Abstract
Purpose
The immunomodulatory effect of lenvatinib (a multikinase inhibitor) on tumor microenvironments may contribute to antitumor activity when combined with programmed death receptor-1 (PD-1) signaling inhibitors in hepatocellular carcinoma (HCC). We report results from a phase Ib study of lenvatinib plus pembrolizumab (an anti-PD-1 antibody) in unresectable HCC (uHCC).Patients and methods
In this open-label multicenter study, patients with uHCC received lenvatinib (bodyweight ≥ 60 kg, 12 mg; < 60 kg, 8 mg) orally daily and pembrolizumab 200 mg intravenously on day 1 of a 21-day cycle. The study included a dose-limiting toxicity (DLT) phase and an expansion phase (first-line patients). Primary objectives were safety/tolerability (DLT phase), and objective response rate (ORR) and duration of response (DOR) by modified RECIST (mRECIST) and RECIST version 1.1 (v1.1) per independent imaging review (IIR; expansion phase).Results
A total of 104 patients were enrolled. No DLTs were reported (n = 6) in the DLT phase; 100 patients (expansion phase; included n = 2 from DLT phase) had received no prior systemic therapy and had Barcelona Clinic Liver Cancer stage B (n = 29) or C disease (n = 71). At data cutoff, 37% of patients remained on treatment. Median duration of follow-up was 10.6 months (95% CI, 9.2 to 11.5 months). Confirmed ORRs by IIR were 46.0% (95% CI, 36.0% to 56.3%) per mRECIST and 36.0% (95% CI, 26.6% to 46.2%) per RECIST v1.1. Median DORs by IIR were 8.6 months (95% CI, 6.9 months to not estimable [NE]) per mRECIST and 12.6 months (95% CI, 6.9 months to NE) per RECIST v1.1. Median progression-free survival by IIR was 9.3 months per mRECIST and 8.6 months per RECIST v1.1. Median overall survival was 22 months. Grade ≥ 3 treatment-related adverse events occurred in 67% (grade 5, 3%) of patients. No new safety signals were identified.Conclusion
Lenvatinib plus pembrolizumab has promising antitumor activity in uHCC. Toxicities were manageable, with no unexpected safety signals.Free full text

Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma
Abstract
PURPOSE
The immunomodulatory effect of lenvatinib (a multikinase inhibitor) on tumor microenvironments may contribute to antitumor activity when combined with programmed death receptor-1 (PD-1) signaling inhibitors in hepatocellular carcinoma (HCC). We report results from a phase Ib study of lenvatinib plus pembrolizumab (an anti–PD-1 antibody) in unresectable HCC (uHCC).
PATIENTS AND METHODS
In this open-label multicenter study, patients with uHCC received lenvatinib (bodyweight ≥ 60 kg, 12 mg; < 60 kg, 8 mg) orally daily and pembrolizumab 200 mg intravenously on day 1 of a 21-day cycle. The study included a dose-limiting toxicity (DLT) phase and an expansion phase (first-line patients). Primary objectives were safety/tolerability (DLT phase), and objective response rate (ORR) and duration of response (DOR) by modified RECIST (mRECIST) and RECIST version 1.1 (v1.1) per independent imaging review (IIR; expansion phase).
RESULTS
A total of 104 patients were enrolled. No DLTs were reported (n = 6) in the DLT phase; 100 patients (expansion phase; included n = 2 from DLT phase) had received no prior systemic therapy and had Barcelona Clinic Liver Cancer stage B (n = 29) or C disease (n = 71). At data cutoff, 37% of patients remained on treatment. Median duration of follow-up was 10.6 months (95% CI, 9.2 to 11.5 months). Confirmed ORRs by IIR were 46.0% (95% CI, 36.0% to 56.3%) per mRECIST and 36.0% (95% CI, 26.6% to 46.2%) per RECIST v1.1. Median DORs by IIR were 8.6 months (95% CI, 6.9 months to not estimable [NE]) per mRECIST and 12.6 months (95% CI, 6.9 months to NE) per RECIST v1.1. Median progression-free survival by IIR was 9.3 months per mRECIST and 8.6 months per RECIST v1.1. Median overall survival was 22 months. Grade ≥ 3 treatment-related adverse events occurred in 67% (grade 5, 3%) of patients. No new safety signals were identified.
CONCLUSION
Lenvatinib plus pembrolizumab has promising antitumor activity in uHCC. Toxicities were manageable, with no unexpected safety signals.
INTRODUCTION
Hepatocellular carcinoma (HCC) is estimated to be the sixth most prevalent cancer worldwide and the fourth leading cause of cancer-related death.1 Despite advances in early detection, a majority of patients with HCC present with advanced disease.2
Patients with advanced HCC or tumor progression after locoregional treatment can benefit from systemic treatment.3 Sorafenib demonstrated a statistically significant survival benefit versus placebo in 2 randomized phase III studies in advanced HCC (SHARP study4 and Asia-Pacific study5). Lenvatinib, a multikinase inhibitor of vascular endothelial growth factor (VEGF) receptors 1 to 3, fibroblast growth factor (FGF) receptors 1 to 4, platelet-derived growth factor receptor-α (PDGFRα), RET, and KIT,6-9 was later approved for first-line treatment of unresectable HCC (uHCC) based on the phase III REFLECT study.10 In REFLECT, lenvatinib met its primary end point of overall survival (OS) by statistical confirmation of noninferiority to sorafenib (median OS, 13.6 months with lenvatinib v 12.3 months with sorafenib; hazard ratio [HR], 0.92; 95% CI, 0.79 to 1.06).10 Lenvatinib also resulted in significant and clinically meaningful improvements versus sorafenib in objective response rate (ORR; including unconfirmed responses), progression-free survival (PFS), and time to progression (TTP).10 Specifically, ORR by blinded independent imaging review (IIR) was significantly higher with lenvatinib versus sorafenib per RECIST version 1.1 (RECIST v1.1; 18.8% v 6.5%; P < .0001) and modified RECIST11 (mRECIST; 40.6% v 12.4%; P < .0001).10 PFS (by IIR per RECIST v1.1 and mRECIST) was also significantly longer with lenvatinib versus sorafenib (median PFS, 7.3 v 3.6 months; P < .0001 for both RECIST v1.1 and mRECIST).10
Immunotherapies, including immune checkpoint inhibitors, have had promising results in patients with advanced HCC, likely in part because of the contribution of both inflammation and suppressed immune microenvironments to the pathogenesis of HCC.12,13 The potential importance of programmed death receptor-1 (PD-1)/PD-1 ligand (PD-L1) blockade in HCC has been further underscored by the US Food and Drug Administration (FDA) decision to grant accelerated approvals of pembrolizumab and nivolumab (PD-1 monoclonal antibodies) for second-line HCC treatment after the results of phase II studies.14-17 The approvals of pembrolizumab and nivolumab were based on the therapeutic benefits of each drug (observed by ORR and duration of response [DOR]) in their respective phase II studies (CheckMate-040 for nivolumab; KEYNOTE-224 for pembrolizumab).14-17 In KEYNOTE-240, a phase III study evaluating pembrolizumab versus placebo as a second-line treatment option for HCC, pembrolizumab reduced the risk of death by 22% and improved PFS versus placebo; however, pembrolizumab did not reach its primary end points (ie, OS and PFS did not reach statistical significance per prespecified criteria).18
Combination therapies involving PD-1 inhibitors are being studied for a variety of malignancies, including non–small-cell lung cancer, renal cell carcinoma, and endometrial cancer.19,20 In March 2020, the FDA granted ipilimumab plus nivolumab accelerated approval as a second-line treatment option for HCC.16,21 In addition, the combination of lenvatinib plus pembrolizumab was granted accelerated approval for the treatment of patients with advanced endometrial carcinoma that is not microsatellite instability high or mismatch repair deficient, who have disease progression after systemic therapy, and who are not candidates for curative surgery or radiotherapy.15,22 The rationale for combining lenvatinib with pembrolizumab is based on the ability of lenvatinib to inhibit the proneoangiogenic and immunosuppressive effects of tumor microenvironments; such inhibition would improve the clinical benefit of PD-1 antibodies by boosting the antitumor immune response.23,24 Preclinical data have suggested that this combination may be effective in HCC; in a mouse model of HCC, lenvatinib combined with PD-1 signaling blockade resulted in promising antitumor activity compared with either monotherapy.25 Specifically, in a Hepa1-6 mouse HCC syngeneic tumor model, lenvatinib alone decreased proportions of monocytes and macrophages, and in combination with a PD-1 antibody, lenvatinib increased the percentage of early-activated CD8+ T cells.25 These encouraging results led to the phase Ib study, which was conducted to assess the tolerability, safety, and efficacy profiles of lenvatinib plus pembrolizumab in uHCC, reported here.
