Abstract
Free full text

In-depth virological assessment of kidney transplant recipients with COVID-19
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread widely, causing coronavirus disease 2019 (COVID-19) and significant mortality. However, data on viral loads and antibody kinetics in immunocompromised populations are lacking. We aimed to determine nasopharyngeal and plasma viral loads via reverse transcription-polymerase chain reaction and SARS-CoV-2 serology via enzyme-linked immunosorbent assay and study their association with severe forms of COVID-19 and death in kidney transplant recipients. In this study, we examined hospitalized kidney transplant recipients with nonsevere (n = 21) and severe (n = 19) COVID-19. SARS-CoV-2 nasopharyngeal and plasma viral load and serological response were evaluated based on outcomes and disease severity. Ten recipients (25%) displayed persistent viral shedding 30 days after symptom onset. The SARS-CoV-2 viral load of the upper respiratory tract was not associated with severe COVID-19, whereas the plasma viral load was associated with COVID-19 severity (P = .010) and mortality (P = .010). All patients harbored antibodies during the second week after symptom onset that persisted for 2 months. We conclude that plasma viral load is associated with COVID-19 morbidity and mortality, whereas nasopharyngeal viral load is not. SARS-CoV-2 shedding is prolonged in kidney transplant recipients and the humoral response to SARS-CoV-2 does not show significant impairment in this series of transplant recipients.
1. INTRODUCTION
In December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in China, and it has since spread widely across the world.1 The resulting coronavirus disease 2019 (COVID-19) has led to a high death toll. Scientific knowledge on SARS-CoV-2 has evolved rapidly since the outbreak, but little is known about responses to the virus in immunocompromised populations. Infection with respiratory viruses has been shown to be particularly concerning in transplant recipients due to prolonged viral shedding and a higher risk of complications.2 , 3 However, there have been no reports indicating whether SARS-CoV-2 infection presents the same risks for transplant recipients as other respiratory viruses. Determining viral loads and antibody kinetics in immunocompromised individuals is necessary to protect this highly immunocompromised population. Because prolonged viral shedding and/or a lack of protective immunity could lead to significant viral spread in patients’ environments, protective measures may need to be increased.
We thus conducted a retrospective cohort study in kidney transplant recipients (KTR) in Alsace, Grand-Est, France, to determine the dynamics of nasopharyngeal and plasma viral loads and SARS-CoV-2 serology and to study their association with mortality and severe forms of COVID-19.
2. PATIENTS AND METHODS
2.1. Study population
A total of 40 adult KTR hospitalized with COVID-19 were consecutively recruited at our transplant center between March 4 and April 7, 2020. COVID-19 was diagnosed in these patients based on their clinical symptoms and positive reverse transcription-polymerase chain reaction (RT-PCR) results obtained using nasopharyngeal swabs and/or typical lung lesions observed through chest computed tomography (CT). Patient characteristics were retrieved from digital medical records from the day of admission through the date of last follow-up (May 13, 2020). Data for the following parameters were collected: demographic variables, symptoms and time of presentation, immunosuppressive therapy and management, laboratory parameters, chest CT findings, administered drugs, death, and intensive care unit (ICU) admissions. In accordance with the published literature,4, 5, 6, 7 patients were considered to have severe COVID-19 in the presence of at least 1 of the following criteria: oxygen requirement >6 L/min; need for ICU admission; and death. The study protocol was reviewed and approved by the local Institutional Review Board (approval number: DC-2013-1990).
2.2. Virological diagnosis and follow-up of SARS-CoV-2 infection
In keeping with the COVID-HUS study protocol (ClinicalTrials.gov identifier: NCT04405726), quantitative RT-PCR tests for SARS-CoV-2 nucleic acid were performed using nasopharyngeal swabs and plasma samples obtained at admission and on a weekly basis during follow-up until discharge for all but 1 patient, whose nasopharyngeal swab was collected at another laboratory at the time of diagnosis. Afterward, nasopharyngeal swab testing was performed at 30, 45, and 60 days after the date of symptom onset. Primer and probe sequences targeted 2 regions on the RNA-dependent RNA polymerase gene specific to SARS-CoV-2. SARS-CoV-2 RNA was quantified using serial 10-fold dilutions of SARS-CoV-2 RNA transcript at a concentration of 109 copies/µL. The assay’s sensitivity was ≈10 RNA copies per reaction. A SARS-CoV-2 RT-PCR result was considered positive when at least 1 of the targets was amplified (Supplementary Material).8 Viral clearance was defined as at least 1 negative RT-PCR test in nasopharyngeal swabs.
2.3. SARS-CoV-2 serological assessment
Immunoglobulin (Ig) M and IgG antibody responses to SARS-CoV-2 recombinant nucleocapsid and spike antigens were tested using a commercially available enzyme-linked immunosorbent assay (DIA.PRO Diagnostic BioProbes Srl, Sesto San Giovanni, Italy) according to the manufacturer’s instructions. Antibody levels are presented as measured absorbance values divided by the cut-off. The cut-off value was defined as the mean absorbance values of the 3 negative controls plus 0.250. IgM and IgG specificity and sensitivity reached an overall value of ≥98%. Seronegative patients were negative for IgM and IgG.
