Abstract
Purpose
To identify risk factors associated with lens opacities in Chinese Americans.Methods
A cross-sectional population-based study of 4,582 Chinese Americans ≥50 years residing in Monterey Park, California. Participants completed a comprehensive clinical examination with lens assessment using the Lens Opacities Classification System II, with lens opacities defined by a grade ≥2 in either eye. Participants were considered to have nuclear-only, cortical-only, or posterior subcapsular (PSC)-only if that was the only type of opacity present in both eyes.Results
Cortical-only opacity was associated with older age, diabetes mellitus (OR 1.5, 95%CI 1.1-2.1), and family history of cataracts (OR 1.5, 95%CI 1.2-1.9). Nuclear-only opacity was associated with older age, diabetes mellitus (OR 1.4, 95%CI 1.1-1.9), greater waist-to-hip ratio (OR 1.2, 95%CI 1.1-1.4), and high-density lipoprotein (OR 1.1, 95%CI 1.02-1.2). Mixed-type opacities were associated with older age, greater waist-to-hip ratio (OR 1.3, 95%CI 1.1-1.6), and higher HbA1 c (OR 1.3, 95%CI 1.1-1.4). Taller height (OR 0.7, 95%CI 0.6-0.8), greater weight (OR 0.98, 95%CI 0.97-0.99), and higher diastolic pressure (OR 0.98, 95%CI 0.96-0.99) were protective.Conclusion
CHES identified a strong, dose-response association between age and all types of prevalent lens opacities, which suggests an increasing cataract burden in Chinese Americans based on aging populations. CHES results demonstrate general consistency with previous population-based studies in regard to more sedentary lifestyle exposures (e.g., Westernized lifestyle) and prevalent cortical-only, nuclear-only, and mixed-type opacities, yet also identified further sedentary lifestyle exposures associated with prevalent lens opacities. Improved glycemic control and a more active lifestyle that minimizes factors contributing to metabolic syndrome may help reduce the burden of vision loss associated with lens opacities.Free full text

Factors Associated with Prevalent Lens Opacities in Chinese American Adults: The Chinese American Eye Study
Abstract
Purpose:
To identify risk factors associated with lens opacities in Chinese Americans.
Methods:
A cross-sectional population-based study of 4,582 Chinese Americans ≥50 years residing in Monterey Park, California. Participants completed a comprehensive clinical examination with lens assessment using the Lens Opacities Classification System II, with lens opacities defined by a grade ≥2 in either eye. Participants were considered to have nuclear-only, cortical-only, or posterior subcapsular (PSC)-only if that was the only type of opacity present in both eyes.
Results:
Cortical-only opacity was associated with older age, diabetes mellitus (OR 1.5, 95%CI 1.1–2.1), and family history of cataracts (OR 1.5, 95%CI 1.2–1.9). Nuclear-only opacity was associated with older age, diabetes mellitus (OR 1.4, 95%CI 1.1–1.9), greater waist-to-hip ratio (OR 1.2, 95%CI 1.1–1.4), and high-density lipoprotein (OR 1.1, 95%CI 1.02–1.2). Mixed-type opacities were associated with older age, greater waist-to-hip ratio (OR 1.3, 95%CI 1.1–1.6), and higher HbA1c (OR 1.3, 95%CI 1.1–1.4). Taller height (OR 0.7, 95%CI 0.6–0.8), greater weight (OR 0.98, 95%CI 0.97–0.99) and higher diastolic pressure (OR 0.98, 95%CI 0.96–0.99) were protective.
Conclusion:
CHES identified a strong, dose-response association between age and all types of prevalent lens opacities, which suggests an increasing cataract burden in Chinese Americans based on aging populations. CHES results demonstrate general consistency with previous population-based studies in regards more sedentary lifestyle exposures (e.g., Westernized lifestyle) and prevalent cortical-only, nuclear-only, and mixed-type opacities, yet also identified further sedentary lifestyle exposures associated with prevalent lens opacities. Improved glycemic control and a more active lifestyle that minimizes factors contributing to metabolic syndrome may help reduce the burden of vision loss associated with lens opacities.
INTRODUCTION
Lens opacities known as age-related cataracts are a leading cause of blindness worldwide and visual impairment in the US.1 US prevalence estimates are 70% in adults older than 75 years, versus 50% in adults between 65–74 years.2 Comparison of prevalence estimates across racial/ethnic groups, particularly among various opacity types, suggests differences by racial/ethnic group.3–7 Reasons are unclear and may include: 1) variation in genetic susceptibility; 2) dissimilarities in research methodology (e.g., case ascertainment); and 3) differences in the exposure to, and prevalence of, risk factors by racial/ethnic group.
Asian Americans, of which Chinese Americans represent the majority, are the fastest growing US demographic over the past 2 decades. Between 2000 and 2010, the Asian American population increased 43.3% to 14.7 million (4.8% of the population), according to the US Census Bureau.8,9 When considering this demographic in the context of the aging US population and increased life expectancy, it is important to assess factors associated with disease to better understand the underlying biology and develop preventative measures.