PATIENTS AND METHODS
Study Design and Participants
Study 116 is an ongoing phase Ib multicenter open-label study of lenvatinib plus pembrolizumab in patients with uHCC. The study consists of 2 phases: a dose-limiting toxicity (DLT) phase and an expansion phase. Patients received lenvatinib 12 mg (if bodyweight ≥ 60 kg) or 8 mg (if bodyweight < 60 kg) orally once daily and pembrolizumab 200 mg intravenously on day 1 of a 21-day treatment cycle (for up to 2 years after cycle 1 day 1). If no DLTs were reported in the DLT phase, the expansion phase would be initiated using the recommended dose from the DLT phase. Treatment was continued until disease progression, development of unacceptable toxicity, or withdrawal of consent. Additional details regarding continued pembrolizumab treatment are provided in the Appendix (online only).
Key inclusion criteria comprised the following: histologically or cytologically confirmed HCC (excluding fibrolamellar, sarcomatoid, and mixed cholangio-HCC tumors) or clinically confirmed HCC according to the American Association for the Study of Liver Diseases criteria; stage B (not suitable for transarterial chemoembolization) or C categorization based on the Barcelona Clinic Liver Cancer (BCLC) staging system; at least 1 measurable target lesion according to mRECIST per investigator assessment; Child-Pugh class A (score, 5-6); and Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1. Patients were excluded if they had clear invasion of the bile duct (classified clinically as a cholestatic type of HCC in which a patient’s initial manifestation is obstructive jaundice resulting from tumor thrombosis/compression/diffuse infiltration into the biliary tract); portal vein invasion with Vp426; prior blood-enhancing treatment (including blood transfusion, blood products, or agents that stimulate blood cell production [eg, granulocyte colony-stimulating factor]) within 28 days before first dose of study drugs; prior treatment with lenvatinib or any anti–PD-1, anti–PD-L1, or anti–PD-L2 agent; and imaging findings with HCC having ≥ 50% liver occupation. Additionally, patients were excluded from the expansion phase if they had received prior systemic therapy for uHCC.
Written informed consent was provided by all patients before undergoing any study-specific procedures. The study protocol was approved by the relevant institutional review boards/independent ethics committees, and the study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines.
End Points and Clinical Assessments
Tolerability and safety of the combination regimen (the primary objectives of the DLT phase) were initially evaluated by assessing DLTs during the first treatment cycle using a 3 + 3 design. In the expansion phase, the primary end points were ORR and DOR by mRECIST and RECIST v1.1 per IIR. Tumor assessment scans were performed every 6 weeks until week 24 and then every 9 weeks; treatment decisions were based on mRECIST per investigator assessment. Additional information regarding tumor assessments is included in the Appendix. Secondary end points were ORR and DOR by investigator assessment per mRECIST. Additional secondary end points included PFS, TTP, time to response (TTR), and OS. Safety assessments consisted of the monitoring and recording of adverse events (AEs) according to Common Terminology Criteria for Adverse Events version 4.03, laboratory evaluations, vital signs, electrocardiograms, and echocardiograms or multigated acquisition scans.
Statistical Analyses
The safety and efficacy analysis sets included all first-line patients from the DLT phase and the expansion phase who received at least 1 dose of study drug. A brief overview of the expansion of enrollment and sample size estimation can be found in the Appendix. ORR was calculated with 95% CI using the Clopper-Pearson method. DOR, PFS, TTP, and OS were estimated using the Kaplan-Meier method. DOR and TTR were analyzed for patients with confirmed complete response (CR) or partial response. Duration of follow-up was calculated by the reverse Kaplan-Meier estimate of OS.27 All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Patients
Overall, 104 patients were enrolled in the study (DLT and expansion phases) between February 27, 2017, and April 11, 2019. The primary analysis set included 100 patients who were treated in the first-line setting (4 patients from the DLT phase were excluded because of prior sorafenib treatment). At the data cutoff date (October 31, 2019), all patients in the primary analysis set had an opportunity for a minimum follow-up period of ≥ 6 months; 37 patients (37%) were still receiving treatment (both study drugs, n = 34; lenvatinib only, n = 3); 63 patients (63%) had discontinued treatment; and 26 of the 63 patients remained in survival follow-up (Appendix Table A1, online only). The primary reasons for treatment discontinuation are listed in Appendix Table A1. Data on subsequent anticancer medications during survival follow-up are summarized in Appendix Table A2 (online only).
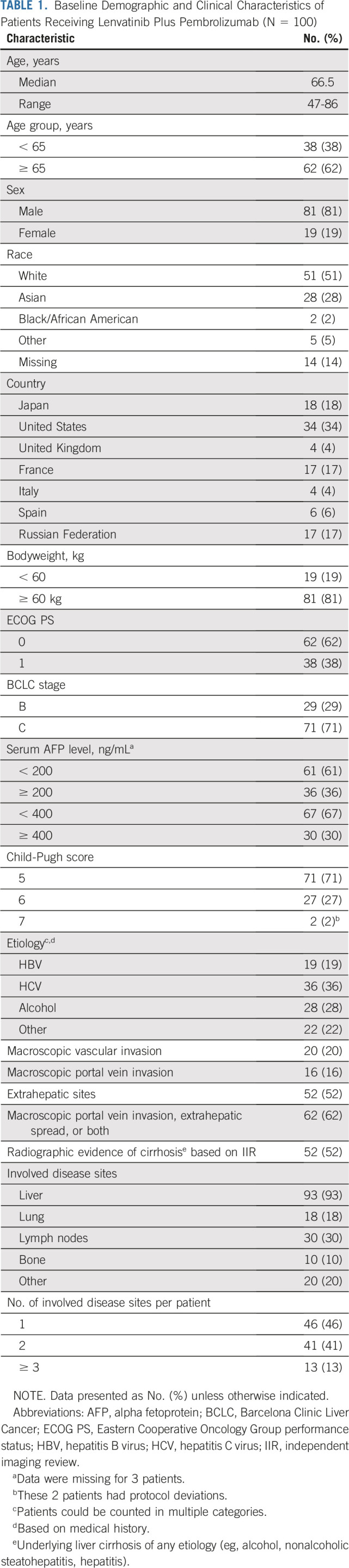
Median patient age in the first-line setting was 66.5 years (range, 47-86 years), and most patients were White (51%) or Asian (28%). At baseline, 62% and 38% of patients had ECOG PS of 0 and 1, respectively. BCLC stage B was noted in 29% of patients and stage C in 71%. Child-Pugh scores of 5, 6, and 7 were reported for 71%, 27%, and 2% of patients, respectively. Proportions of patients with macroscopic portal vein invasion and extrahepatic spread at baseline were 16% and 52%, respectively. Etiology of HCC included hepatitis B (19%), hepatitis C (36%), and alcohol (28%); and 30% of patients had an alpha fetoprotein level ≥ 400 ng/mL (Table 1).
TABLE 1.
Baseline Demographic and Clinical Characteristics of Patients Receiving Lenvatinib Plus Pembrolizumab (N = 100)
Study Drug Exposure
In the first-line setting, at the time of data cutoff, median duration of exposure was 7.9 months (range, 0.2-31.1 months) for lenvatinib plus pembrolizumab (lenvatinib: median, 7.6 months; range, 0.2-31.1 months; pembrolizumab: median, 7.4 months; range, 0.03-23.5 months). Median received dose as a percentage of the planned starting dose of lenvatinib was 69% (range, 23%-100%). Median number of pembrolizumab administrations was 11 (range, 1-33 administrations).
Safety
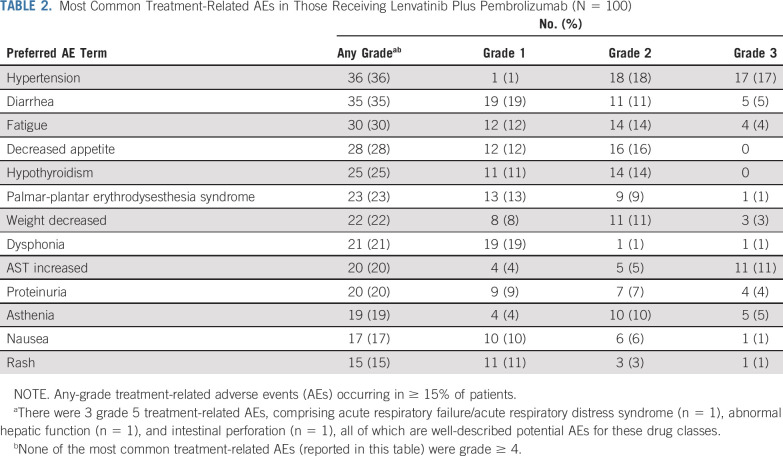
No DLTs were reported in the 6 patients enrolled in the DLT phase of the study. In the first-line setting, most patients (99%) experienced an AE (the most common AEs are reported in Appendix Table A3, online only), and 95% of patients experienced ≥ 1 treatment-related AE. The most common any-grade treatment-related AEs (Table 2) were hypertension (36%), diarrhea (35%), fatigue (30%), decreased appetite (28%), and hypothyroidism (25%). Grade ≥ 3 treatment-related AEs occurred in 67% of patients (grade 3, 63% [n = 63]; grade 4, 1% [n = 1]; grade 5, 3% [n = 3]). The most common grade 3 treatment-related AE was hypertension (17%). Leukopenia/neutropenia was the only grade 4 treatment-related AE.