2.4. Statistical analysis
Continuous data are presented as medians and interquartile ranges (IQR) and were analyzed using the nonparametric Mann-Whitney U test. Categorical variables are expressed as counts and percentages and were compared using the Fisher exact test. The associations between maximum nasopharyngeal and plasma SARS-CoV-2 viral loads and clinical, demographic, and laboratory variables were determined using Spearman’s correlation coefficient (ρ) values. Receiver operating characteristic (ROC) curves were generated to investigate the viral loads in the upper respiratory tract and plasma with respect to disease severity and mortality. Survival plots of severe COVID-19-free survival and COVID-19-specific survival were graphically represented with Kaplan-Meier curves according to RNAemia (ie, positive plasma viral load), using the log-rank test to compare differences in survival. Patients were censored at the time of last follow-up. Statistical analyses were performed using GraphPad Prism 8.0 (GraphPad Software, La Jolla, CA, USA). A P value < .05 (2-tailed) was considered statistically significant.
3. RESULTS
3.1. General characteristics of the study patients
A total of 40 patients were included in this study. Their demographic and clinical characteristics are presented in Table 1. The patients were mainly men (77.5%), with a median age of 63.8 years (IQR: 54.6-68.2 years) and a median body mass index of 29.5 kg/m2 (IQR: 24-33 kg/m2). The median time after kidney transplantation was 6.6 years (IQR: 2.8-14.6 years). At the time of COVID-19 diagnosis, 35 (87.5%) patients were receiving calcineurin inhibitors (CNI). Mycophenolate mofetil or mycophenolic acid was being taken by 34 patients (85%), mammalian target of rapamycin inhibitors were being administered to 6 (15%) patients, and steroids were taken by 23 (57.5%) patients. The median interval between the onset of symptoms and COVID-19 diagnosis was 4 days (IQR: 3-7 days). All but 2 patients had a fever. Respiratory (n = 34, 85%) and gastrointestinal (n = 31, 77.5%) symptoms were the most common clinical manifestations. Twenty-one patients had a nonsevere clinical presentation, whereas 19 had a severe clinical course. During follow-up, the mortality rate was found to be 22.5% (9/40). The management of immunosuppression and the antiviral and immunomodulatory drugs administered to the study patients upon COVID-19 diagnosis are described in Table 2.
TABLE 1
Demographics and clinical characteristics of patients according to disease severity
| All patients (n = 40) | Nonsevere patients (n = 21) | Severe patients (n = 19) | P | |
|---|---|---|---|---|
| Men | 31 (77.5%) | 19 (90.5%) | 12 (63.1%) | .06 |
| Age, y | 63.8 [54.6-68.2] | 58.4 [50.9-64.3] | 65.5 [62.6-69.9] | .02 |
| >60 y | 25 (62.5%) | 9 (42.9%) | 16 (84.2%) | .01 |
| Comorbidities | ||||
| BMI, kg/m2 | 29.5 [24-33] | 25 [23-32] | 31 [27-33] | .07 |
| <25 | 13 (32.5%) | 11 (52.4%) | 2 (10.5%) | .02 |
| 25-30 | 8 (20%) | 4 (19.1%) | 4 (21.1%) | |
| >30 | 20 (50%) | 7 (33.3%) | 13 (68.4%) | |
| Cardiovascular disease | 16 (40%) | 8 (38.1%) | 8 (42.1%) | 1 |
| Respiratory disease | 9 (22.5%) | 5 (23.8%) | 4 (21%) | 1 |
| Obstructive sleep apnea | 7 (17.1%) | 4 (19.1%) | 3 (15%) | 1 |
| Diabetes | 19 (47.5%) | 8 (38.1%) | 11 (57.9%) | .34 |
| Active cancer | 0 | 0 | 0 | |
| Hypertension | 33 (82.5%) | 15 (71.4%) | 18 (94.7%) | .09 |
| RAAS inhibitor use | 15 (37.5%) | 7 (33.3%) | 8 (42.1%) | .75 |
| ACE inhibitor use | 9 (22.5%) | 3 (14.3%) | 6 (31.6%) | .26 |
| ARB use | 6 (15%) | 4 (19.1%) | 2 (10.5%) | .66 |
| Interval from kidney transplantation (y) | 6.6 [2.8-14.6] | 3.8 [2.1-12.6] | 7.7 [5.2-14.9] | .22 |
| Immunosuppressive therapy | ||||
| Induction immunosuppression | ||||
| Anti-thymocyte globulin | 18 (43.9%) | 10 (47.6%) | 8 (42.1%) | .9 |
| Anti-CD25 | 19 (46.3%) | 9 (42.9%) | 10 (52.6%) | |
| No induction | 3 (7.3%) | 2 (9.5%) | 1 (5%) | |
| Maintenance immunosuppression | ||||
| Tacrolimus | 21 (52.5%) | 10 (47.6%) | 11 (57.9%) | .54 |
| Cyclosporin | 14 (35%) | 7 (33.3%) | 7 (36.8%) | 1 |
| MMF/MPA | 34 (85%) | 19 (90.5%) | 15 (78.9%) | .40 |
