Abstract
Free full text

Platelet and Vascular Biomarkers Associate With Thrombosis and Death in Coronavirus Disease
The SARS-CoV-2 (SARS-CoV-2 severe acute respiratory syndrome coronavirus 2) coronavirus disease (COVID-19) is a global pandemic. Laboratory testing suggests a coagulopathy with up to 30% of hospitalized patients with COVID-19 developing thrombotic events.1 Platelets are central protagonists in both arterial and venous thrombosis, and virus-platelet interactions contribute to thrombotic risk, promoting an overall procoagulant and inflammatory states during viral infection.2 Furthermore, recent studies report platelets to be hyperactivated in subjects with COVID-19.3
We speculated that in COVID-19, biomarkers of platelet activation are associated with incident thrombosis or death. Thus, we investigated in vivo surrogate biomarkers of platelet activation and vascular inflammation collected in the early phase of COVID-19 hospitalization. Plasma levels of soluble CD40 ligand (sCD40L), P-selectin, the metabolite of thromboxane A2, thromboxane B2 (TxB2), and mean platelet volume (MPV) were assessd. Venous blood samples were collected within 24 hours of hospital admission to NYU Langone Health between March 15 and April 20, 2020, in accordance with the policies of the NYU Langone Health Institutional Review Board. Plasma was collected by centrifugation of PST tubes; MPV was measured during routine care from EDTA tubes on a SYSMEX analyzer.
Among 100 randomly selected hospitalized patients with COVID-19, median age was 65 years, 39 were female, 48 were White, 53 had hypertension, and at admission, 27 were on antiplatelet therapy, of whom 24 (89%) were on aspirin. Thrombosis or death occurred in 32 subjects (24 deaths and 14 thrombotic events [8 VTE (venous thromboembolism), 5 myocardial infarction, and 1 VTE and myocardial infarction]), 6 subjects experienced a thrombotic event and subsequently died. Patients with thrombosis or death were older and more likely to have chronic obstructive pulmonary disease than those without an event. There was no significant difference in platelet count or the use of antiplatelet therapy at presentation between groups (Table (Table11).
Table 1.
Baseline Characteristics of Patients With COVID-19 Stratified by the Incidence of Thrombosis or Death
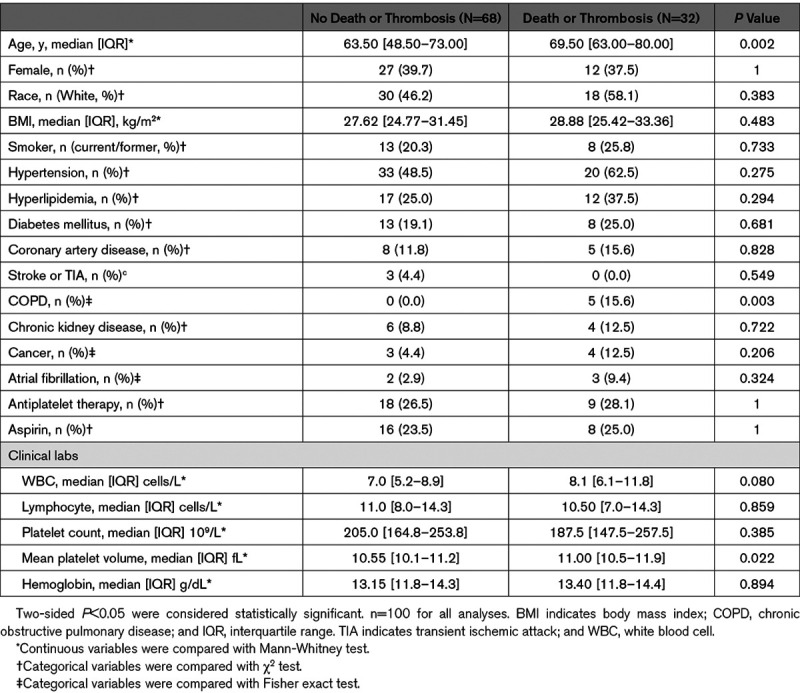
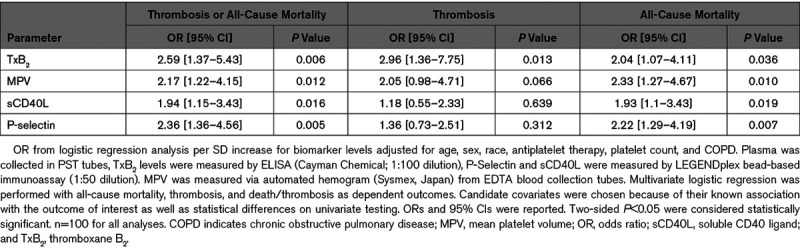
We analyzed banked samples collected on the day of COVID-19 diagnosis to investigate in vivo platelet activity and vascular health biomarkers. Following adjustment for age, sex, race/ethnicity, antiplatelet therapy, platelet count, and chronic obstructive pulmonary disease, TxB2 (P=0.006), P-selectin (P=0.005), sCD40L (P=0.016), and MPV (P=0.012) were independently associated with the composite of thrombosis or death. Of the 14 patients who experienced a thrombotic event, only TxB2 was associated with thrombosis after multivariable adjustment (P=0.013). Of the 24 patients who died TxB2 (P=0.006), P-selectin (P=0.005), sCD40L (P=0.016), and MPV (P=0.012) were associated with all-cause mortality after multivariable adjustment (Table (Table2).2). Aspirin was associated with a lower TxB2 (P<0.001; data not shown) and was not associated with other measured biomarkers. In a sensitivity analysis, the association between TxB2 and thrombosis or death remained robust (P=0.012) after excluding subjects on aspirin.