TABLE 2.
Most Common Treatment-Related AEs in Those Receiving Lenvatinib Plus Pembrolizumab (N = 100)
Serious AEs (SAEs) were reported in 65 patients (65%); treatment-related SAEs were reported in 36 patients (36%). During the study, 13 (13%) grade 5 AEs occurred: 10 deaths were considered unrelated to study treatment, and 3 deaths were considered treatment related (acute respiratory failure/acute respiratory distress syndrome [n = 1] on day 124; abnormal hepatic function [n = 1] on day 127; and intestinal perforation [n = 1] on day 60).
Treatment-related AEs led to treatment interruption, dose reduction, and treatment discontinuation of lenvatinib in 62 (62%), 52 (52%), and 14 patients (14%), respectively; treatment-related AEs led to treatment interruption and treatment discontinuation of pembrolizumab in 43 (43%) and 10 patients (10%), respectively. Discontinuation of both lenvatinib and pembrolizumab because of treatment-related AEs occurred in 6 patients (6%).
Efficacy
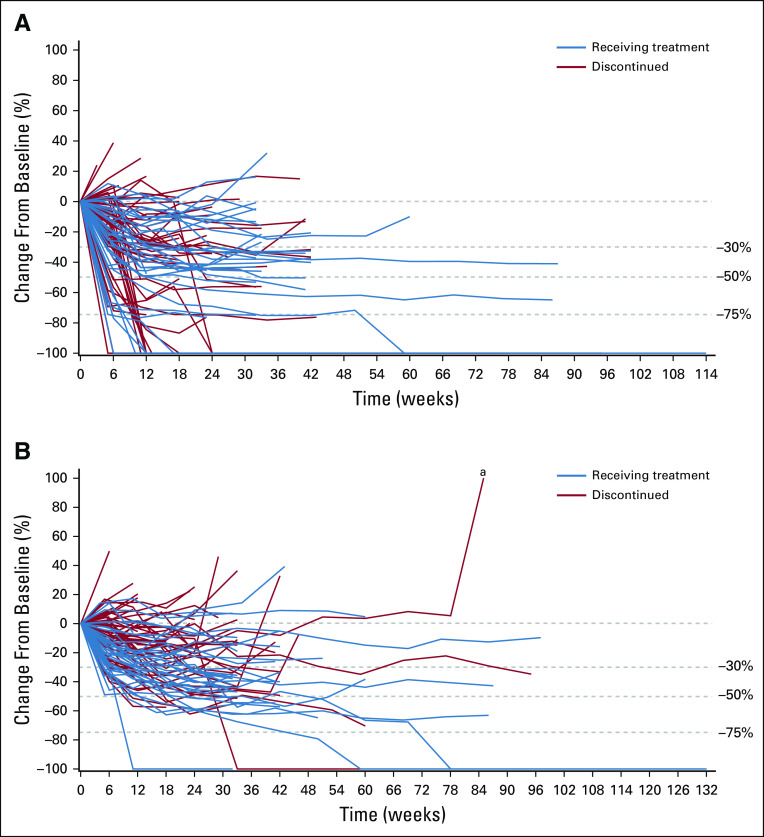
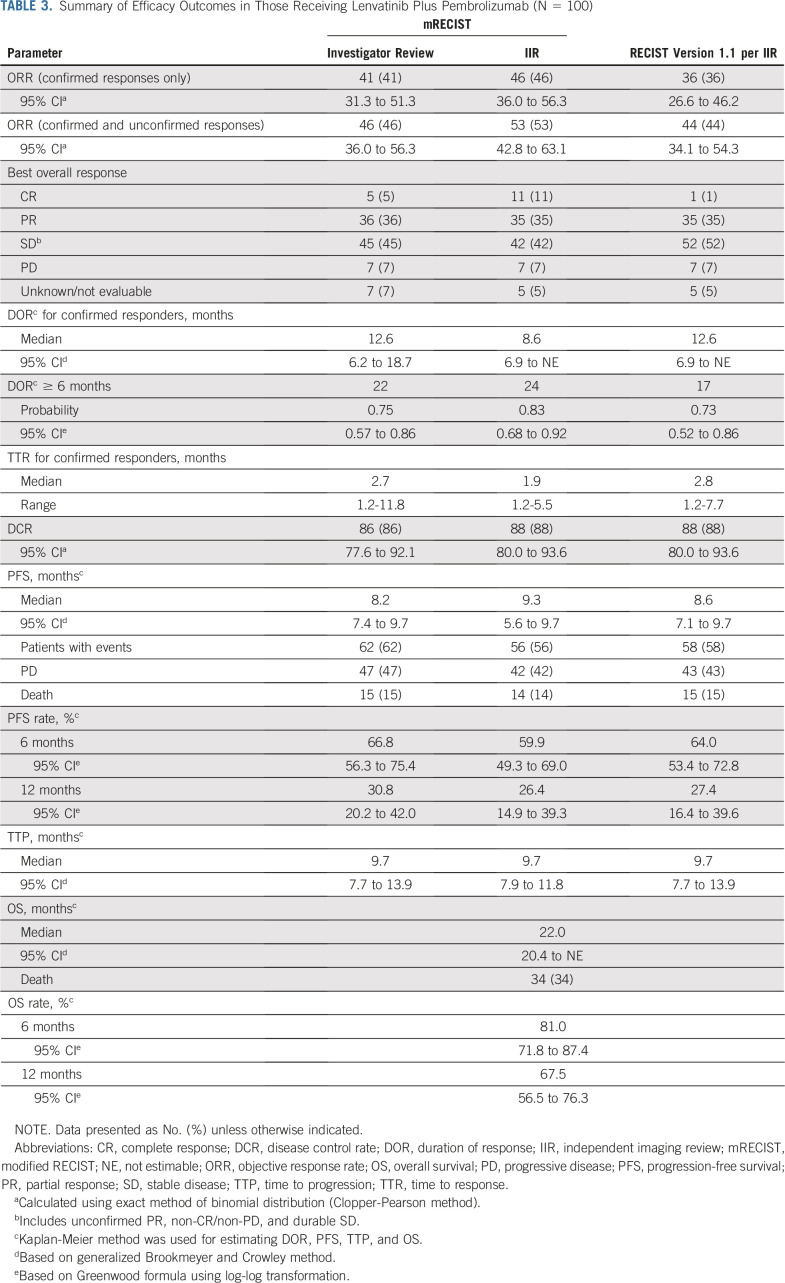
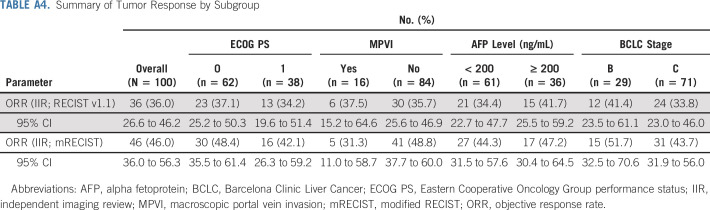
Efficacy data according to investigator assessment by mRECIST, IIR by mRECIST, and IIR by RECIST v1.1 are summarized in Table 3. The following results for tumor assessments (among patients treated in the first-line setting) are based on IIR. Median duration of follow-up was 10.6 months (95% CI, 9.2 to 11.5 months). ORR (including confirmed responses only) was 46.0% (95% CI, 36.0% to 56.3%) by mRECIST and 36.0% (95% CI, 26.6% to 46.2%) by RECIST v1.1. CRs (as best overall response) were observed in 11 patients (11%) by mRECIST and 1 patient (1%) by RECIST v1.1. Median DOR for confirmed responders was 8.6 months (95% CI, 6.9 months to not estimable [NE]) by mRECIST and 12.6 months (95% CI, 6.9 months to NE) by RECIST v1.1 (Appendix Fig A1, online only). ORRs were consistent across various subgroups, including those with poor prognostic features, such as ECOG PS of 1, macroscopic portal vein invasion, high alpha fetoprotein level, and BCLC stage C (Appendix Table A4, online only). Median TTR for confirmed responders was 1.9 months by mRECIST and 2.8 months by RECIST v1.1 (Table 3). Median PFS was 9.3 months (95% CI, 5.6 to 9.7 months) by mRECIST (Table 3; Fig 1A) and 8.6 months by RECIST v1.1 (95% CI, 7.1 to 9.7 months; Table 3; Fig 1B). Median OS was 22.0 months (95% CI, 20.4 months to NE; Table 3; Fig 1C). Reductions in tumor size per IIR by mRECIST and RECIST v1.1 were reported in 89% (83 of 93) and 83% (78 of 94) of evaluable patients, respectively (Fig 2), and the reductions seemed to be durable (Fig 3; Appendix Fig A2, online only). Median TTP was 9.7 months (95% CI, 7.9 to 11.8 months) by mRECIST per IIR and 9.7 months (95% CI, 7.7 to 13.9 months) by RECIST v1.1 per IIR (Table 3; Appendix Fig A3, online only).