| mTOR inhibitors | 6 (15%) | 4 (19.1%) | 2 (10.5%) | .66 |
| Azathioprine | 1 (2.5%) | 0 | 1 (5.3%) | .47 |
| Steroids | 23 (57.5%) | 12 (57.1%) | 11 (57.9%) | 1 |
| Belatacept | 2 (5%) | 2 (9.5%) | 0 | .49 |
| Eculizumab | 1 (2.5%) | 0 | 1 (5.3%) | .47 |
| Clinical symptoms during hospitalization | ||||
| Dyspnea | 28 (70%) | 9 (42.9%) | 19 (100%) | <.001 |
| Cough | 31 (77.5%) | 15 (71.4%) | 16 (84.2%) | .46 |
| Fever | 38 (95%) | 20 (95.2%) | 18 (94.7%) | 1 |
| Myalgia | 22 (55%) | 14 (66.7%) | 8 (42.1%) | .20 |
| Headache | 12 (30%) | 9 (42.8%) | 3 (15.8%) | .09 |
| Diarrhea | 31 (77.5%) | 19 (90.5%) | 12 (63.2%) | .06 |
| Vomiting | 7 (17.5%) | 5 (23.8%) | 2 (10.5%) | .41 |
| Anosmia/ageusia | 8 (20%) | 6 (28.6%) | 2 (10.5%) | .24 |
| Neurological manifestations | 15 (37.5%) | 8 (38.1%) | 7 (36.9%) | 1 |
Continuous variables are presented as median (interquartile range), whereas categorical variables are presented as count (percentage).
ACE, angiotensin converting enzyme; ARB, angiotensin receptor blockers; BMI, body mass index; MMF, mycophenolate mofetil; MPA, mycophenolic acid; mTOR, mammalian target of rapamycin; RAAS, renin-angiotensin-aldosterone system.
TABLE 2
Drugs administered to hospitalized patients stratified according to disease severity
| All patients (n = 40) | Nonsevere patients (n = 21) | Severe patients (n = 19) | |
|---|---|---|---|
| Azithromycin | 26 (65%) | 15 (71.4%) | 11 (57.9%) |
| Other antibiotics | 40 (100%) | 21 (100%) | 19 (100%) |
| Azole antifungals | 1 (2.5%) | 0 (0%) | 1 (5.3%) |
| Lopinavir-ritonavir | 4 (10%) | 1 (4.8%) | 3 (15.8%) |
| Hydroxychloroquine | 15 (37.5%) | 8 (38.1%) | 7 (36.9%) |
| Tocilizumab | 4 (10%) | 1 (4.8%) | 3 (15.8%) |
| High-dose corticosteroidsa | 14 (35%) | 5 (23.8%) | 9 (47.4%) |
| Management of immunosuppression | |||
| MMF/MPA withdrawal | 34/34 (100%) | 17 (100%) | 15 (100%) |
| Calcineurin inhibitors withdrawal | 15/35 (42.6%) | 2 (11.8%) | 13 (72.2%) |
| mTOR inhibitors withdrawal | 6/6 (100%) | 4 (100%) | 2 (100%) |
| Delayed belatacept administration | 1 (2.5%) | 1 (4.8%) | 0 (0%) |
MMF, mycophenolate mofetil; MPA, mycophenolic acid; mTOR, mammalian target of rapamycin.
3.2. Viral load in nasopharyngeal swabs
A total of 118 upper respiratory specimens were analyzed (median of 3 swabs per patient [IQR: 2-3 swabs]). The median viral load was 5.17 log10 copies/reaction (IQR: 3.80-6.69) at diagnosis (between day 0 and day 14 after symptom onset). A total of 29 patients (74.4%) had their peak viral load at admission, and 2 patients hospitalized at D10 and D11 after symptom onset had negative RT-PCR results at admission and during the follow-up. In these 2 cases, COVID-19 was initially diagnosed based on the presence of typical pulmonary lesions on chest CT and subsequently confirmed by positive SARS-CoV-2 serology tests. The viral load at admission was not statistically different between severe and nonsevere patients (6.22 log10 copies/reaction vs 5.17 log10 copies/reaction, respectively, P = .29) and was not predictive of disease severity (area under the ROC curve = 0.595, P = .31) at admission, at the peak of viral load ( Figure 1A,B), or during the course of the disease ( Figure 2). Recipient age (ρ = 0.23, P = .16, Figure 3) and sex (P = .05) were marginally associated with the viral load. Notably, patients receiving steroid therapy and not presenting gastrointestinal symptoms displayed a higher viral load ( Table 3). There was no correlation between the time from symptom onset and viral loads in nasopharyngeal swabs at admission (ρ = 0.261, P = .11). Similar results were observed for SARS-CoV-2 RNAemia (ρ = 0.336, P = .15). Moreover, no correlation was evident between maximum viral load and inflammatory markers, including interleukin (IL)-6 (ρ = 0.041, P = .81) and C-reactive protein (ρ = 0.001, P = .99). Among patients with an initial positive RT-PCR test result (n = 37), no patient showed a viral clearance before D21. Fifteen (38.5%) patients displayed a positive viral load >3 log10 copies/reaction after D10, and 10 patients (24.4%) showed persistent viral shedding after D30 (Figure 2).
