Abstract
Free full text

Interleukin 1 induces a shock-like state in rabbits. Synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition.
Abstract
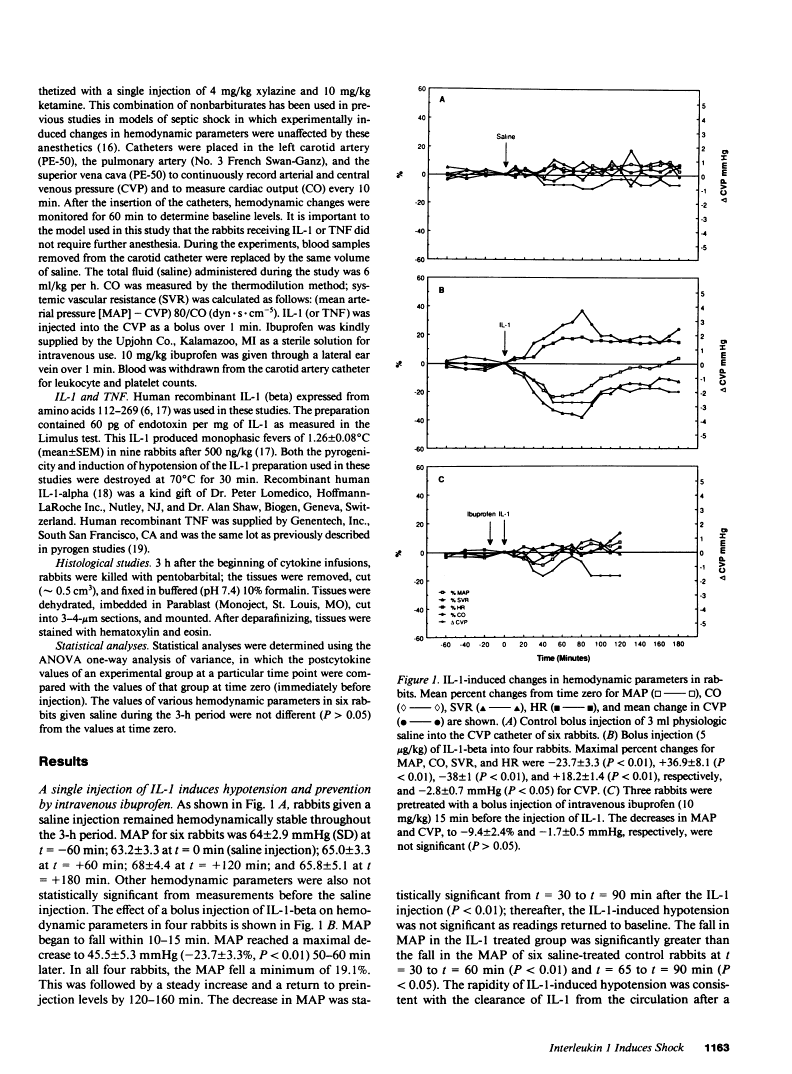
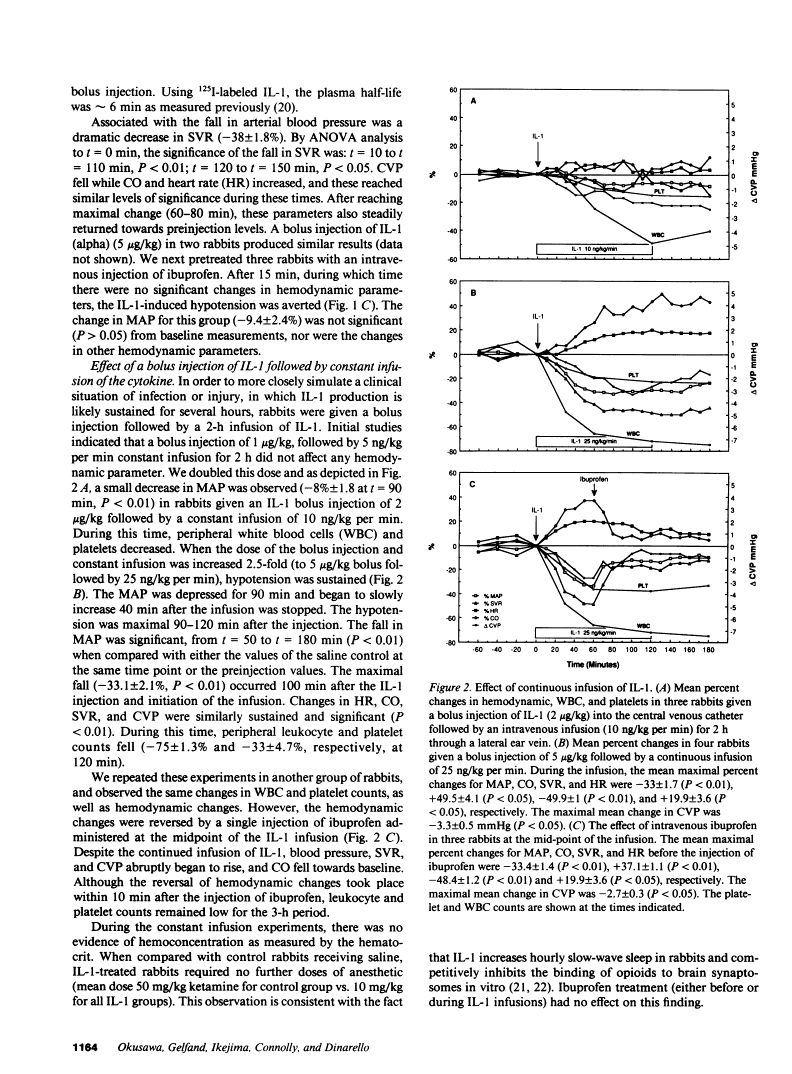
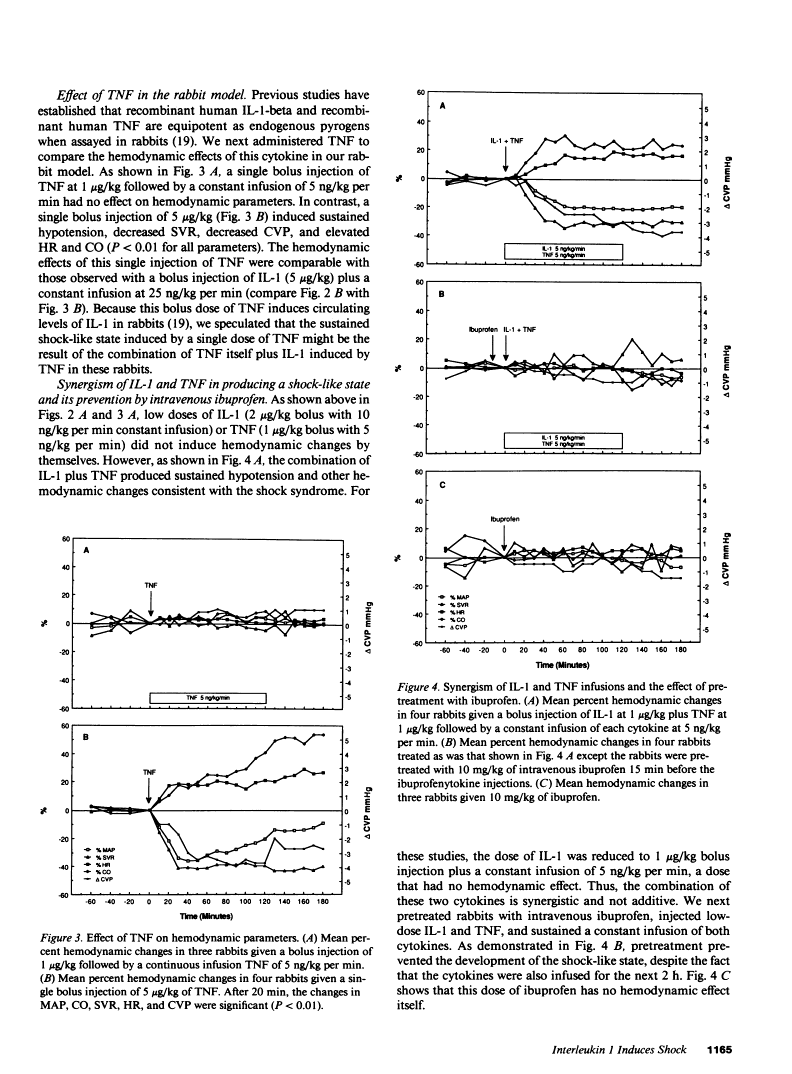
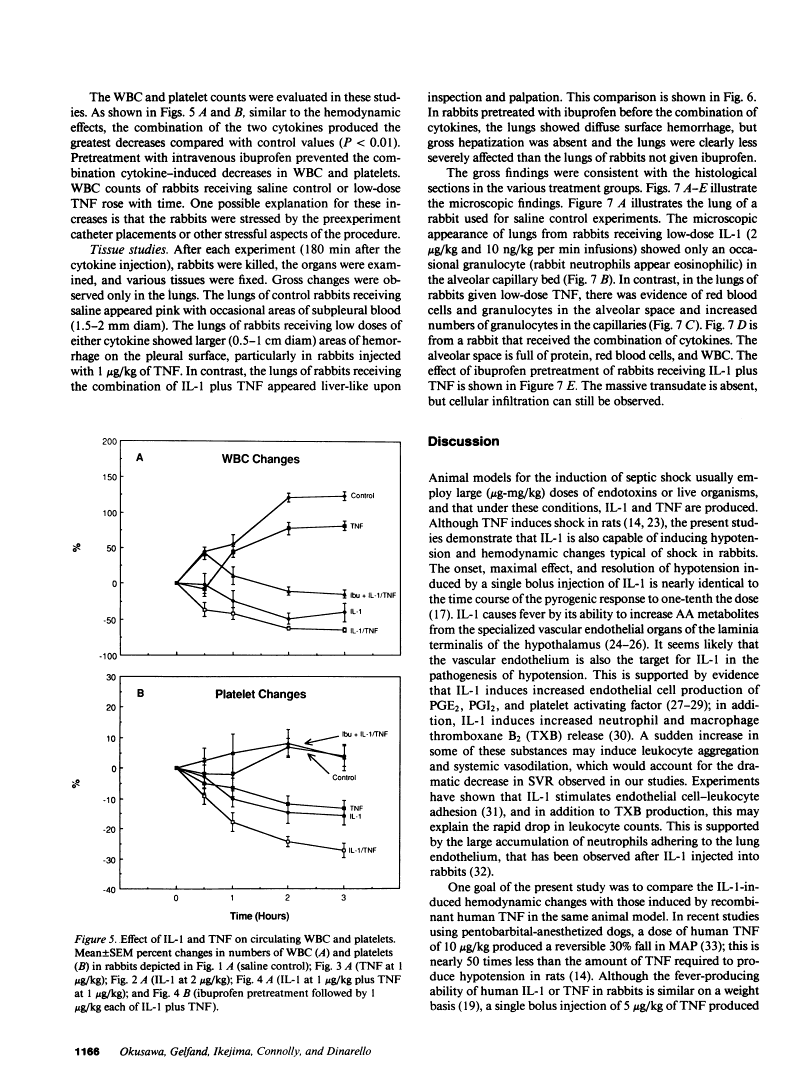
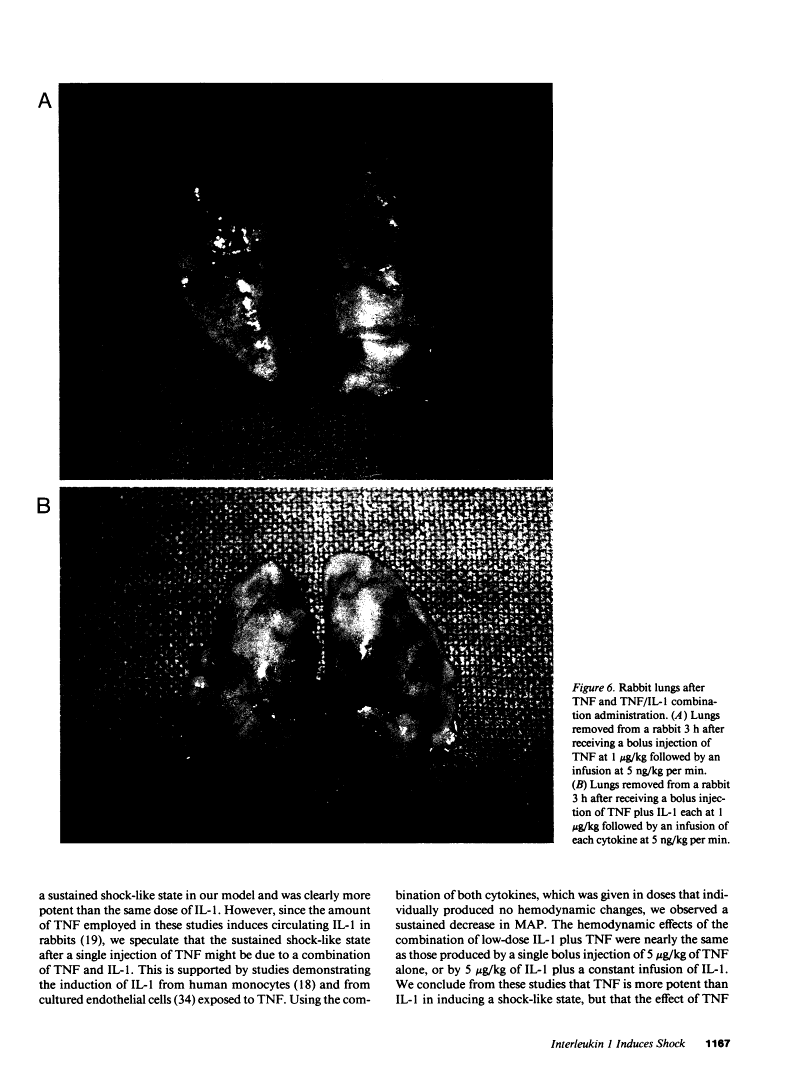
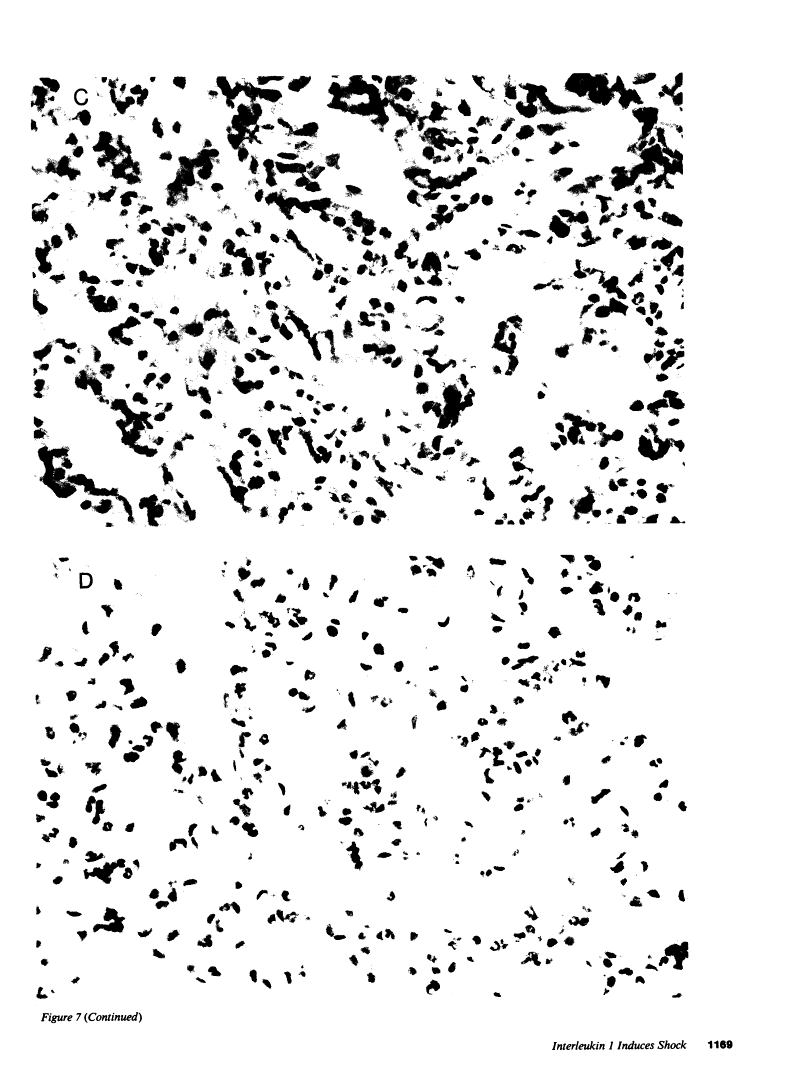
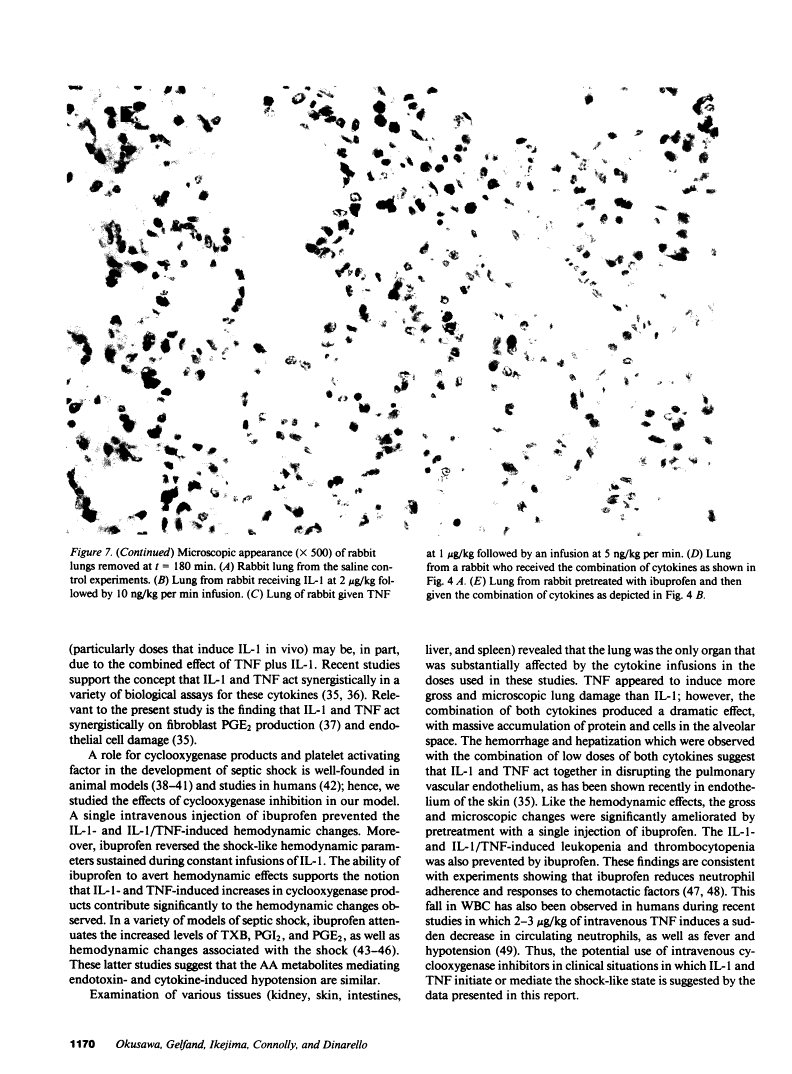
In addition to activating T and B lymphocytes, interleukin 1 (IL-1) induces several hematologic and metabolic changes typical of host responses to infection and injury. We now report a new biological property, namely, the induction of hypotension. Rabbits given a single intravenous injection of recombinant human IL-1-beta (5 micrograms/kg) rapidly developed decreased systemic arterial pressure, which reached the lowest levels after 50-60 min and slowly returned to pre-IL-1 values after 3 h. Associated with the hypotension, systemic vascular resistance and central venous pressure fell, while cardiac output and heart rate increased. These responses were prevented by ibuprofen given 15 min before the IL-1. A bolus injection of IL-1 followed by a 2-h infusion sustained the hypotension and was associated with leukopenia and thrombocytopenia. Ibuprofen given at the mid-point of the infusion reversed the changes in all hemodynamic parameters, but had no effect on the leukopenia or thrombocytopenia. Tumor necrosis factor (TNF) also induced a shock-like state in rabbits. When the dose of IL-1 or TNF was reduced to 1 microgram/kg, no hemodynamic changes were observed; however, the combination of these low doses of both cytokines resulted in a profound shock-like state including histological evidence of severe pulmonary edema and hemorrhage. Pretreatment with ibuprofen prevented the hemodynamic, leukocyte, and