Abstract
Background
Patients with COVID-19 usually manifest fever and respiratory symptoms. However, some patients also experience gastrointestinal (GI) symptoms such as diarrhea, vomiting and abdominal pain. In addition, SARS-CoV-2 RNA has been detected in feces of infected patients. Currently there is huge evolving research interest in this potentially lethal disease. We systematically reviewed and meta-analyzed the evidence suggesting involvement of the digestive system in COVID-19.Methods
PubMed, Medline and Embase databases were searched up to 10 April 2020, using suitable keywords. Individual and pooled prevalence rates with 95% confidence intervals (CI) were calculated using the fixed- or random-effects model as appropriate. Heterogeneity between studies was calculated employing the Cochran Q test and I2 values, whereas the possibility of publication bias was examined by constructing funnel plots. Additionally, subgroup and sensitivity analyses were performed.Results
In adult COVID-19 patients, the prevalence rates (95%CI) for all GI symptoms, and separately for diarrhea, nausea/vomiting, and abdominal discomfort/pain were 9.8% (6.4-14.7), 10.4% (95%CI 7.7-13.9), 7.7% (95%CI 4.8-12.1), and 6.9% (95%CI 3.9-11.9) respectively. The prevalence rates for children were 9.6% (95%CI 6.3-14.3) for all symptoms, 9.6% (95%CI 6.3-14.3) for diarrhea, and 6.8% (95% CI 4.2-11) for nausea/vomiting. In 30.3% (95%CI 10.5-61.6) of the patients SARS-CoV-2 RNA was detected in feces.Conclusions
A percentage of patients with COVID-19 will manifest symptoms from the digestive system. The GI tract may be a target organ and potential transmission route of SARS-CoV-2, with important implications for disease management and transmission.Free full text

Gastrointestinal involvement in COVID-19: a systematic review and meta-analysis
Abstract
Background:
Patients with COVID-19 usually manifest fever and respiratory symptoms. However, some patients also experience gastrointestinal (GI) symptoms such as diarrhea, vomiting and abdominal pain. In addition, SARS-CoV-2 RNA has been detected in feces of infected patients. Currently there is huge evolving research interest in this potentially lethal disease. We systematically reviewed and meta-analyzed the evidence suggesting involvement of the digestive system in COVID-19.
Methods:
PubMed, Medline and Embase databases were searched up to 10 April 2020, using suitable keywords. Individual and pooled prevalence rates with 95% confidence intervals (CI) were calculated using the fixed- or random-effects model as appropriate. Heterogeneity between studies was calculated employing the Cochran Q test and I2 values, whereas the possibility of publication bias was examined by constructing funnel plots. Additionally, subgroup and sensitivity analyses were performed.
Results:
In adult COVID-19 patients, the prevalence rates (95%CI) for all GI symptoms, and separately for diarrhea, nausea/vomiting, and abdominal discomfort/pain were 9.8% (6.4-14.7), 10.4% (95%CI 7.7-13.9), 7.7% (95%CI 4.8-12.1), and 6.9% (95%CI 3.9-11.9) respectively. The prevalence rates for children were 9.6% (95%CI 6.3-14.3) for all symptoms, 9.6% (95%CI 6.3-14.3) for diarrhea, and 6.8% (95% CI 4.2-11) for nausea/vomiting. In 30.3% (95%CI 10.5-61.6) of the patients SARS-CoV-2 RNA was detected in feces.
Conclusions:
A percentage of patients with COVID-19 will manifest symptoms from the digestive system. The GI tract may be a target organ and potential transmission route of SARS-CoV-2, with important implications for disease management and transmission.
Introduction
At the end of 2019, a cohort of unidentified viral pneumonia cases was first reported in Wuhan, the capital city of Hubei Province in China [1]. Soon, the virus responsible was identified [2] and named SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) by the International Committee on Taxonomy of Viruses. SARS-CoV-2 belongs to the beta-coronavirus genus, which also includes the SARS-CoV and the Middle East Respiratory Syndrome Coronavirus (MERS-CoV), and is a single-stranded RNA virus that genetically strongly resembles the bat coronaviruses [3]. However, the intermediate reservoir has yet to be found [3,4]. Consequently, the disease caused by this novel virus was named COVID-19 (coronavirus disease 2019) by the World Health Organization [5]; it is the third coronavirus identified to cause severe lung disease in humans, like the other two previous coronaviruses, i.e., SARS-CoV and MERS-CoV [5,6]. After Wuhan, SARS-CoV, because of its high infectivity due to nonexistent immunity in the community, rapidly spread to the rest of China and then the rest of the world, causing a major pandemic and undoubtedly an ongoing global health crisis.