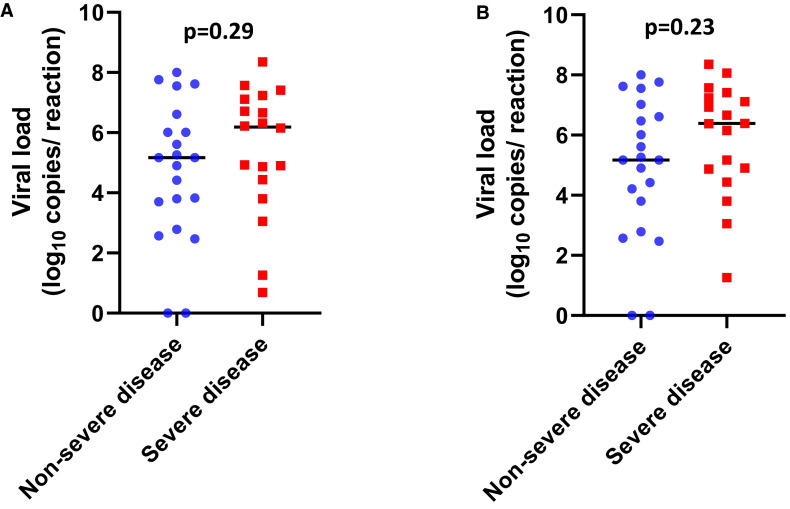
Severe acute respiratory syndrome coronavirus 2 viral load distribution in nasopharyngeal swabs according to disease severity. A, Scatter plots with the medians (black lines) of the viral loads at admission in nonsevere (blue circles) and severe patients (red squares). B, Scatter plots with the medians (black lines) of the maximum viral loads during the follow-up in nonsevere (blue circles) and severe patients (red squares) [Colour figure can be viewed at wileyonlinelibrary.com]
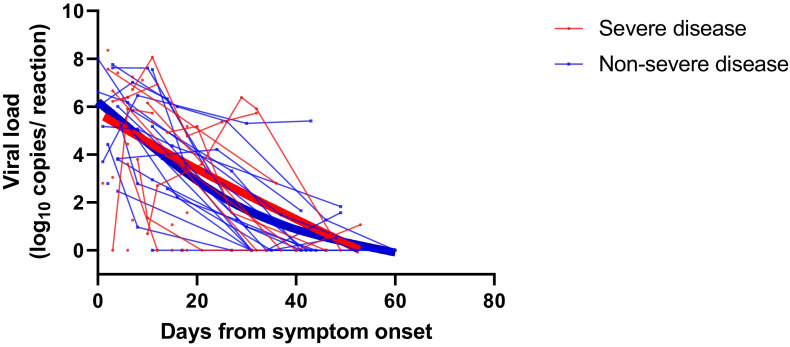
Severe acute respiratory syndrome coronavirus 2 viral load kinetics analyzed using nasopharyngeal swabs. Patients are stratified according to nonsevere disease (blue) and severe disease (red). The thick lines show the trend in viral load using smoothing splines
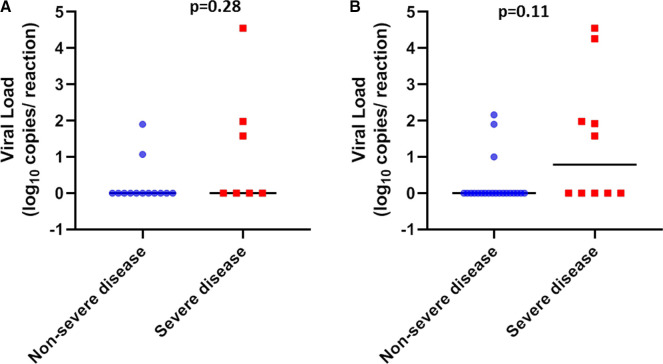
Severe acute respiratory syndrome coronavirus 2 viral load distribution in plasma according to disease severity. A, Scatter plots with the medians (black lines) of the viral loads at admission in nonsevere (blue circles) and severe patients (red squares). B, Scatter plots with the medians (black lines) of the maximum viral loads during the follow-up in nonsevere (blue circles) and severe patients (red squares) [Colour figure can be viewed at wileyonlinelibrary.com]
TABLE 3
SARS-CoV-2 nasopharyngeal viral load in log10 copies/reaction according to demographic and clinical characteristics of hospitalized kidney transplant recipients (N = 39)
| n | Nasopharyngeal maximal viral load | P | |
|---|---|---|---|
| COVID-19 severity | .23 | ||
| Nonsevere disease | 21 | 5.17 [3.80-6.61] | |
| Severe disease | 18 | 6.38 [4.88-7.21] | |
| Recipient age, y | .70 | ||
| <60 | 24 | 5.26 [3.42-6.92] | |
| ≥60 | 15 | 5.88 [4.43-7.04] | |
| Sex | .05 | ||
| Female | 8 | 7.34 [5.74-8.02] | |
| Male | 31 | 5.17 [4.32-6.63] | |
| BMI, kg/m2 | .08 | ||
| <30 | 20 | 6.50 [5.10-7.32] | |
| ≥30 | 19 | 4.90 [3.43-6.42] | |
| Obstructive sleep apnea | .57 | ||
| No | 32 | 5.81 [4.71-7.14] | |
| Yes | 7 | 4.44 [3.50-4.44] | |
| Cardiovascular disease | .84 | ||
| No | 23 | 6.01 [4.88-6.84] | |
| Yes | 16 | 5.04 [3.80-7.50] | |
| Respiratory disease | .31 | ||
| No | 24 | 6.26 [4.62-7.28] | |
| Yes | 15 | 4.90 [4.32-6.70] | |
| Diabetes | .11 | ||
| No | 21 | 6.01 [4.90-7.55] | |
| Yes | 18 | 5.04 [3.24-6.45] | |
| Hypertension | .84 | ||
| No | 7 | 5.17 [4.35-6.82] | |
| Yes | 32 | 5.81 [4.37-7.14] | |
| RAAS inhibitor | .17 | ||
| No | 25 | 6.38 [4.90-7.11] | |