platelet changes induced by the low-dose cytokine combination, and ameliorated the pulmonary tissue damage. These results demonstrate that IL-1, like TNF, possesses the ability to induce hemodynamic and hematological changes typical of septic shock, and that the combination of IL-1 and TNF is more potent than either agent alone. These effects seem to require cyclooxygenase products, and suggest that intravenous cyclooxygenase inhibitors may be of therapeutic value in patients with IL-1/TNF-mediated shock.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (4.2M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Kampschmidt RF. Infection, inflammation, and interleukin 1 (IL-1). Lymphokine Res. 1983;2(3):97–102. [Abstract] [Google Scholar]
- Besedovsky H, del Rey A, Sorkin E, Dinarello CA. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science. 1986 Aug 8;233(4764):652–654. [Abstract] [Google Scholar]
- Dinarello CA. Interleukin-1: amino acid sequences, multiple biological activities and comparison with tumor necrosis factor (cachectin). Year Immunol. 1986;2:68–89. [Abstract] [Google Scholar]
- Lomedico PT, Gubler U, Hellmann CP, Dukovich M, Giri JG, Pan YC, Collier K, Semionow R, Chua AO, Mizel SB. Cloning and expression of murine interleukin-1 cDNA in Escherichia coli. Nature. 312(5993):458–462. [Abstract] [Google Scholar]
- Auron PE, Webb AC, Rosenwasser LJ, Mucci SF, Rich A, Wolff SM, Dinarello CA. Nucleotide sequence of human monocyte interleukin 1 precursor cDNA. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7907–7911. [Europe PMC free article] [Abstract] [Google Scholar]
- Duff GW, Atkins E. The detection of endotoxin by in vitro production of endogenous pyrogen: comparison with limulus amebocyte lysate gelation. J Immunol Methods. 1982 Aug 13;52(3):323–331. [Abstract] [Google Scholar]