Table 2.
Multivariable Regression Models Predicting Thrombosis or All-Cause Mortality, Thrombosis, and All-Cause Mortality
Patients hospitalized with COVID-19 are at increased risk for thrombosis, and autopsy data from our group and others demonstrate micro- and macro-thrombi across vascular beds in patients with and without clinical thrombosis.4,5 Randomized trials are ongoing testing dosing strategies of anticoagulation. However, emerging data suggest that patients continue to accrue thrombotic events even on high-dose anticoagulation.
We report for the first time that biomarkers of platelet activity and vascular health, are significantly associated with the composite outcome of thrombosis or death in hospitalized patients with COVID-19. A composite outcome was used due to a competing risk of death and likely underdiagnosing of thrombotic events in hospitalized patients with COVID-19. These findings suggest that multiple platelet-related processes contribute to thrombosis and mortality in patients with COVID-19. Increased plasma TxB2 levels indicate the activation of platelets via COX-1. Moreover, both platelet P-selectin and sCD40L contribute to thrombosis by supporting platelet-myeloid heteroaggregate formation and thrombi stability by interaction with PSGL-1 and αIIbβ3, respectively. Our current study does not characterize the cellular source of measured biomarkers, thus in addition to platelets, plasma P-selectin and sCD40L may originate from alternate sources including endothelial cells and T cells, respectively. However, consistent with our surrogate biomarkers of platelet activity is the similar association obtained with the platelet-specific marker, MPV, a biomarker of platelet hyperactivity.
Our findings are consistent with recent reports of platelet hyperactivity in patients with COVID-19.3 We extend those finding and demonstrate that biomarkers of platelet activation are associated with thrombosis or death in patients hospitalized with COVID-19. Our findings suggest platelet activation mechanisms may contribute to adverse events and warrant further investigation into the mechanistic role of platelets in COVID-19 pathogenesis and highlight the potential role of antiplatelet therapy.
Sources of Funding
This study was supported by National Institutes of Health (R35HL144993, J.S. Berger), American Society of Hematology (18-A0-00-1001884, T.J. Barrett), and the NYU CTSA grant UL1TR001445.
References
Full text links
Read article at publisher's site: https://doi.org/10.1161/circresaha.120.317803
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7478197
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Targeting GPVI with glenzocimab in COVID-19 patients: Results from a randomized clinical trial.
PLoS One, 19(6):e0302897, 17 Jun 2024
Cited by: 0 articles | PMID: 38885234 | PMCID: PMC11182546
Effects of Pycnogenol® in people with post-COVID-19 condition (PYCNOVID): study protocol for a single-center, placebo controlled, quadruple-blind, randomized trial.
Trials, 25(1):385, 15 Jun 2024
Cited by: 0 articles | PMID: 38879571 | PMCID: PMC11179231
Reactive oxygen species in endothelial signaling in COVID-19: Protective role of the novel peptide PIP-2.
PLoS One, 19(5):e0289854, 21 May 2024
Cited by: 0 articles | PMID: 38771750 | PMCID: PMC11108150
Predictive ability of complete blood count, mean platelet ratio, mean platelet volume, and neutrophil/lymphocyte ratio for severe pneumonia among RT-PCR or radiologically proven COVID-19 patients.
J Family Med Prim Care, 13(5):1856-1862, 24 May 2024
Cited by: 0 articles | PMID: 38948551
Does SARS-CoV-2 infect platelets?
Front Immunol, 15:1392000, 23 Apr 2024
Cited by: 0 articles | PMID: 38715614 | PMCID: PMC11074452
Go to all (72) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Nox2 activation in Covid-19.
Redox Biol, 36:101655, 25 Jul 2020
Cited by: 97 articles | PMID: 32738789 | PMCID: PMC7381406
Coagulation abnormalities and thrombosis in patients with COVID-19.
Lancet Haematol, 7(6):e438-e440, 11 May 2020
Cited by: 889 articles | PMID: 32407672 | PMCID: PMC7213964
COVID-19 and thrombosis: Beyond a casual association.
Med Clin (Barc), 155(1):44, 11 May 2020
Cited by: 3 articles | PMID: 32446683 | PMCID: PMC7211593
Platelets in Coronavirus Disease 2019.
Semin Thromb Hemost, 46(7):823-825, 30 Apr 2020
Cited by: 28 articles | PMID: 32356294 | PMCID: PMC7645810
Review Free full text in Europe PMC
Funding
Funders who supported this work.
American Society of Hematology (1)
Grant ID: 18-A0-00-1001884
HHS | NIH | National Heart, Lung, and Blood Institute (1)
Grant ID: R35HL144993
NCATS NIH HHS (2)
Grant ID: UL1 TR001445
Grant ID: UL1 TR000038
NHLBI NIH HHS (1)
Grant ID: R01 HL139909

 1,4
1,4