| Yes | 14 | 6.88 [3.34-6.53] | |
| Immunosuppressive induction therapy | .20 | ||
| Anti-thymocyte globulin | 18 | 6.38 [2.59-7.61] | |
| Anti-CD25 | 18 | 5.17 [3.96-6.45] | |
| No induction | 3 | 4.90 [3.85-6.07] | |
| Immunosuppressive maintenance therapy | |||
| CNI | .71 | ||
| No | 5 | 4.90 [4.42-7.76] | |
| Yes | 34 | 5.81 [3.96-7.00] | |
| MMF/MPA | .15 | ||
| No | 6 | 7.08 [6.34-7.81] | |
| Yes | 33 | 5.17 [4.21-6.66] | |
| mTOR | .92 | ||
| No | 33 | 5.61 [4.21-7.02] | |
| Yes | 6 | 5.92 [4.54-7.16] | |
| Steroids | .03 | ||
| No | 17 | 4.87 [3.05-6.38] | |
| Yes | 22 | 6.50 [5.17-7.53] | |
| Clinical symptoms | |||
| Dyspnea | .23 | ||
| No | 12 | 4.79 [2.73-6.71] | |
| Yes | 27 | 6.15 [4.88-7.17] | |
| Diarrhea | .02 | ||
| No | 9 | 6.93 [6.38-7.41] | |
| Yes | 30 | 5.17 [3.80-7.83] | |
| Positive RNAemia | .35 | ||
| No | 18 | 5.17 [4.26-6.88] | |
| Yes | 9 | 6.61 [5.26-7.24] | |
Nasopharyngeal maximal viral load is presented as median (interquartile range).
BMI, body mass index; CNI, calcineurin inhibitor; COVID-19, coronavirus disease 2019; MMF, mycophenolate mofetil; MPA, mycophenolic acid; mTOR, mammalian target of rapamycin; RAAS, renin-angiotensin-aldosterone system; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
3.3. SARS-CoV-2 RNAemia
SARS-CoV-2 loads were measured in 73 plasma samples obtained from 31 patients (21 in the nonsevere group and 10 in the severe group). Plasma viral loads ranged from 1 to 4.55 log10 copies/reaction. Three patients had positive viremia at admission (Figure 3A) and 8 showed at least 1 positive RNAemia during the follow-up period (Figure 3B). Severe patients showed a higher frequency of RNAemia compared with nonsevere patients (50% vs 14.3%, respectively, P = .01, Figure 4A). Moreover, RNAemia was found to be associated with mortality (Figure 4B). Accordingly, RNAemia was positive in 3 of the 4 nonsurvivors tested; in contrast, only 5 of 29 (24%) tested survivors had positive RNAemia (P = .035). Furthermore, 2 nonsurvivors harbored high viral loads (4.55 log10 copies/reaction and 4.26 log10 copies/reaction), whereas other patients were characterized by low RNAemia (<2.16 log10 copies/reaction). With regard to immunosuppressive therapy, patients receiving CNI tended to have more positive RNAemia albeit not significantly so (8/26 vs 0/5, respectively, P = .29). There were no correlations between SARS-CoV-2 RNAemia and viral loads in nasopharyngeal swabs both at admission (ρ = 0.375, P = .13) and during follow-up (ρ = 0.159, P = .41).