- Ikejima T, Dinarello CA, Gill DM, Wolff SM. Induction of human interleukin-1 by a product of Staphylococcus aureus associated with toxic shock syndrome. J Clin Invest. 1984 May;73(5):1312–1320. [Europe PMC free article] [Abstract] [Google Scholar]
- Okusawa S, Dinarello CA, Yancey KB, Endres S, Lawley TJ, Frank MM, Burke JF, Gelfand JA. C5a induction of human interleukin 1. Synergistic effect with endotoxin or interferon-gamma. J Immunol. 1987 Oct 15;139(8):2635–2640. [Abstract] [Google Scholar]
- Cannon JG, Dinarello CA. Increased plasma interleukin-1 activity in women after ovulation. Science. 1985 Mar 8;227(4691):1247–1249. [Abstract] [Google Scholar]
- Lonnemann G, Bingel M, Koch KM, Shaldon S, Dinarello CA. Plasma interleukin-1 activity in humans undergoing hemodialysis with regenerated cellulosic membranes. Lymphokine Res. 1987 Spring;6(2):63–70. [Abstract] [Google Scholar]
- Cannon JG, Kluger MJ. Endogenous pyrogen activity in human plasma after exercise. Science. 1983 May 6;220(4597):617–619. [Abstract] [Google Scholar]
- Beutler B, Milsark IW, Cerami AC. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. [Abstract] [Google Scholar]
- Tracey KJ, Beutler B, Lowry SF, Merryweather J, Wolpe S, Milsark IW, Hariri RJ, Fahey TJ, 3rd, Zentella A, Albert JD, et al. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. [Abstract] [Google Scholar]
- Wolff SM. Biological effects of bacterial endotoxins in man. J Infect Dis. 1973 Jul;128(Suppl):259–264. [Abstract] [Google Scholar]
- Simon GL, Gelfand JA, Connolly RA, O'Donnell TF, Jr, Gorbach SL. Experimental Bacteroides fragilis bacteremia in a primate model: evidence that Bacteroides fragilis does not promote the septic shock syndrome. J Trauma. 1985 Dec;25(12):1156–1162. [Abstract] [Google Scholar]