TABLE 3.
Summary of Efficacy Outcomes in Those Receiving Lenvatinib Plus Pembrolizumab (N = 100)
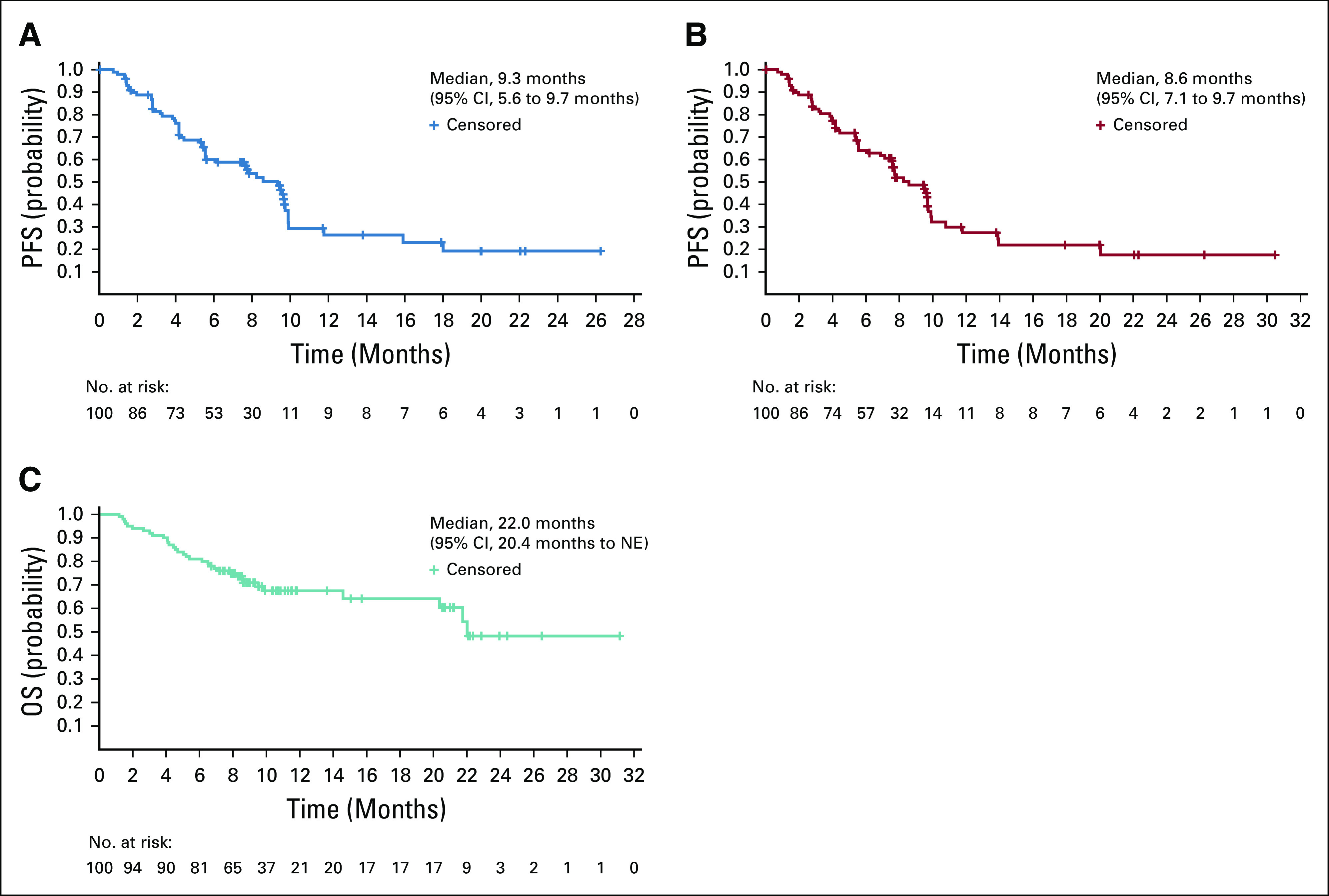
Kaplan-Meier estimates of (A) progression-free survival (PFS) by modified RECIST per independent imaging review (IIR), (B) PFS by RECIST version 1.1 per IIR, and (C) overall survival (OS; efficacy analysis set). NE, not estimable.
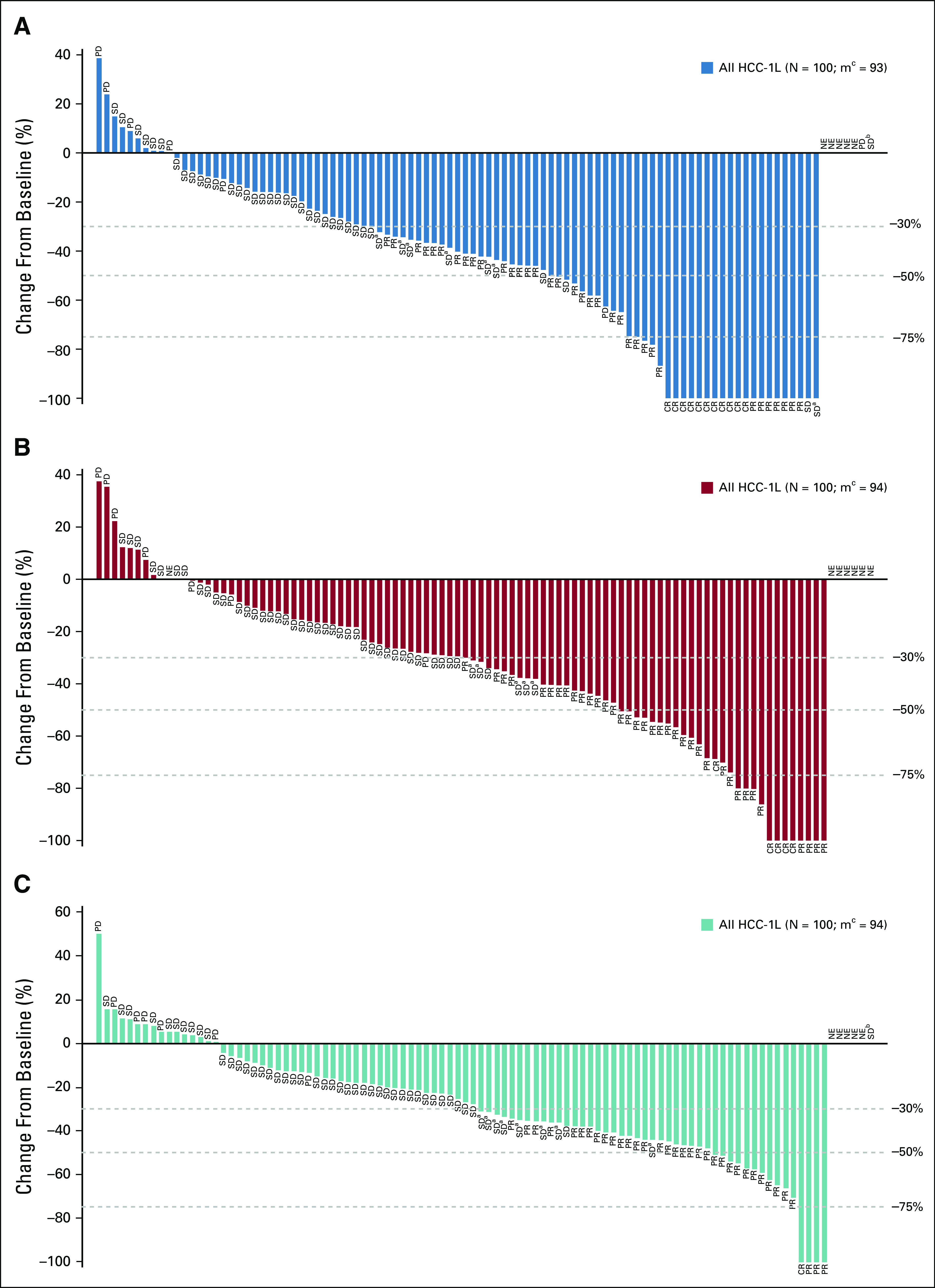
Percentage change from baseline in sums of diameters of target lesions by (A) modified RECIST (mRECIST) per independent imaging review (IIR), (B) mRECIST per investigator review, and (C) RECIST version 1.1 per IIR. CR, complete response; HCC-1L, hepatocellular carcinoma first line; NE, not estimable; PD, progressive disease; PR, partial response; SD, stable disease. (a) Unconfirmed PR. (b) Non-CR/non-PD. (c) No. of patients with both baseline and postbaseline sums of diameters of target lesions.