Most COVID-19 patients typically present with fever and respiratory symptoms [1,4,6]. However, some patients also have gastrointestinal (GI) manifestations, such as diarrhea, nausea, vomiting and abdominal pain [4,6]; furthermore, in some COVID-19 patients SARS-CoV-2 RNA was identified in anal/rectal swabs and stool specimens [7-10]. Interestingly, in some COVID-19 patients, SARS-CoV-2 RNA was detected in feces even after the clearance of the virus in the upper respiratory tract [7,8,11]. Moreover, former studies [12] have shown that SARS-CoV receptor ACE2 (angiotensin-converting enzyme 2) is expressed in small intestine epithelial cells. All the above suggest that SARS-CoV-2 can actively infect and replicate in the GI tract, with important implications for disease management, transmission and infection control. Therefore, reviews and critical evaluations of the rapidly evolving research evidence on this potentially lethal disease are vitally necessary. In this study we systematically reviewed and meta-analyzed the current evidence suggesting involvement of the digestive system in COVID-19.
Materials and methods
Study identification and extraction of data
PubMed, MEDLINE and Embase database medical literature searches for human studies were performed up to the 20th of April 2020, using suitable keywords, i.e., ((“COVID-19”[All Fields] OR “COVID-2019”[All Fields] OR “severe acute respiratory syndrome coronavirus 2”[Supplementary Concept] OR “severe acute respiratory syndrome coronavirus 2”[All Fields] OR “2019-nCoV”[All Fields] OR “SARS-CoV-2”[All Fields] OR “2019nCoV”[All Fields] OR ((“Wuhan”[All Fields] AND (“coronavirus”[MeSH Terms] OR “coronavirus”[All Fields])) AND (2019/12[PDAT] OR 2020[PDAT]))) OR (“severe acute respiratory syndrome coronavirus 2”[Supplementary Concept] OR “severe acute respiratory syndrome coronavirus 2”[All Fields] OR “sars cov 2”[All Fields])) AND GASTROINTESTINAL[All Fields] AND (MANIFESTATIONS[All Fields] OR INVOLVEMENT[All Fields] OR (“gastrointestinal tract”[MeSH Terms] OR (“gastrointestinal”[All Fields] AND “tract”[All Fields]) OR “gastrointestinal tract”[All Fields] OR (“gi”[All Fields] AND “tract”[All Fields]) OR “gi tract”[All Fields])). In addition, a manual search of all review articles, editorials and retrieved original studies was also performed. The meta-analysis was performed in accordance with PRISMA [13].
Selection criteria
Inclusion and exclusion criteria were defined prior to the literature search. Hence, the following criteria had to be met for studies to be included in this meta-analysis: 1) published as full articles or letters to the Editor; 2) written in English; and 3) cohort studies, with extractable data concerning the involvement of the digestive system in SARS-Cov-2 infection, including at least 15 patients. Exclusion criteria were the following: 1) studies not meeting the inclusion criteria; 2) studies without data for retrieval; 3) duplicate studies; and 4) the less informative publication of two on the same study. Concerning quality, the cohort studies included in this meta-analysis were assessed by using the Newcastle-Ottawa Quality Assessment Scale for cohort studies [14]. This scale assesses the main sources of bias, including the selection of study cohorts, comparability of cohorts, and outcome assessment. Furthermore, it uses a “star system” to judge a study on these three broad perspectives, with a maximum possible score of 9. According to the score, and therefore the relevant risk of bias, studies were classified as low, fair, and high quality.