- Dinarello CA, Cannon JG, Mier JW, Bernheim HA, LoPreste G, Lynn DL, Love RN, Webb AC, Auron PE, Reuben RC, et al. Multiple biological activities of human recombinant interleukin 1. J Clin Invest. 1986 Jun;77(6):1734–1739. [Europe PMC free article] [Abstract] [Google Scholar]
- Gubler U, Chua AO, Stern AS, Hellmann CP, Vitek MP, DeChiara TM, Benjamin WR, Collier KJ, Dukovich M, Familletti PC, et al. Recombinant human interleukin 1 alpha: purification and biological characterization. J Immunol. 1986 Apr 1;136(7):2492–2497. [Abstract] [Google Scholar]
- Dinarello CA, Cannon JG, Wolff SM, Bernheim HA, Beutler B, Cerami A, Figari IS, Palladino MA, Jr, O'Connor JV. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. [Europe PMC free article] [Abstract] [Google Scholar]
- Dinarello CA, Ikejima T, Warner SJ, Orencole SF, Lonnemann G, Cannon JG, Libby P. Interleukin 1 induces interleukin 1. I. Induction of circulating interleukin 1 in rabbits in vivo and in human mononuclear cells in vitro. J Immunol. 1987 Sep 15;139(6):1902–1910. [Abstract] [Google Scholar]
- Krueger JM, Walter J, Dinarello CA, Wolff SM, Chedid L. Sleep-promoting effects of endogenous pyrogen (interleukin-1). Am J Physiol. 1984 Jun;246(6 Pt 2):R994–R999. [Abstract] [Google Scholar]
- Ahmed MS, Llanos-Q J, Dinarello CA, Blatteis CM. Interleukin 1 reduces opioid binding in guinea pig brain. Peptides. 1985 Nov-Dec;6(6):1149–1154. [Abstract] [Google Scholar]
- Beutler B, Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. [Abstract] [Google Scholar]
- Blatteis CM, Bealer SL, Hunter WS, Llanos-Q J, Ahokas RA, Mashburn TA., Jr Suppression of fever after lesions of the anteroventral third ventricle in guinea pigs. Brain Res Bull. 1983 Nov;11(5):519–526. [Abstract] [Google Scholar]
- Stitt JT. Evidence for the involvement of the organum vasculosum laminae terminalis in the febrile response of rabbits and rats. J Physiol. 1985 Nov;368:501–511. [Abstract] [Google Scholar]