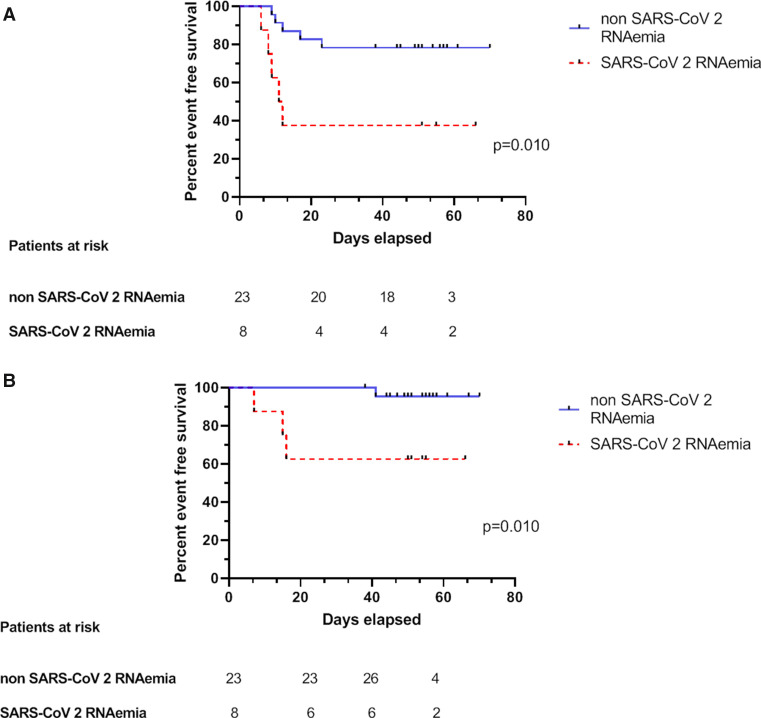
Association of positive SARS-CoV-2 RNAemia with COVID-19 severity and mortality. A, Kaplan-Meier plots of COVID-19-free survival according to SARS-CoV-2 RNAemia. Presence of SARS-CoV-2 RNAemia (dotted red curve) vs its absence (solid blue curve), P = .010. B, Kaplan-Meier plots of severe COVID-19-free survival according to SARS-CoV-2 RNAemia. Presence of SARS-CoV-2 RNAemia (dotted red curve) vs its absence (solid blue curve), P = .01. COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2 [Colour figure can be viewed at wileyonlinelibrary.com]
3.4. SARS-CoV-2 serological findings
A total of 116 samples from 35 patients were analyzed, with a median of 3 sera tested per patient (IQR: 2-4 sera). All survivors were seropositive at follow-up. Four nonsurvivors had negative serology at their time of death, which occurred on D7, D10, D15, and D16. The 2 patients with a negative SARS-CoV-2 RT-PCR result had positive serology, with 1 patient showing positive serology at the time of diagnosis. Among the 25 samples tested before D8, 6 (24%) were seropositive, whereas all samples tested after D14 were seropositive ( Figure 5). The kinetics of the antibodies showed that a stable titer of IgG antibodies was maintained until D59, suggesting persistence of immunity for at least until 2 months after infection ( Figure 6). Notably, IgM and IgG antibody levels and delays in seroconversion were not correlated with COVID-19 severity ( Figure 7A,B).
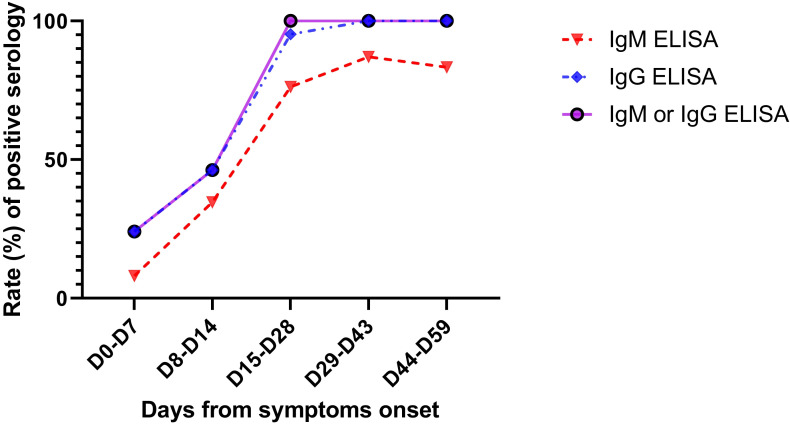
Rate of positive SARS-CoV-2 IgM (dotted red curve with triangles), IgG (dotted blue curve with rhombus), and IgM or IgG (solid purple curve) tested by an ELISA according to the days from symptom onset. A total of 116 samples from 35 patients were tested. From day 15 (D15) onwards, all samples were positive for IgM or IgG. ELISA, enzyme-linked immunosorbent assay; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2 [Colour figure can be viewed at wileyonlinelibrary.com]
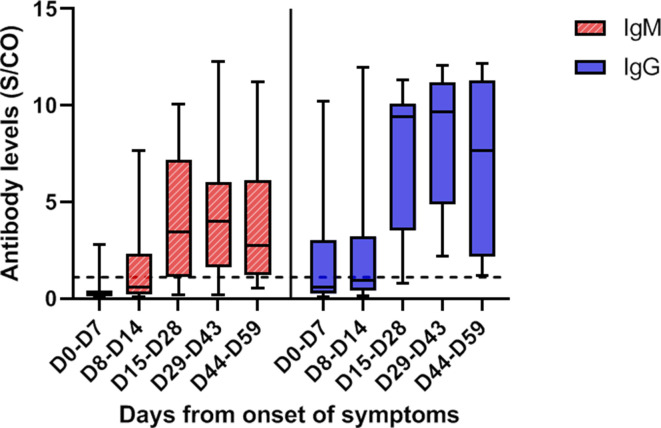
SARS-CoV-2 IgM (hatched red plots) and IgG (filled blue plots) titers tested by an ELISA, according to the days from symptom onset. IgM and IgG levels increased significantly over time. Antibody levels are presented as the measured S/CO. The dotted line represents the cutoff value (1.1). The boxplots show medians (middle line) and first and third quartiles (boxes), whereas the whiskers indicate minimum and maximum values. ELISA, enzyme-linked immunosorbent assay; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; S/CO, absorbance values divided by the cutoff [Colour figure can be viewed at wileyonlinelibrary.com]
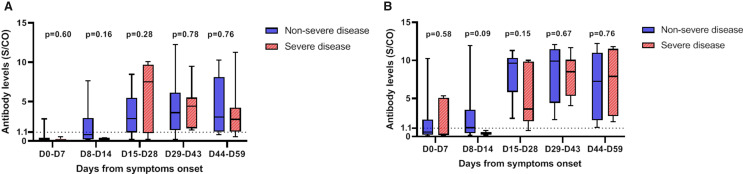
Severe acute respiratory syndrome coronavirus 2 IgM (A) and IgG (B) titers tested by an enzyme-linked immunosorbent assay according to the days from symptom onset and stratified by disease severity (severe [hatched red plots] vs nonsevere [filled blue plots]). IgM and IgG antibody levels did not differ according to disease severity (P > .05). Antibody levels are presented as the measured S/CO. The dotted line represents the cutoff value (1.1). The boxplots show medians (middle line) and first and third quartiles (boxes), whereas the whiskers indicate minimum and maximum values. S/CO, absorbance values divided by the cutoff [Colour figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
In this retrospective study conducted in a sample of 40 immunocompromised KTR hospitalized for COVID-19, we precisely determined the temporal evolution of nasopharyngeal and plasma SARS-CoV-2 loads, as well as the serological response to the virus. All parameters were correlated with patient characteristics, disease severity, and clinical outcomes. In our study, the viral load in the respiratory specimens of most patients was at the peak at the time of diagnosis. This finding is in line with those previously reported for the general population.9, 10, 11 Based on the data we analyzed, the viral load at the time of diagnosis did not predict the severity of the disease. Moreover, viral loads were not related to inflammatory markers that have been previously associated with COVID-19 severity.12 Reports on the relationship between viral load and disease severity are contradictory; 3 studies showed no correlation between the severity of the disease and the viral load in respiratory specimens,10 , 11 , 13 whereas Liu et al14 described a higher viral load in patients with more severe disease.
