Oblon DJ, Gross MA, Anderson L, Weiner RS. Interleukin 1 stimulates fibroblasts to produce granulocyte-macrophage colony-stimulating activity and prostaglandin E2. J Clin Invest. 1986 Jun;77(6):1857–1863. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Infection and Immunity are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/iai.56.1.247-251.1988
Read article for free, from open access legal sources, via Unpaywall:
https://iai.asm.org/content/iai/56/1/247.full.pdf
Free after 4 months at iai.asm.org
http://iai.asm.org/cgi/reprint/56/1/247
Free to read at iai.asm.org
http://iai.asm.org/cgi/content/abstract/56/1/247
Citations & impact
Impact metrics
Citations of article over time
Article citations
The granulocyte colony-stimulating factor produced during Streptococcus suis infection controls neutrophil recruitment in the blood without affecting bacterial clearance.
Front Immunol, 15:1403789, 02 Aug 2024
Cited by: 0 articles | PMID: 39156897 | PMCID: PMC11327821
Neutrophils cultured ex vivo from CD34<sup>+</sup> stem cells are immature and genetically tractable.
J Transl Med, 22(1):526, 31 May 2024
Cited by: 2 articles | PMID: 38822352 | PMCID: PMC11143668
Role of granulocyte colony stimulating factor in the treatment of cirrhosis of liver: a systematic review.
J Int Med Res, 51(11):3000605231207064, 01 Nov 2023
Cited by: 0 articles | PMID: 37946367 | PMCID: PMC10637184
Review Free full text in Europe PMC
Novel Insights into the Role of Kras in Myeloid Differentiation: Engaging with Wnt/β-Catenin Signaling.
Cells, 12(2):322, 14 Jan 2023
Cited by: 1 article | PMID: 36672256 | PMCID: PMC9857056
Review Free full text in Europe PMC
The Effect of Pegbovigrastim Injection on Phagocytic and Oxidative Burst Activities of Peripheral Blood Granulocytes and Monocytes in Calves Challenged with Mycoplasma bovis.
Pathogens, 11(11):1317, 09 Nov 2022
Cited by: 1 article | PMID: 36365068 | PMCID: PMC9693237
Go to all (133) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Effect of interleukin-4 on the growth of granulocyte-macrophage progenitor cells stimulated by hematopoietic growth factors.
J UOEH, 12(2):163-174, 01 Jun 1990
Cited by: 3 articles | PMID: 1697091
Synergistic myelopoietic actions in vivo after administration to mice of combinations of purified natural murine colony-stimulating factor 1, recombinant murine interleukin 3, and recombinant murine granulocyte/macrophage colony-stimulating factor.
Proc Natl Acad Sci U S A, 84(11):3871-3875, 01 Jun 1987
Cited by: 33 articles | PMID: 3495800 | PMCID: PMC304978
Proliferative effects of purified granulocyte colony-stimulating factor (G-CSF) on normal mouse hemopoietic cells.
J Cell Physiol, 116(2):198-206, 01 Aug 1983
Cited by: 108 articles | PMID: 6602806
Specificity of action of colony-stimulating factors in the differentiation of granulocytes and macrophages.
Ciba Found Symp, 118:7-28, 01 Jan 1986
Cited by: 13 articles | PMID: 3015515
Review