- Coceani F, Bishai I, Lees J, Sirko S. Prostaglandin E2 and fever: a continuing debate. Yale J Biol Med. 1986 Mar-Apr;59(2):169–174. [Europe PMC free article] [Abstract] [Google Scholar]
- Dejana E, Breviario F, Erroi A, Bussolino F, Mussoni L, Gramse M, Pintucci G, Casali B, Dinarello CA, Van Damme J, et al. Modulation of endothelial cell functions by different molecular species of interleukin 1. Blood. 1987 Feb;69(2):695–699. [Abstract] [Google Scholar]
- Albrightson CR, Baenziger NL, Needleman P. Exaggerated human vascular cell prostaglandin biosynthesis mediated by monocytes: role of monokines and interleukin 1. J Immunol. 1985 Sep;135(3):1872–1877. [Abstract] [Google Scholar]
- Conti P, Cifone MG, Alesse E, Reale M, Fieschi C, Dinarello CA. In vitro enhanced thromboxane B2 release by polymorphonuclear leukocytes and macrophages after treatment with human recombinant interleukin 1. Prostaglandins. 1986 Jul;32(1):111–115. [Abstract] [Google Scholar]
- Bevilacqua MP, Pober JS, Wheeler ME, Cotran RS, Gimbrone MA., Jr Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985 Nov;76(5):2003–2011. [Europe PMC free article] [Abstract] [Google Scholar]
- Goldblum SE, Cohen DA, Gillespie MN, McClain CJ. Interleukin-1-induced granulocytopenia and pulmonary leukostasis in rabbits. J Appl Physiol (1985) 1987 Jan;62(1):122–128. [Abstract] [Google Scholar]
- Tracey KJ, Lowry SF, Fahey TJ, 3rd, Albert JD, Fong Y, Hesse D, Beutler B, Manogue KR, Calvano S, Wei H, et al. Cachectin/tumor necrosis factor induces lethal shock and stress hormone responses in the dog. Surg Gynecol Obstet. 1987 May;164(5):415–422. [Abstract] [Google Scholar]
- Libby P, Ordovas JM, Auger KR, Robbins AH, Birinyi LK, Dinarello CA. Endotoxin and tumor necrosis factor induce interleukin-1 gene expression in adult human vascular endothelial cells. Am J Pathol. 1986 Aug;124(2):179–185. [Europe PMC free article] [Abstract] [Google Scholar]