In our immunocompromised population with a median follow-up of 53 days, the duration of viral detection in the respiratory tract was longer compared to that in the general population. More than a third of our patients displayed a high viral load after D10. Furthermore, almost a quarter of them still had viral shedding at D30; in contrast, in immunocompetent populations, the median duration of viral shedding was 20 days.15 Isolation was stopped after 2 consecutively negative RT-PCR tests in nasopharyngeal swabs. Notably, this approach generally resulted in prolonged isolation periods. Only 8 of the 40 study patients had evidence of 2 consecutively negative RT-PCR tests in nasopharyngeal swabs during follow-up (median duration: 53 days). These figures seem longer that those reported for the general population. Notably, the Centers for Disease Control and Prevention recommend 10-day isolation for the immunocompetent population.16 Thus, even if a positive RT-PCR result does not indicate an infectious virus, we should be cautious about viral spread in immunocompromised populations and extend isolation after a SARS-CoV-2 infection.
SARS-CoV-2 RNAemia was positive in 32% of our KTR with COVID-19. To our knowledge, SARS-CoV-2 RNAemia in immunocompromised patients had not been previously described. Nonetheless, this finding is in accordance with what was observed in the general population (ranging from 10.4% to 41% positivity for RNAemia).12 , 15 , 17 Our study shows that RNAemia is associated with disease severity and mortality in KTR patients. However, published data on the subject are mixed. In studies conducted by Huang et al15 and Zheng et al,17 RNAemia was not associated with COVID-19 severity, whereas in the cohort study carried out by Chen et al,12 positive RNAemia was correlated with highly elevated IL-6 plasma level and was only detected in critically ill patients. In the study by Hadjadj et al,13 patients with severe and critical COVID-19 had higher plasma viral loads than those with mild and moderate disease. The pathophysiological mechanism of the association between COVID-19 severity and plasma viral load is still unclear; perhaps the cytokine storm that affects COVID-19 severity could enhance RNAemia by causing increased vascular permeability, or a significant viral load could trigger the cytokine storm.12
Regarding the SARS-CoV-2 humoral response, this is the largest study with the longest follow-up in an immunocompromised population. All but 4 patients had positive serology during the follow-up. The 4 negative patients were tested early in the course of the disease and died shortly after. A total of 13 (43.3%) patients displayed positive serology before D15, supporting the potential usefulness of serology tests for acute diagnosis. All patients harbored SARS-CoV-2 antibodies from D15 onwards. Data on the serological response to SARS-CoV-2 in immunocompromised populations remain scanty. In a study from the United States, 9 patients infected with SARS-CoV-2 after a solid organ transplant had positive IgG serology, with a delay of seroconversion between D6 and D27.18 Zhao et al19 reported a delayed antibody response in a patient co-infected with COVID-19, HIV, and hepatitis C. In the general population, studies have reported seroconversion for all patients between the third and fourth week after symptom onset, which is concordant with our findings. These results suggest that the anti-SARS-CoV-2 humoral response is not significantly impaired in our immunocompromised population.20, 21, 22 Notably, the delay after transplantation was long in our cohort, and only 1 patient had undergone depleting induction therapy during the year preceding COVID-19. The level of IgG remained steady until 2 months after onset of symptoms. Although the neutralizing effect of the antibodies was not studied here, it is encouraging that IgG levels are correlated with a neutralizing effect in the general population.10 , 20
To our knowledge, this is the first report that provides a precise assessment of SARS-CoV-2 virological and antibody response kinetics in an immunocompromised population, with a follow-up for 2 months after symptom onset. However, several caveats need to be considered. First, the small sample size poses a limitation regarding the ability to generalize our conclusions. Second, the lack of data from some patients represents a potential bias. Finally, retrospective cohort studies are prone to unavoidable confounding or residual confounding on unmeasured variables.