DISCUSSION
Treatment options for advanced HCC have rapidly evolved over the past several years. After a decade with sorafenib as the only available treatment in advanced disease, new options are now available to treat patients in various settings (eg, first and second lines).3,28 Although single-agent immune checkpoint inhibitors have demonstrated long-term disease control with manageable toxicity in a subset of patients,12,13 phase III studies have failed to meet their primary end points in the first-line setting versus sorafenib29 and second-line setting versus placebo.18
In this phase Ib single-arm study of 100 patients in the first-line setting, lenvatinib plus pembrolizumab yielded confirmed response rates (46% by mRECIST; 36% by RECIST v1.1) per IIR, median PFS of 9.3 months (by mRECIST; 8.6 months by RECIST v1.1) per IIR, and median OS of 22.0 months. Moreover, responses were durable (median DOR, 8.6 months by mRECIST and 12.6 months by RECIST v1.1) per IIR. Together, these numbers indicate that multikinase inhibition (ie, VEGF receptors 1-3, FGF receptors 1-4, PDGFRα, RET, and KIT)6-9 with lenvatinib plus PD-1 inhibition with pembrolizumab results in improved antitumor activity. Although the exact mechanism driving these higher response rates is still not well understood, preclinical data suggest that the immunomodulatory effect of lenvatinib complements pembrolizumab activity, thereby increasing sensitivity of tumors to this combination therapy.24,25 Similar observations have been described in other immune checkpoint inhibitor combination studies in advanced HCC. In the phase III IMbrave150 study,30 atezolizumab (a PD-L1 antibody) plus bevacizumab (a VEGF inhibitor) treatment resulted in improved OS compared with sorafenib (HR, 0.58; 95% CI, 0.42 to 0.79; P = .0006), as well as response rates (ORR and disease control rate [DCR]) > 27% per RECIST v1.1. This combination is now included in the National Comprehensive Cancer Network guidelines for hepatobiliary cancer.32 Moreover, ipilimumab (a CTLA-4 antibody) plus nivolumab (a PD-1 antibody) as second-line agents for HCC33 had response rates (ORR and DCR) > 30% by RECIST v1.1. Similar to outcomes reported with lenvatinib plus pembrolizumab, these combinations yielded durable responses.
In this study, with patients who had uHCC but well-preserved liver function, there were no new or unexpected toxicities resulting from lenvatinib plus pembrolizumab combination therapy. Treatment-related AEs with the combination of lenvatinib and pembrolizumab were consistent with the known AEs of each individual agent,10,17,34 and there have been no reported cases of viral hepatitis flares with pembrolizumab to date.17,18 The most frequent any-grade treatment-related AEs were hypertension, diarrhea, fatigue, decreased appetite, and hypothyroidism; however, only grade 3 hypertension and elevated AST occurred in > 10% of patients, and the only grade 4 treatment-related AE was leukopenia/neutropenia (1%). There were 3 deaths (each occurred early during the study) considered to be treatment related by the investigator, attributed to acute respiratory failure/acute respiratory distress syndrome (n = 1), abnormal hepatic function (n = 1), and intestinal perforation (n = 1), all of which are well-described potential AEs for these drug classes. Overall, treatment-related AEs led to discontinuation of both lenvatinib and pembrolizumab in 6 patients (6%). These rates were comparable to those reported in monotherapy studies of each drug in patients with HCC, suggesting that the toxicity profile of this combination is manageable with appropriate monitoring, treatment interruption, and/or dose modification (this latter option applies to lenvatinib only).10,17,18,34
Lenvatinib plus pembrolizumab resulted in a DCR of > 85% (irrespective of RECIST category) in this study, but further refinement of the population selection criteria to target those most likely to benefit from this combination therapy would be valuable. To date, serum and tissue biomarker analyses of patients with advanced HCC who were treated with either lenvatinib35 or checkpoint inhibitors17,18,36 have not clearly defined predictive markers of response or resistance. Importantly, in this current study, efforts were made to collect archival tumor tissue or a newly obtained biopsy before the first dose of study drug in the expansion phase, and these tissue samples will provide analytic material for future mechanism-based biomarker analyses.
Despite recent advances in treatment, advanced HCC is still associated with poor prognosis and median OS remains approximately 1 year.3 The competing risk of death from both underlying liver disease and malignancy adds significant complexity to the clinical management of these patients because AEs must be balanced with efficacy. Median OS and TTP for approved first-line treatments, such as sorafenib and lenvatinib, range from 11 to 14 and 4 to 9 months, respectively.3,4,10 Although most agents available to treat advanced HCC have improved survival, response rates remain low.28 ORR rates after lenvatinib and sorafenib treatment have ranged from 19% to 41% and 7% to 12%, respectively.10 In this study of lenvatinib plus pembrolizumab, improvements in both ORR and DCR were observed.
The current data are limited because of the nature of this study (ie, single arm and open label). However, the sample size, multicenter design, and AE profile of the study and its use of blinded IIR support the conclusion that lenvatinib plus pembrolizumab demonstrated promising antitumor activity with acceptable tolerability. Moreover, on the basis of interim data from this study, the FDA granted lenvatinib plus pembrolizumab a breakthrough therapy designation for the first-line treatment of uHCC that is not amenable to locoregional therapy. An ongoing double-blind randomized controlled phase III study of lenvatinib plus pembrolizumab versus lenvatinib plus placebo as first-line treatment of uHCC (LEAP-002; ClinicalTrials.gov identifier: NCT03713593) should confirm the efficacy and safety of this combination in patients with uHCC.
ACKNOWLEDGMENT
We thank the patients, their families, the investigators, and the teams who participated in this study.
Appendix
Pembrolizumab Treatment
Patients who stopped study treatment after receiving 35 administrations of pembrolizumab for reasons other than progressive disease or intolerability and patients who achieved a complete response and stopped study treatment were eligible to receive a second course of treatment of up to 17 additional administrations of pembrolizumab (approximately 1 year).
Tumor Responses
As of February 2018, all tumor assessment scans (including scans previously reviewed by investigators) were sent to an imaging core laboratory for independent imaging review by RECIST version 1.1 and modified RECIST. Tumor assessments of complete or partial response were confirmed ≥ 4 weeks after initial response.
Expansion of Enrollment and Sample Size Determination
A protocol amendment allowed for the expansion phase to be further expanded by approximately 94 evaluable patients. Interim analyses were planned to take place when 20 (6 patients for the DLT phase plus 14 patients for the expansion phase) and 56 patients (6 patients for the DLT phase plus 50 patients for the expansion phase) had sufficient follow-up to be evaluated for response. The decision to expand enrollment was based on the results of these 2 interim analyses, which spent β = 0.012 and β = 0.024 at the first and second interim analyses, respectively.
On the basis of an assumption of H0: 25% objective response rate (ORR) and H1: 45% ORR, the 100-patient design with 2 futility analyses had approximately 96% statistical power at 2-sided α = 0.02 (corresponding to 1-sided α = 0.01). At the first interim analysis (n = 20), if there were > 5 responses, approximately 36 additional patients would be enrolled. At the second interim analysis (n = 56), if there were > 16 responses, approximately 44 additional patients would be enrolled. If there were ≤ 5 responses at the first interim analysis (n = 20) or ≤ 16 responses at the second interim analysis (n = 56), the sponsor would decide whether to expand enrollment based on clinical outcome (eg, duration of response). The 2 interim analyses were not formally conducted, because the numbers of responses required to enable expansion enrollment were reached before the planned interim analyses at n = 20 and n = 56, respectively.
Data-Sharing Statement
The data will not be available for sharing at this time, because the data are commercially confidential. However, Eisai will consider written requests to share the data on a case-by-case basis.
FIG A1.