Statistical analysis
The statistical analyses used were described in detail in our previous publications [15,16]. Briefly, the individual and the pooled prevalence rates, with 95% confidence intervals (CI), were derived by employing the fixed-effects or the random-effects model, as appropriate [17,18], and were depicted in constructed forest plots for visual display. Heterogeneity was measured with the Cochran Q test and the I2 statistic [19,20]. In addition, we used cumulative meta-analysis to explore whether the calculated prevalence rates remained steady [19]. The Comprehensive Meta-Analysis software, version 2 (BIOSTAT INC., Englewood, NJ, USA) and Stata statistics (Stata 13.2, StataCorp, College Station, TX) were used throughout this meta-analysis.
Sensitivity analyses and publication bias
In case of significant heterogeneity, sensitivity analyses were employed to check the consistency of the results. Thus, to explore any significant influence of single studies on the pooled prevalence rates, we performed the exclusion method by repeating the meta-analysis after excluding single studies one at a time [19]. The possibility of publication bias was examined by constructing funnel plots [21,22]. Their symmetry was measured by Egger’s regression test and the Begg and Mazumdar adjusted rank correlation test [23,24].
Results
Descriptive assessment and characteristics of the studies
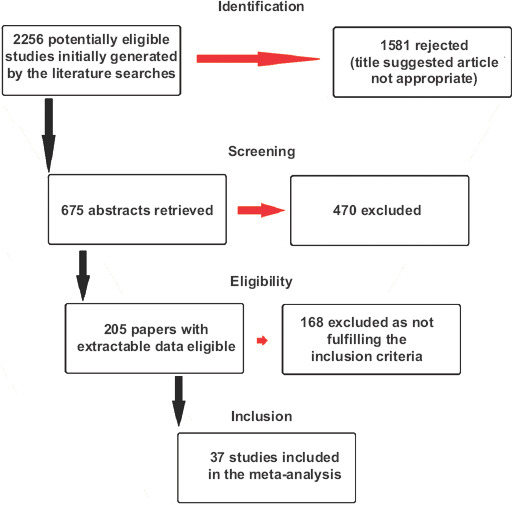
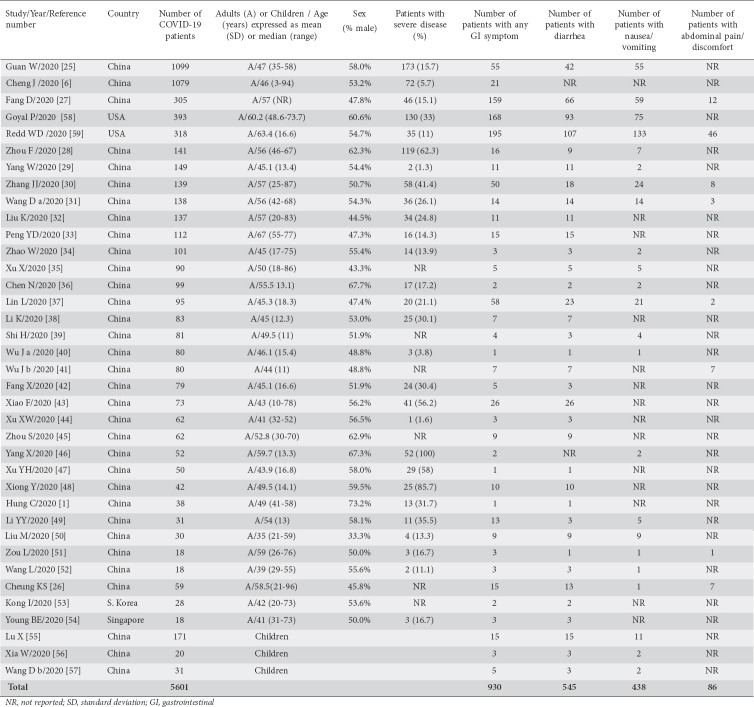
A flowchart depicting the study selection is shown in Fig. 1. Of the 2256 titles initially found by the literature searches, 37 cohort studies were found suitable for meta-analysis [1,6,25-59]. There were 33 studies from China [1,25-52,55-57], 1 from South Korea [53], 1 from Singapore [54], and 2 from the USA [58,59], including a total of 5601 patients. Thirty-four studies [1,6,25-54,58,59] reported data from adults and 3 studies [55-57] from children. The main characteristics of the 37 studies included in the meta-analysis are shown in Table 1. According to the Newcastle-Ottawa Quality Assessment Scale, 5 studies (13.5%) were classified as high quality, 10 (27%) as fair, and 22 (59.5%) as low quality.