- Elias JA, Gustilo K, Baeder W, Freundlich B. Synergistic stimulation of fibroblast prostaglandin production by recombinant interleukin 1 and tumor necrosis factor. J Immunol. 1987 Jun 1;138(11):3812–3816. [Abstract] [Google Scholar]
- Halushka PV, Cook JA, Wise WC. Beneficial effects of UK 37248, a thromboxane synthetase inhibitor, in experimental endotoxic shock in the rat. Br J Clin Pharmacol. 1983;15 (Suppl 1):133S–139S. [Europe PMC free article] [Abstract] [Google Scholar]
- Slotman GJ, Quinn JV, Burchard KW, Gann DS. Thromboxane, prostacyclin, and the hemodynamic effects of graded bacteremic shock. Circ Shock. 1985;16(4):395–404. [Abstract] [Google Scholar]
- Wise WC, Cook JA, Halushka PV. Implications for thromboxane A2 in the pathogenesis of endotoxic shock. Adv Shock Res. 1981;6:83–91. [Abstract] [Google Scholar]
- Chang SW, Feddersen CO, Henson PM, Voelkel NF. Platelet-activating factor mediates hemodynamic changes and lung injury in endotoxin-treated rats. J Clin Invest. 1987 May;79(5):1498–1509. [Europe PMC free article] [Abstract] [Google Scholar]
- Slotman GJ, Burchard KW, Williams JJ, D'Arezzo A, Yellin SA. Interaction of prostaglandins, activated complement, and granulocytes in clinical sepsis and hypotension. Surgery. 1986 Jun;99(6):744–751. [Abstract] [Google Scholar]
- Jacobs ER, Soulsby ME, Bone RC, Wilson FJ, Jr, Hiller FC. Ibuprofen in canine endotoxin shock. J Clin Invest. 1982 Sep;70(3):536–541. [Europe PMC free article] [Abstract] [Google Scholar]
- Almqvist PM, Kuenzig M, Schwartz SI. Treatment of experimental canine endotoxin shock with ibuprofen, a cyclooxygenase inhibitor. Circ Shock. 1984;13(3):227–232. [Abstract] [Google Scholar]