In summary, our data indicate that (1) SARS-CoV-2 shedding from the upper respiratory tract is prolonged in KTR patients, indicating the requirement for prolonged protective measures for these patients; (2) the SARS-CoV-2 plasma load is associated with COVID-19 severity and mortality, whereas the viral load of the upper respiratory tract is not; and (3) based on the presence of antibodies in all samples collected by us after the second week of symptom onset, the SARS-CoV-2 humoral response in our immunocompromised population does not show serious impairment and the antibodies persist until 2 months after COVID-19 symptom onset.
ACKNOWLEDGMENTS
This study was supported by the Strasbourg University Hospital (COVID-HUS study-HUS N°7760).
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Dr Caillard reports personal fees and nonfinancial support from Novartis, nonfinancial support from Sanofi, and nonfinancial support from Astellas, unrelated to the current study. The other authors have no conflicts of interest to disclose.
DATA AVAILABILITY STATEMENT
Data supporting the findings from this study are available from the corresponding author upon reasonable request.
Notes
Funding information Strasbourg University Hospital (COVID-HUS study-HUS N°7760)
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
Supplementary Material
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.1111/ajt.16251
Read article for free, from open access legal sources, via Unpaywall:
http://www.amjtransplant.org/article/S1600613522215639/pdf
Citations & impact
Impact metrics
Article citations
SARS-CoV-2 viral load is linked to remdesivir efficacy in severe Covid-19 admitted to intensive care.
Sci Rep, 14(1):20825, 06 Sep 2024
Cited by: 0 articles | PMID: 39242658 | PMCID: PMC11379941
SARS-CoV-2-specific immune responses converge in kidney disease patients and controls with hybrid immunity.
NPJ Vaccines, 9(1):93, 28 May 2024
Cited by: 0 articles | PMID: 38806532 | PMCID: PMC11133345
Comprehensive immune profiling of SARS-CoV-2 infected kidney transplant patients.
Front Transplant, 2:1261023, 20 Nov 2023
Cited by: 0 articles | PMID: 38993862
Evaluation of the Humoral and Cellular Immune Response Post COVID-19 Infection in Kidney Transplant Recipients.
J Clin Med, 12(12):3900, 07 Jun 2023
Cited by: 0 articles | PMID: 37373595 | PMCID: PMC10298929
Method for determining predictor factor for worse outcomes in kidney transplant recipients infected with coronavirus disease 2019 in a systematic review and meta-analysis research.
MethodsX, 11:102250, 11 Jun 2023
Cited by: 0 articles | PMID: 37325705 | PMCID: PMC10257946
Go to all (64) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT04405726
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Viral Clearance and Serological Response to SARS-CoV-2 in Kidney Transplant Recipients.
Transplant Proc, 53(4):1180-1186, 17 Dec 2020
Cited by: 9 articles | PMID: 33419577 | PMCID: PMC7834639
The kinetics of viral load and antibodies to SARS-CoV-2.
Clin Microbiol Infect, 26(12):1690.e1-1690.e4, 06 Sep 2020
Cited by: 37 articles | PMID: 32898715 | PMCID: PMC7474805
Kinetics of torquetenovirus DNA load in a recent kidney transplant recipient with mild SARS-CoV-2 infection and a failed antibody response.
Transpl Infect Dis, 23(2):e13524, 06 Dec 2020
Cited by: 8 articles | PMID: 33226684 | PMCID: PMC7744855
Case report of a neonate with high viral SARSCoV-2 loads and long-term virus shedding.
J Infect Public Health, 13(12):1878-1884, 27 Oct 2020
Cited by: 10 articles | PMID: 33158806 | PMCID: PMC7590917
Review Free full text in Europe PMC
Funding
Funders who supported this work.