Table 1
The main characteristics of the studies included in the meta-analysis
Prevalence rates of GI manifestations
a. All GI symptoms
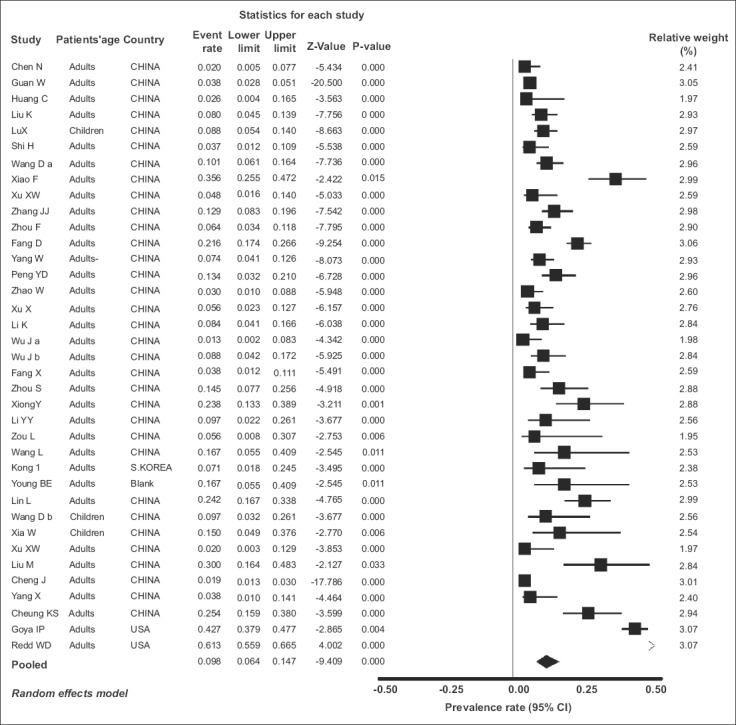
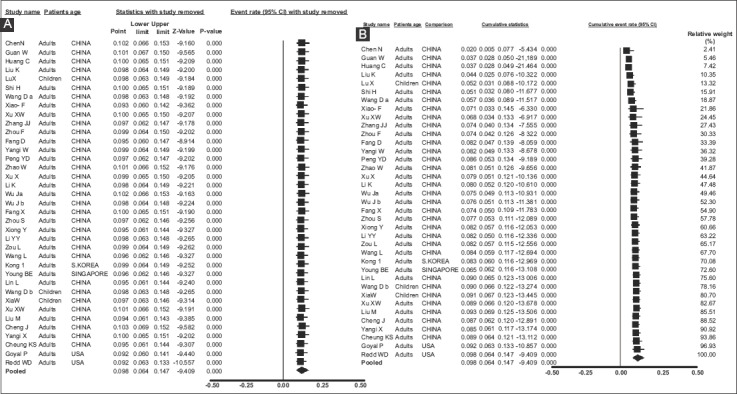
All 37 included studies [1,6,25-59] reported one or more GI symptom in their total of 5601 COVID-19 patients. The individual and pooled prevalence rates for all symptoms are shown in Fig. 2. Since there was significant heterogeneity in the included studies [Q=833.67, df(Q)= 36, I2=95.68%, P<0.001], a random-effects model was applied, which yielded a pooled prevalence rate of 9.8% (95%CI 6.4-14.7) with a test for overall effect Z=-9.409, P<0.001. In addition, significant publication bias was found [asymmetry estimation P (2-tailed) value 0.001 by Egger’s regression test], as seen in the funnel plot (Fig. 3). Furthermore, sensitivity analyses were utilized to detect the presence of any biases related to the inclusion of different population studies. Thus, the exclusion of any study did not alter the summary results substantially (Fig. 4A). Similarly, the cumulative meta-analysis did not alter the results significantly (Fig. 4B).