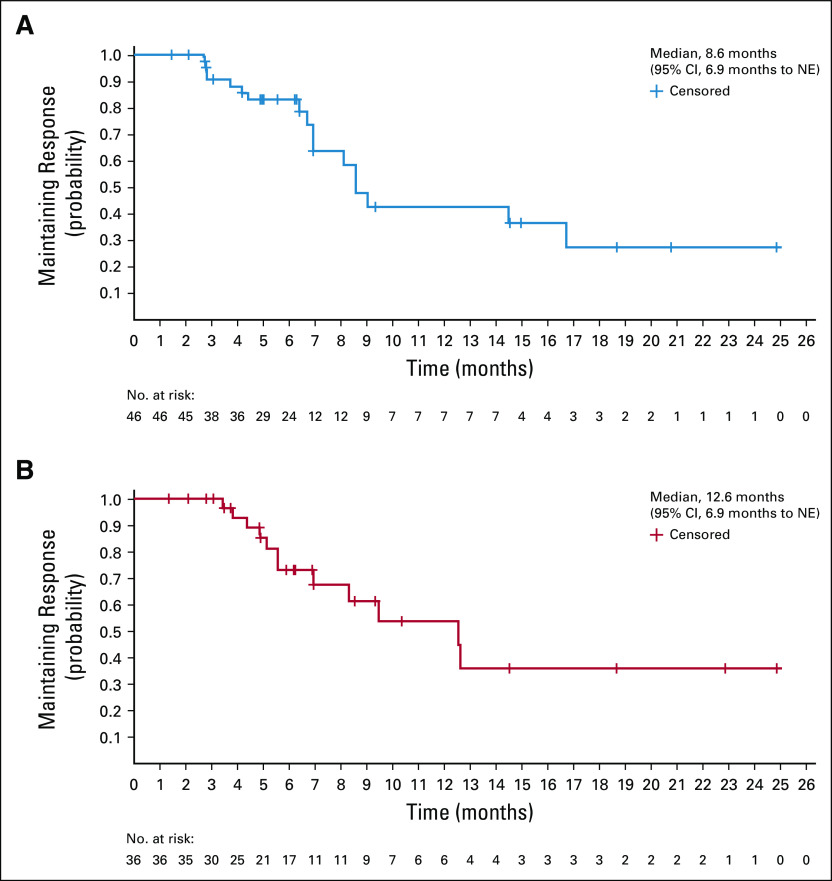
Kaplan-Meier estimate of duration of response by (A) modified RECIST and (B) RECIST version 1.1 per independent imaging review. NE, not estimable.
FIG A2.
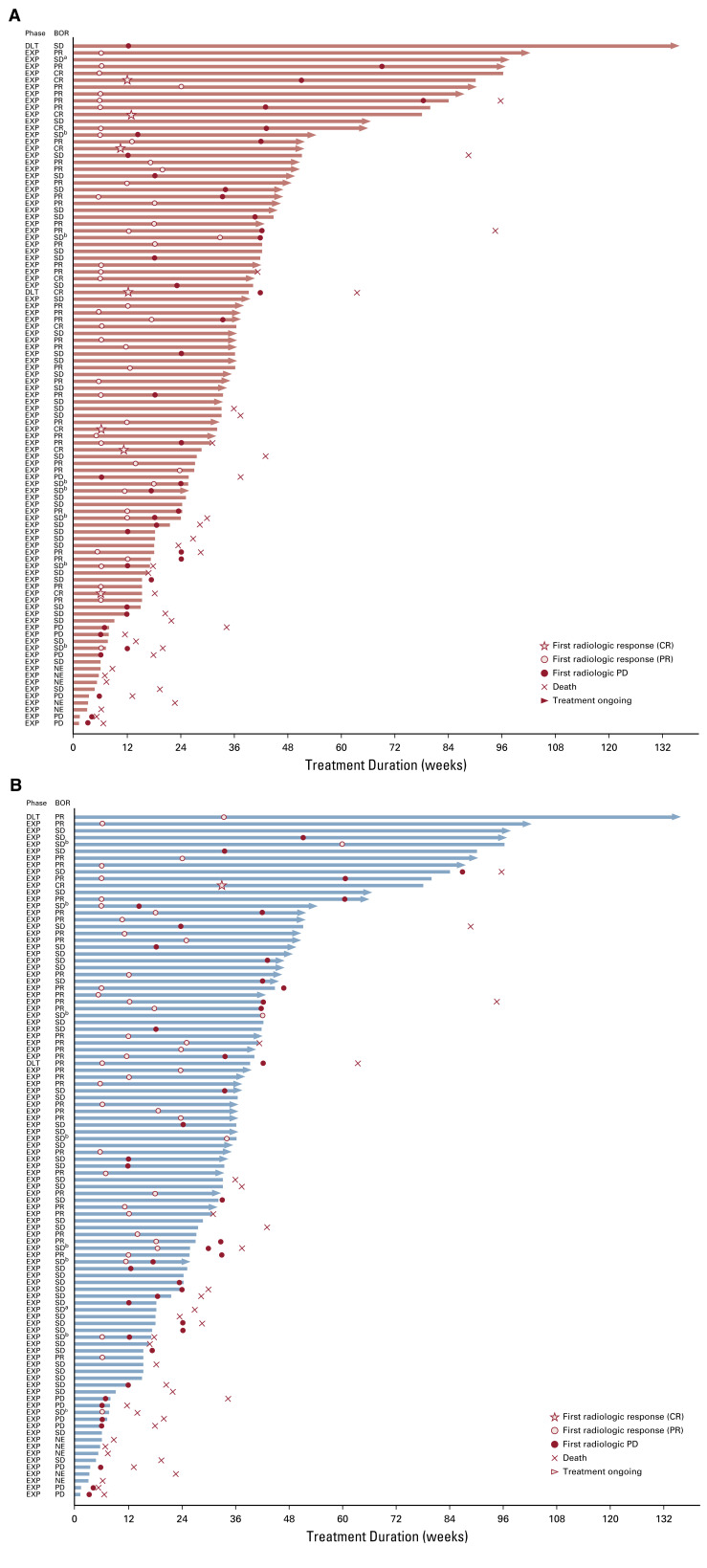
Duration of treatment and response assessments by (A) modified RECIST and (B) RECIST version 1.1 per independent imaging review. BOR, best overall response; CR, complete response; DLT, dose-limiting toxicity; EXP, expansion; NE, not estimable; PD, progressive disease; PR, partial response; SD, stable disease. (a) Non-CR/non-PD. (b) Unconfirmed PR.
FIG A3.
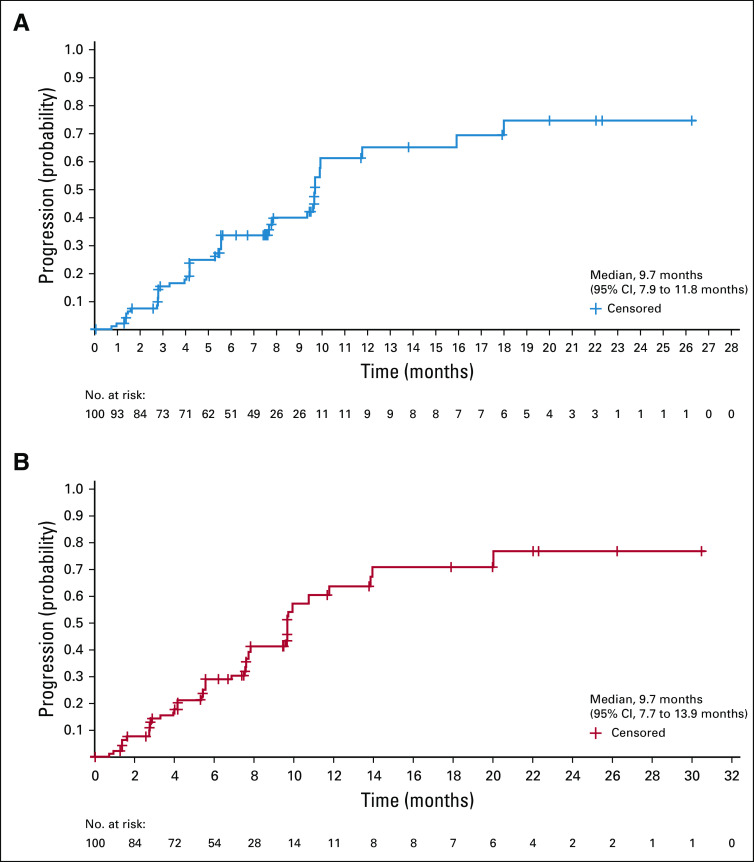
Kaplan-Meier estimate of time to progression (TTP) by (A) modified RECIST and (B) RECIST version 1.1 per independent imaging review.