- Toth PD, Hamburger SA, Judy WV. The effects of vasoactive mediator antagonists on endotoxic shock in dogs. I. Circ Shock. 1984;12(4):277–286. [Abstract] [Google Scholar]
- Fink MP, MacVittie TJ, Casey LC. Inhibition of prostaglandin synthesis restores normal hemodynamics in canine hyperdynamic sepsis. Ann Surg. 1984 Nov;200(5):619–626. [Abstract] [Google Scholar]
- Maderazo EG, Breaux SP, Woronick CL. Protective effects of ibuprofen and methylprednisolone on chemotactic factor-induced transcutaneous hypoxia. J Pharmacol Exp Ther. 1986 Aug;238(2):453–456. [Abstract] [Google Scholar]
- Venezio FR, DiVincenzo C, Pearlman F, Phair JP. Effects of the newer nonsteroidal anti-inflammatory agents, ibuprofen, fenoprofen, and sulindac, on neutrophil adherence. J Infect Dis. 1985 Oct;152(4):690–694. [Abstract] [Google Scholar]
- Chapman PB, Lester TJ, Casper ES, Gabrilove JL, Wong GY, Kempin SJ, Gold PJ, Welt S, Warren RS, Starnes HF, et al. Clinical pharmacology of recombinant human tumor necrosis factor in patients with advanced cancer. J Clin Oncol. 1987 Dec;5(12):1942–1951. [Abstract] [Google Scholar]
Associated Data
Articles from The Journal of Clinical Investigation are provided here courtesy of American Society for Clinical Investigation
Full text links
Read article at publisher's site: https://doi.org/10.1172/jci113431
Read article for free, from open access legal sources, via Unpaywall:
http://www.jci.org/articles/view/113431/files/pdf
Citations & impact
Impact metrics
Article citations
A pairwise cytokine code explains the organism-wide response to sepsis.
Nat Immunol, 25(2):226-239, 08 Jan 2024
Cited by: 8 articles | PMID: 38191855 | PMCID: PMC10834370
Lactobacillus reuteri Ameliorates Lipopolysaccharide-Induced Acute Lung Injury by Modulating the Gut Microbiota in Mice.
Nutrients, 15(19):4256, 04 Oct 2023