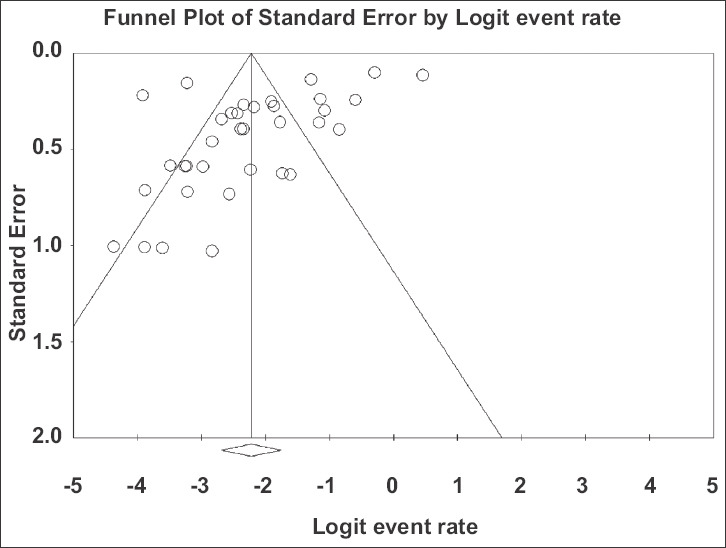
Funnel plot of all studies included in the meta-analysis. There is evidence of publication bias (P=0.001 by regression test)
b. Diarrhea
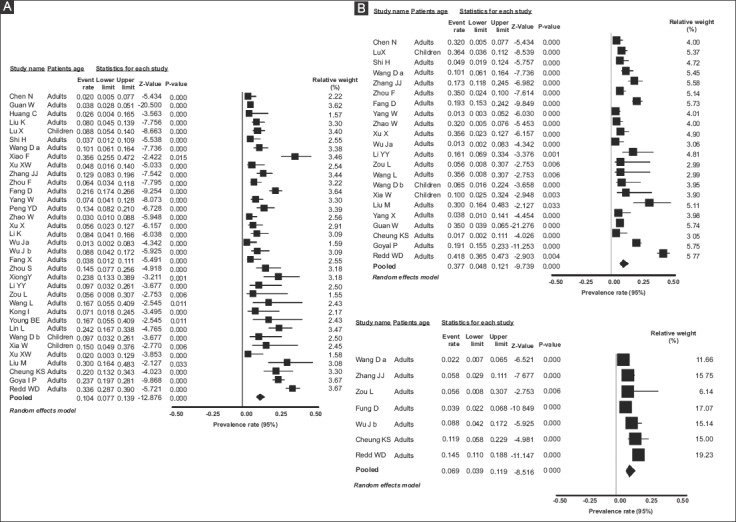
Thirty-five studies [1,25-45,47-59] reported diarrhea in their COVID-19 patients. The individual and pooled prevalence rates are shown in Fig. 5A. Since there was significant heterogeneity in the included studies [Q=329.15, df(Q)= 34, I2=89.27%, P<0.001], a random-effects model was applied, which yielded a pooled prevalence rate of 10.4% (95%CI 7.7-13.9) with a test for overall effect Z=-12.87, P<0.001.
c. Nausea/vomiting
Twenty-two studies [25-31,34-36,39,40,46,49-52,55-59] reported nausea/vomiting in their COVID-19 patients. The individual and pooled prevalence rates for this symptom are shown in Fig. 5B. Similarly to diarrhea, there was significant heterogeneity in the included studies [Q=326.56, df(Q)= 21, I2=93.57%, P<0.001]; therefore, a random-effects model was applied, which yielded a pooled prevalence rate of 7.7% (95%CI 4.8-12.1) with a test for overall effect Z=-9.74, P<0.001.
d. Abdominal discomfort/pain
Seven studies [26,27,30,31,41,51,59] reported abdominal discomfort/pain in their COVID-19 patients. The individual and pooled prevalence rates for all symptoms are shown in Fig. 5C. Since there was significant heterogeneity in the included studies [Q=29.12, df(Q)=6, I2=79.45%, P<0.001], a random-effects model was applied, which yielded a pooled prevalence rate of 6.9% (95%CI 3.9-11.9) with a test for overall effect Z=-8.51, P<0.001.
e. Fecal detection of SARS-CoV-2 RNA
Three studies [26,30,43] reported fecal detection of SARS-CoV-2 RNA in their COVID-19 patients. A total of 147 patients were included in these studies and in 52 viral RNA was detected in feces. Thus, the prevalence rate for SARS-CoV-2 RNA positivity was 30.3% (95%CI 10.5-61.6) as shown in Fig. 6A.