TABLE A1.
Patient Disposition and Reasons for Discontinuation From Treatment at Data Cutoff Date of October 31, 2019, Among Those Receiving Lenvatinib Plus Pembrolizumab (N = 100)
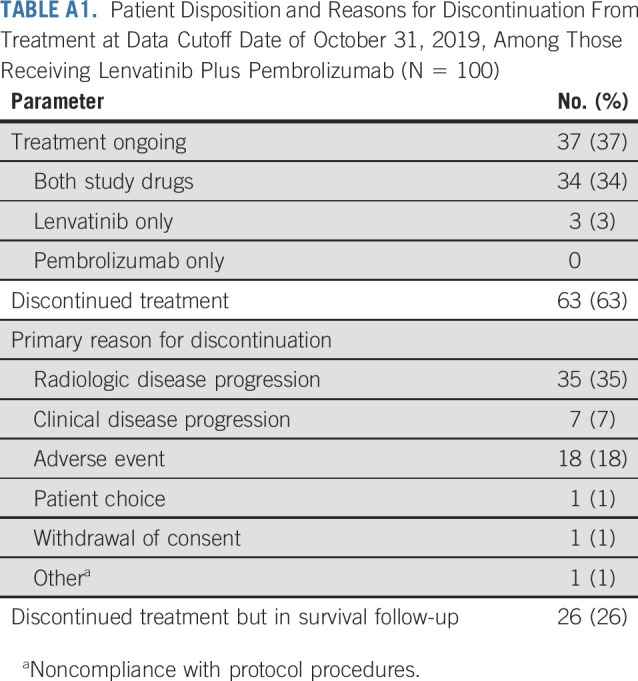
TABLE A2.
Anticancer Medications During Survival Follow-Up Among Those Receiving Lenvatinib Plus Pembrolizumab (N = 100)
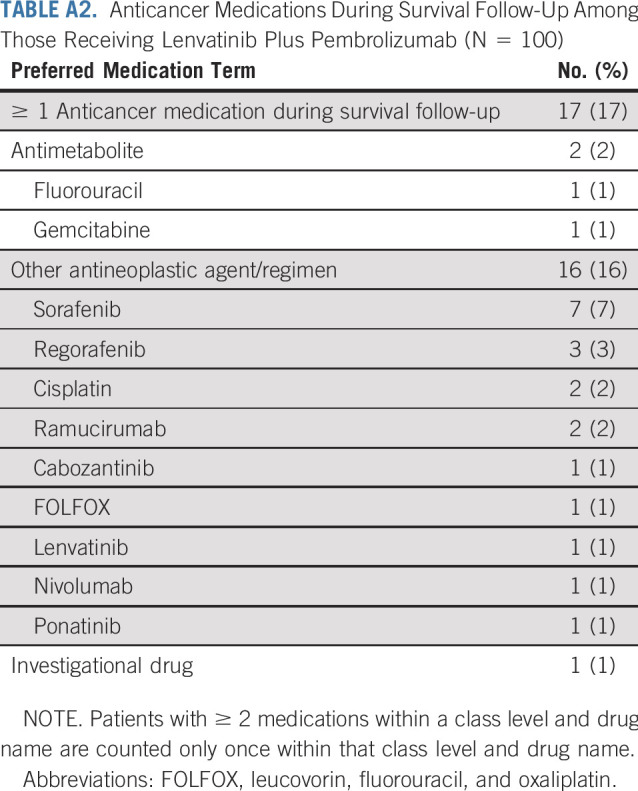
TABLE A3.
Most Common Treatment-Emergent AEs Among Those Receiving Lenvatinib Plus Pembrolizumab (N = 100)
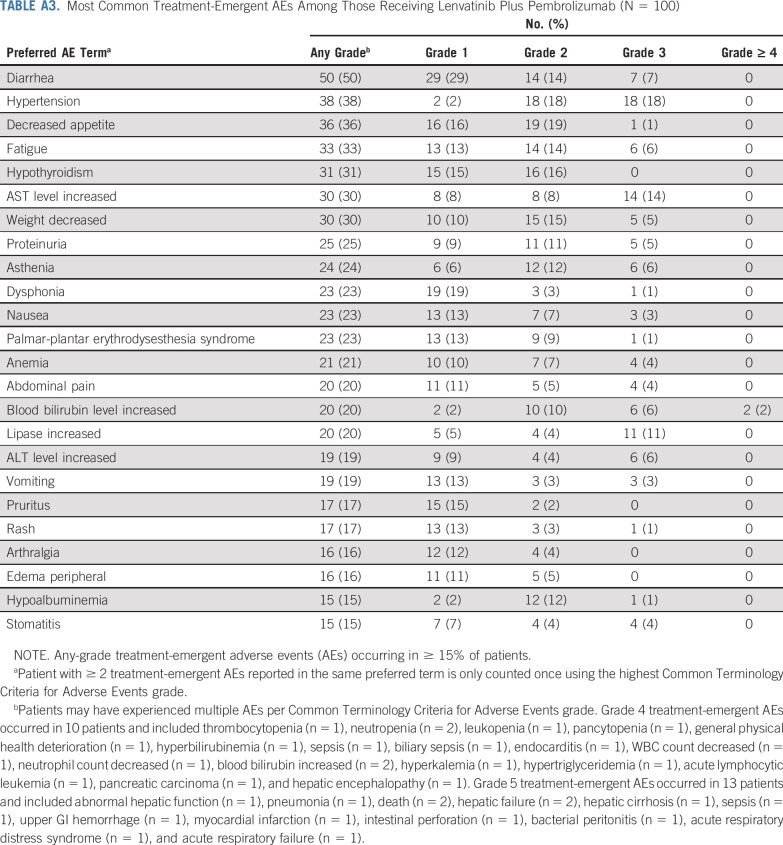
TABLE A4.
Summary of Tumor Response by Subgroup
These authors contributed equally to the study and publication and are listed in alphabetical order: R.S.F., M.I., and A.X.Z.
PRIOR PRESENTATION
Presented in abstract form at the ASCO Annual Meeting, Chicago, IL, June 1-5, 2018; American Association for Cancer Research Annual Meeting, Atlanta, GA, March 29-April 3, 2019; and European Society for Medical Oncology Annual Meeting, Barcelona, Spain, September 27-October 1, 2019.
SUPPORT
Supported by Eisai (Woodcliff Lake, NJ) and Merck Sharp & Dohme a subsidiary of Merck & Co (Kenilworth, NJ), both of which also funded medical writing support provided by Oxford PharmaGenesis (Newtown, PA).
CLINICAL TRIAL INFORMATION
Processed as a Rapid Communication manuscript.