Cited by: 0 articles | PMID: 37836540 | PMCID: PMC10574429
Role of SARS-CoV-2-induced cytokine storm in multi-organ failure: Molecular pathways and potential therapeutic options.
Int Immunopharmacol, 113(pt b):109428, 07 Nov 2022
Cited by: 21 articles | PMID: 36379152 | PMCID: PMC9637536
Review Free full text in Europe PMC
The noncanonical inflammasome in health and disease.
Infect Med (Beijing), 1(3):208-216, 11 Sep 2022
Cited by: 0 articles | PMID: 38077630 | PMCID: PMC10699704
Review Free full text in Europe PMC
Targeting the NOD-Like Receptor Pyrin Domain Containing 3 Inflammasome to Improve Healing of Diabetic Wounds.
Adv Wound Care (New Rochelle), 12(11):644-656, 13 Jan 2022
Cited by: 7 articles | PMID: 34841901 | PMCID: PMC10701516
Review Free full text in Europe PMC
Go to all (529) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Interleukin-1 induces a shock-like state in rabbits: synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition.
Prog Clin Biol Res, 286:243-263, 01 Jan 1989
Cited by: 3 articles | PMID: 2494673
Interleukin-1 (IL-1) receptor antagonist prevents Staphylococcus epidermidis-induced hypotension and reduces circulating levels of tumor necrosis factor and IL-1 beta in rabbits.
Infect Immun, 61(8):3342-3350, 01 Aug 1993
Cited by: 33 articles | PMID: 8335365 | PMCID: PMC281009
The influence of antibodies to TNF-alpha and IL-1beta on haemodynamic responses to the cytokines, and to lipopolysaccharide, in conscious rats.
Br J Pharmacol, 125(7):1543-1550, 01 Dec 1998
Cited by: 23 articles | PMID: 9884083 | PMCID: PMC1565745
[Cytokines and antagonists in septic shock].
Schweiz Med Wochenschr, 123(11):480-491, 01 Mar 1993
Cited by: 3 articles | PMID: 8386392
Review
Funding
Funders who supported this work.
NIAID NIH HHS (1)
Grant ID: AI-15614
NICHD NIH HHS (1)
Grant ID: HD-19675
NIGMS NIH HHS (1)
Grant ID: GM-21700