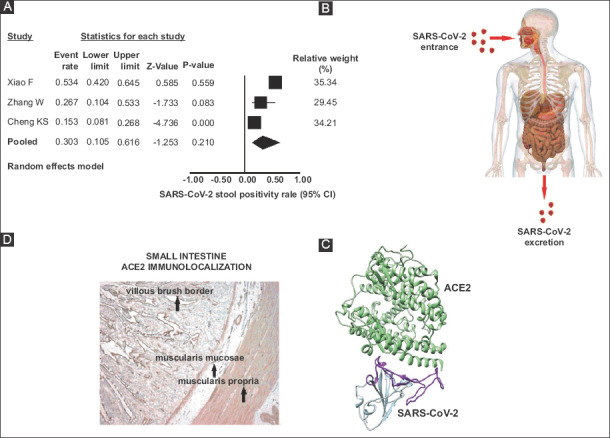
(A) Forest plot showing individual and pooled prevalence ratios (95% confidence interval) of SARS-CoV-2 RNA detection in feces (B) Cartoon representation of the SARS-CoV-2 fecal route transmission. (C) Ribbon diagram depicting the SARS-CoV-2 attachment to ACE2 receptors (modified from ref. 66) (D) ACE2 small intestine immunolocalization (modified from ref. 12)
ACE2, angiotensin-converting enzyme 2
Subgroup analyses
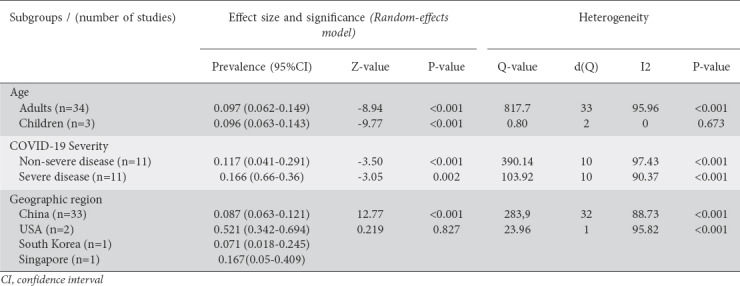
In order to examine the influence of factors such as the patient’s age, COVID-19 severity and geographic region on the results of this meta-analysis, we performed the respective subgroup analyses and the results are shown in detail in Table 2. Thus, the prevalence rate concerning symptoms was similar in adults and children. The prevalence rate (95%CI) for all symptoms was 9.8% (6.4-14.7) in adults and 9.6% (6.3-14.3) in children. Concerning disease severity, in patients with severe COVID-19 the prevalence of GI symptoms was 16.6% (6.6-35.9), higher than in patients with non-severe COVID-19, who showed a prevalence of 11.7% (4.1-29.1).
Table 2
Subgroup analyses of prevalence of gastrointestinal symptoms in COVID-19 patients by age, disease severity and geographic region
Discussion
The novel SARS-CoV-2, because of its high infectivity, is currently causing a major pandemic and undoubtedly constitutes a global health crisis. Consequently, in the short 3-month period (January to March) since December 2019, we have been faced with an explosion of publications concerning COVID-19 cohorts of patients. COVID-19 patients commonly develop fever and respiratory illness. However, some patients also report GI symptoms such as diarrhea, vomiting and abdominal pain. This meta-analysis pooled current individual data concerning the involvement of the digestive system in COVID-19 and showed that a percentage of the COVID-19 patients will manifest GI symptoms. Thus, the pooled prevalence rate for all symptoms was 8.9%, while the separate rates were 9.6% for diarrhea, 6.6% for nausea/vomiting and 5.8% for abdominal discomfort/pain. In the subgroup analysis in adult patients these rates were 8.7%, 9.6%, 6.5% and 5.8%, respectively. In children the pooled figures were 9% for all symptoms, 9% for diarrhea and 6.8% for nausea/vomiting. In addition, the prevalence of GI symptoms in patients with severe COVID-19 (16.6%) was higher than in non-severe COVID-19 (11.7%). Finally, in over 30% of patients SARS-CoV-2 RNA was detected in feces by reverse-transcriptase polymerase-chain-reaction (RT-PCR).