AUTHOR CONTRIBUTIONS
Conception and design: Richard S. Finn, Masafumi Ikeda, Andrew X. Zhu, Takuji Okusaka, Hiromitsu Kumada, Tomoki Kubota, Corina E. Dutcus, Kenichi Saito, Abby B. Siegel, Kalgi Mody, Josep M. Llovet
Administrative support: Takuji Okusaka
Provision of study material or patients: Richard S. Finn, Masafumi Ikeda, Andrew X. Zhu, Max W. Sung, Ari D. Baron, Masatoshi Kudo, Takuji Okusaka, Masahiro Kobayashi, Marc Pracht, Konstantin Mamontov
Collection and assembly of data: Richard S. Finn, Masafumi Ikeda, Andrew X. Zhu, Max W. Sung, Ari D. Baron, Masatoshi Kudo, Takuji Okusaka, Masahiro Kobayashi, Hiromitsu Kumada, Marc Pracht, Konstantin Mamontov, Tim Meyer, Corina E. Dutcus, Kalgi Mody, Josep M. Llovet
Data analysis and interpretation: Richard S. Finn, Masafumi Ikeda, Andrew X. Zhu, Max W. Sung, Ari D. Baron, Masatoshi Kudo, Takuji Okusaka, Hiromitsu Kumada, Shuichi Kaneko, Marc Pracht, Tim Meyer, Corina E. Dutcus, Kenichi Saito, Abby B. Siegel, Leonid Dubrovsky, Kalgi Mody, Josep M. Llovet
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Richard S. Finn
Consulting or Advisory Role: Pfizer, Bayer, Novartis, Bristol Myers Squibb, Merck, Eisai, Lilly, Genentech/Roche, AstraZeneca, Exelixis, C Stone Pharma
Research Funding: Pfizer (Inst), Bayer (Inst), Novartis (Inst), Eisai (Inst), Lilly (Inst), Merck (Inst), Bristol Myers Squibb (Inst), Roche/Genentech (Inst)
Expert Testimony: Novartis
Masafumi Ikeda
Honoraria: Taiho Pharmaceutical, Novartis, Bayer Yakuhin, Eisai, Lilly Japan, Dainippon Sumitomo Pharma, Teijin Pharma, Kaken Pharmaceutical, EA Pharma, Merck Sharp & Dohme, Gilead Sciences, Mylan, Otsuka Pharmaceutical, Yakult, Nihon Servier, Astellas Pharma, Chugai Pharma
Consulting or Advisory Role: Bayer Yakuhin, Eisai, Novartis, Shire, Daiichi Sankyo, Teijin Pharma, Lilly Japan, ASLAN Pharmaceuticals, Chugai Pharma, Astellas Pharma, Micron, Ono Pharmaceutical, AstraZeneca, Nihon Servier
Research Funding: Bayer Yakuhin (Inst), Yakult (Inst), Lilly Japan (Inst), Ono Pharmaceutical (Inst), Eisai (Inst), AstraZeneca (Inst), Chugai Pharma (Inst), Bristol Myers Squibb (Inst), Merck Serono (Inst), NanoCarrier (Inst), ASLAN Pharmaceuticals (Inst), Novartis (Inst), Merck Sharp & Dohme (Inst), J-Pharma (Inst), Takeda Pharmaceuticals (Inst), Astellas Pharma (Inst), Pfizer (Inst)
Andrew X. Zhu
Consulting or Advisory Role: Eisai, Merck, AstraZeneca, Bayer, Exelixis, Lilly, Roche/Genentech, Sanofi/Aventis
Research Funding: Lilly (Inst), Bayer (Inst), Bristol Myers Squibb (Inst), Novartis (Inst), Merck (Inst)
Max W. Sung
Consulting or Advisory Role: Bayer, Eisai, Exelixis
Ari D. Baron
Speakers’ Bureau: Bristol Myers Squibb, Merck, Lilly, Amgen, Eisai, Johnson & Johnson, AbbVie
Masatoshi Kudo
Honoraria: Merck Sharp & Dohme, Eisai, Bayer, Lilly Japan, EA Pharma, Bristol Myers Squibb Japan
Consulting or Advisory Role: Merck Sharp & Dohme, Eisai, Ono Pharmaceuticals, Bristol Myers Squibb, Roche
Research Funding: Otsuka Pharmaceutical (Inst), Taiho Pharmaceutical (Inst), AbbVie (Inst), Takeda Pharmaceuticals (Inst), Eisai (Inst), Gilead Sciences (Inst), EA Pharma (Inst), Dainippon Sumitomo Pharma (Inst)
Takuji Okusaka
Honoraria: Meiji Seika Kaisha, Merck Sharp & Dohme, Shire, AbbVie, Eisai, Ono Pharmaceutical, Daiichi Sankyo, Taiho Pharmaceutical, Takeda Pharmaceuticals, Teijin Pharma, Lilly, Nippon Shinyaku, Servier, Novartis, Bayer, Pfizer, Mundipharma
Consulting or Advisory Role: Taiho Pharmaceutical, Daiichi Sankyo, Dainippon Sumitomo Pharma, Bristol Myers Squibb, AstraZeneca, Eisai
Research Funding: Novartis (Inst), Eisai (Inst), Dainippon Sumitomo Pharma (Inst), Baxter (Inst), Lilly (Inst), Taiho Pharmaceutical (Inst), AstraZeneca (Inst), Chugai Pharma (Inst), Bristol Myers Squibb, Merck Sharp & Dohme (Inst)
Travel, Accommodations, Expenses: Takara Bio
Masahiro Kobayashi
Honoraria: Eisai Japan
Hiromitsu Kumada
Honoraria: Merck Sharp & Dohme, Dainippon Sumitomo Pharma, AbbVie, Gilead Sciences, Eisai
Shuichi Kaneko
Honoraria: Merck Sharp & Dohme, Eisai, Gilead Sciences, Dainippon Sumitomo Pharma, Bayer, Bristol Myers Squibb, Lilly
Consulting or Advisory Role: Bayer, Merck Sharp & Dohme, Lilly, Eisai
Research Funding: Merck Sharp & Dohme (Inst), Bayer (Inst), Chugai Pharma (Inst), Eisai (Inst)
Marc Pracht
Travel, Accommodations, Expenses: Merck Sharp & Dohme
Konstantin Mamontov
Honoraria: Merck, Sanofi, BioCad
Consulting or Advisory Role: Eisai, Sanofi
Speakers’ Bureau: Sanofi, BioCad, Merck
Tim Meyer
Consulting or Advisory Role: Bristol Myers Squibb, Eisai, Ipsen, BTG, Beigene, Merck Sharp & Dohme, Roche
Research Funding: Ipsen (Inst), Bayer (Inst), BTG (Inst)
Tomoki Kubota
Employment: Eisai
Corina E. Dutcus
Employment: Eisai
Kenichi Saito
Employment: Eisai
Patents, Royalties, Other Intellectual Property: Applying patent for pharmaceutical composition
Abby B. Siegel
Employment: Merck & Co
Stock and Other Ownership Interests: Merck
Leonid Dubrovsky
Employment: Merck & Co
Stock and Other Ownership Interests: Merck Sharp & Dohme
Kalgi Mody
Employment: Eisai
Travel, Accommodations, Expenses: Eisai
Josep M. Llovet
Research support: Eisai, Bristol Myers Squibb, Bayer Pharmaceuticals, Boehringer-Ingelheim and Ipsen
Consultancy: Eisai, Merck, Bayer Pharmaceuticals, Bristol Myers Squibb, Celsion Corporation, Eli Lilly, Roche, Genentech, Ipsen, Glycotest, Nucleix, Biopharma, Sirtex, AstraZeneca
No other potential conflicts of interest were reported.
REFERENCES
Articles from Journal of Clinical Oncology are provided here courtesy of American Society of Clinical Oncology
Citations & impact
Impact metrics
Article citations
Current Status of Immunotherapy in Management of Small Bowel Neuroendocrine Tumors.
Curr Oncol Rep, 28 Oct 2024
Cited by: 0 articles | PMID: 39466478
Review
Immunotherapy after progression to double immunotherapy: pembrolizumab and lenvatinib versus conventional chemotherapy for patients with metastatic melanoma after failure of PD-1/CTLA-4 inhibition.
Front Oncol, 14:1420879, 07 Oct 2024
Cited by: 0 articles | PMID: 39435288 | PMCID: PMC11491429
Gut microbiota as a prognostic biomarker for unresectable hepatocellular carcinoma treated with anti-PD-1 therapy.
Front Genet, 15:1366131, 03 Oct 2024
Cited by: 0 articles | PMID: 39421302 | PMCID: PMC11484251
Real-world outcomes of combined lenvatinib and anti-PD-1 in advanced melanoma: the Lenvamel study, a multicenter retrospective study of the French Group of Skin Cancers (Groupe de Cancérologie Cutanée).
Oncologist, 29(10):e1364-e1372, 01 Oct 2024
Cited by: 0 articles | PMID: 38956747 | PMCID: PMC11449033
Integrating anoikis and ErbB signaling insights with machine learning and single-cell analysis for predicting prognosis and immune-targeted therapy outcomes in hepatocellular carcinoma.
Front Immunol, 15:1446961, 11 Oct 2024
Cited by: 0 articles | PMID: 39464883 | PMCID: PMC11502379
Go to all (645) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials (2)
- (1 citation) ClinicalTrials.gov - NCT03006926
- (1 citation) ClinicalTrials.gov - NCT03713593
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Efficacy and safety of tislelizumab plus lenvatinib as first-line treatment in patients with unresectable hepatocellular carcinoma: a multicenter, single-arm, phase 2 trial.
BMC Med, 22(1):172, 23 Apr 2024
Cited by: 3 articles | PMID: 38650037 | PMCID: PMC11036623
Lenvatinib Plus Pembrolizumab in Patients With Advanced Endometrial Cancer.
J Clin Oncol, 38(26):2981-2992, 13 Mar 2020
Cited by: 236 articles | PMID: 32167863 | PMCID: PMC7479759
Lenvatinib, sintilimab plus transarterial chemoembolization for advanced stage hepatocellular carcinoma: A phase II study.
Liver Int, 44(4):920-930, 30 Jan 2024
Cited by: 3 articles | PMID: 38291865
Lenvatinib: A Review in Hepatocellular Carcinoma.
Drugs, 79(6):665-674, 01 Apr 2019
Cited by: 131 articles | PMID: 30993651
Review
Funding
Funders who supported this work.
Cancer Research UK (1)
HUNTER: Hepatocellular Carcinoma Expediter Network
Professor Ruth Plummer, Newcastle University
Grant ID: 26813