Concerning the quality of the included studies, 13.5% were classified as high, 27% as fair, and 59.5% as low quality. This is expected in meta-analyses of observational studies. While this meta-analysis was in preparation, an “ahead of print e-publication” cohort study, accompanied by meta-analysis, has emerged [26]. This study included almost exclusively Chinese data. At this point it must be stressed that in our meta-analysis, apart from Chinese data, we have included the first data coming from other countries (USA), with a reasonable number of patients [58,59], which differentiates the results. Overall, GI symptoms appeared to be more common in US rather than in Chinese patients. This difference remains to be confirmed by more studies and it could reflect geographic variation or differential reporting. In addition, in our meta-analysis the inclusion threshold was 15 patients whereas the above-mentioned publication included numerous studies even with one patient, which in meta-analysis methodology constitutes a severe limitation.
During the outbreak of SARS-CoV disease in 2003, among other symptoms, approximately 20% of the patients manifested GI symptoms [60,61]. GI symptoms were also noted in MERS-CoV disease [62], with cohorts reporting symptoms in 11.5-32% of the patients. Interestingly, there is evidence showing that coronaviruses show a tropism to the GI tract, which might explain the frequent occurrence of diarrhea in coronavirus infections. Thus, SARS-CoV RNA was detected readily in stool specimens of SARS patients [63], with active viral replications seen by electron microscopy on biopsy or autopsy specimens of small and large intestines [61]. Similarly, it has been shown that MERS-CoV often resulted in enteric infection, with human intestinal epithelial cells being highly susceptible to MERS-CoV [64]. Moreover, sustainable vigorous viral replication was noted. It seems, therefore, that GI tropism could be behind the occurrence of diarrhea in all SARS-CoV infections. Consequently, the SARS-CoV fecal route could lead to virus transmission (Fig. 6B), especially when infective aerosols from the toilet plume are generated [65]. Since there is approximately an 80% genome sequence identity between the novel SARS-CoV-2 and SARS-CoV [3,67], GI infection by SARS-CoV-2 is not surprising. Interestingly, this meta-analysis showed that the prevalence of GI symptoms was higher in patients with severe disease in comparison to those with less severe disease. This finding might convey potential prognostic implications, as COVID-19 patients who manifest GI symptoms may require closer monitoring.
The mechanism related to GI tract involvement in SARS-CoV-2 infection could be explained by the mediation of ACE2 cell receptors. Indeed, previous studies concerning SARS-CoV have shown that both viral glycoproteins, i.e., encoding and expressing the spike (S), could bind to the entry cellular receptor ACE2 (Fig. 6C), thus entering human cells [2, 3,66]. In addition, it has been shown that the receptor-binding domain on SARS-CoV-2 could bind to human ACE2 with high affinity [67,68]. While ACE2 is expressed in abundance in the alveolar cells in the lungs, the receptor is also highly expressed in the GI tract, especially in the small (Fig. 6D) and large intestines [12,69]. These data provide valuable insight into the receptor-mediated entry into the host enteric cells, and furthermore provide a basis for its possible transmission route through the feces.
According to this meta-analysis, in 30.3% (95%CI 10.5-61.6%) of the patients SARS-CoV-2 RNA was detected in feces. Interestingly there are reports on asymptomatic patients positive for SARS-CoV-2 RNA by RT-PCR in a stool specimen, even 17 days after the viral diagnosis [11]. Furthermore, in this report, the positivity in feces remained for 9 additional days, even when the respiratory tract specimens were negative by RT-PCR. All the above suggest that viral shedding from the GI tract may be abundant and may last long after resolution of clinical symptoms. In agreement with this finding are data from previous SARS-CoV studies, which showed that viral RNA was detected after 30 days in stools of SARS patients [70]. However, despite the similarities, it must be stressed that the viral dynamic of SARS-CoV-2 in the GI tract is not well known so far and might not follow that of SARS-CoV as observed in the respiratory tract [51,71,72].
The direct impact of this meta-analysis data refers to the infectivity of the disease. Towards this notion, a recent study showed that SARS-CoV-2 could remain viable in aerosols for hours and could remain stable on plastic and stainless steel for at least 72 h [45]. While more studies are needed in this field, viral excretion in feces and its environmental stability would cause a favorable spread of SARS-CoV-2 among human hosts, as reported during the SARS epidemics in Hong Kong [65]. The GI involvement of COVID-19 might mean the need to consider suitable hospital policies, such as adopting rectal swab SARS-CoV-2 testing prior to a patient’s discharge [73]. Subsequently, protective policies concerning endoscopy suites should be considered [74].
Undoubtedly, the results of this meta-analysis are important and should be taken seriously into account in our battle against COVID-19. The fact that we defined the threshold of 15 patients as an inclusion criterion, as opposed to another study [26] which included numerous studies even with one patient, strengthens the results of this meta-analysis. However, some limitations should be stressed. Apart from the significant heterogeneity and publication bias observed, the main limitation is related to the fact that studies written in languages other than English were not included in this meta-analysis. Moreover, so far, the main core of relevant studies comes from China, with few studies from other countries, thus precluding a more precise estimate of the prevalence of GI manifestations in COVID-19 worldwide.
In conclusion, the digestive system is involved in COVID-19. The prevalence analysis indicates that 8.9% of patients will manifest symptoms, such as diarrhea, vomiting and abdominal pain. It seems, therefore, that the GI tract might be a target organ of SARS-CoV-2 and a potential fecal transmission route should be taken into account. This might have important implications for disease management and transmission.
References
Articles from Annals of Gastroenterology are provided here courtesy of The Hellenic Society of Gastroenterology
Citations & impact
Impact metrics
Article citations
The Impact of Cardiovascular Antecedents on the Prognosis of COVID-19 Critically Ill Patients.
J Clin Med, 13(12):3518, 15 Jun 2024
Cited by: 0 articles | PMID: 38930047
The Aftermath of COVID-19: Exploring the Long-Term Effects on Organ Systems.
Biomedicines, 12(4):913, 20 Apr 2024
Cited by: 2 articles | PMID: 38672267 | PMCID: PMC11048001
Review Free full text in Europe PMC
H. Pylori Treatment in the COVID-19 Era. What Have We Learned So Far?
Curr Gastroenterol Rep, 26(3):86-91, 02 Feb 2024
Cited by: 0 articles | PMID: 38305956
Review
Risks of digestive diseases in long COVID: evidence from a population-based cohort study.
BMC Med, 22(1):14, 10 Jan 2024
Cited by: 8 articles | PMID: 38195495 | PMCID: PMC10777515
Induction of significant neutralizing antibodies against SARS-CoV-2 by a highly attenuated pangolin coronavirus variant with a 104nt deletion at the 3'-UTR.
Emerg Microbes Infect, 12(1):2151383, 01 Dec 2023
Cited by: 1 article | PMID: 36453209 | PMCID: PMC9769135
Go to all (56) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Prevalence of Gastrointestinal Symptoms and Fecal Viral Shedding in Patients With Coronavirus Disease 2019: A Systematic Review and Meta-analysis.
JAMA Netw Open, 3(6):e2011335, 01 Jun 2020
Cited by: 241 articles | PMID: 32525549 | PMCID: PMC7290409
Review Free full text in Europe PMC
Prevalence and Impact of Gastrointestinal Manifestations in COVID-19 Patients: A Systematic Review.
J Community Hosp Intern Med Perspect, 13(3):39-54, 08 May 2023
Cited by: 2 articles | PMID: 37877065 | PMCID: PMC10593163
Newly Reported Studies on the Increase in Gastrointestinal Symptom Prevalence withCOVID-19 Infection: A Comprehensive Systematic Review and Meta-Analysis.
Diseases, 8(4):E41, 10 Nov 2020
Cited by: 10 articles | PMID: 33182651 | PMCID: PMC7709133
Review Free full text in Europe PMC
Gastrointestinal and hepatic manifestations of COVID-19: A systematic review and meta-analysis.
JGH Open, 5(1):107-115, 21 Nov 2020
Cited by: 15 articles | PMID: 33363257 | PMCID: PMC7753450