The Chinese American Eye Study (CHES) is the first comprehensive study designed to evaluate the prevalence of major eye disease in Chinese Americans in the US. The purpose of this analysis is to determine the association between risk factors (e.g., sociodemographic, biological) and the prevalence of nuclear-only, cortical-only, posterior subcapsular (PSC)-only, and mixed-type lens opacities among CHES participants.
MATERIALS AND METHODS
Study Cohort
The Chinese American Eye Study (CHES), a population-based cohort study designed to determine prevalence estimates and assess risk indicators for major eye diseases in Chinese Americans 50 years and older living in Los Angeles County, California, was used in these analyses. Study methods have been described;10 methodology including the LOCS II grading system11 is nearly identical to the Los Angeles Latino Eye Study (LALES).3,4,10,12 Briefly, 4582 participants were recruited between February 2010 and October 2013 from self-identified Chinese Americans from 10 census tracts of Monterey Park, California, where demographic and socioeconomic characteristics mirror Chinese populations in Los Angeles County, California, and the US.10 Eligible residents were identified through a door-to-door census and invited to complete a questionnaire and clinical examination, conducted after obtaining informed consent. Institutional review board approval was obtained from the University of Southern California Health Sciences Institutional Review Board. All study procedures adhered to the principles outlined in the Declaration of Helsinki.
Interview and Clinical Examination
Interview and clinical examination details have been described.10 Briefly, data on sociodemographic, medical, and ocular history, insurance status, access to care, and acculturation on the Suinn-Lew Asian Self-Identity Acculturation (SL-ASIA) scale13 were collected via questionnaire, and participants underwent a comprehensive eye examination by certified CHES staff. Total cholesterol, high- and low-density lipoprotein (HDL, LDL), and triglycerides were measured using the Cholestech LDX system (Alene). Random blood glucose and glycosylated hemoglobin (HbA1c) were measured using the Hemocue B-Glucose Analyzer (Hemocue Inc.) and the DCA Vantage Analyzer (Siemens Healthcare), respectively.
Lens Examination Protocol
The Lens Opacities Classification System II (LOCS II)11 was used to grade nuclear, cortical, and PSC lens opacities by live grading at the slit lamp under maximum dilation with tropicamide 1% and phenylephrine 2.5%.4 LOCS II,11 as opposed to LOCS III methodology,14 was selected when CHES was designed in order to maintain consistency with LALES.3,4,10,12 Opacities of increasing severity were graded according to LOCS II photographic standards with 6 cortical standards (C0, Ctr, CI, CII, CIII, CIV), 5 nuclear standards based on opalescence and color (N0, NI, NII, NIII, NIV), and 5 PSC standards (P0, PI, PII, PIII, PIV).15 Every 6 months, inter-examiner agreement between the two examining ophthalmologists was assessed in a convenience sample of 50 participants using a proportionally-weighted Kappa (κ) statistic with the percent agreement for each opacity type (nuclear, cortical, and PSC opacities).4,10 The weighted kappa was used for summarizing inter-rater agreement on the categorical scale for lens opacity grading (e.g., 4×4, 5×5-agreement table).
Definition of Lens Opacities
Analyses were based on LOCS II standards, including the reference clear lens.11 If 1 eye had undergone cataract surgery or was not gradable, the other eye was graded. Lens opacities were considered present if participants had a LOCS II grade of greater than or equal to 2 in 1 or both eyes. If grading was not possible, reasons were recorded. Lens opacity definitions used in CHES4,10 are consistent with LALES;16–18 however only “Single or Mixed-Types” of lens opacities, defined below, are reported in this analysis:
Single and Mixed-Types of lens opacities: 1 or more opacity type in an individual. Participants were considered to have a single opacity (categorized as PSC-only, nuclear-only, cortical-only) if that was the only type present in both eyes. Participants with more than 1 type were categorized as having mixed opacities; this definition rendered all 4 categories (PSC-only, nuclear-only, cortical-only, mixed-type) mutually exclusive. The prevalence of single and mixed opacity types was based on participants with gradable LOCS II findings for each type. For this definition, if a participant had unilateral cataract extraction, the LOCS II grading from the contralateral phakic eye defined opacity.
For purposes of this manuscript, the term “lens opacities” refers to cataracts.
Risk Factor Assessment
The conceptual model guiding analysis included 4 potential risk factor categories: sociodemographic; lifestyle; anthropometric/clinical; and health care access/utilization. Sociodemographic variables analyzed as potential covariates included: age; gender; country of birth; income; years of education; marital status; and acculturation. Lifestyle factors, or specifically, personal health practice factors, included use of: cigarettes, alcohol, diabetes treatments, anti-inflammatory drugs, statins, and antihypertensive drugs; and for women, oral contraceptives and female hormones. Anthropometric/clinical factors, or more simply known as biologic factors, assessed through interview included a history of diabetes, and hypertension, and a family history of cataracts (any cataract). Biologic factors assessed through the clinical examination included: HbA1c; height; weight; body mass index (BMI: underweight, <18.5 kg/m2; normal weight, 18.5–24.9 kg/m2; overweight, 25.0–29.9 kg/m2; obese, > 30.0 kg/m2); waist-to-hip ratio; systolic blood pressure (SBP); diastolic blood pressure (DBP); total cholesterol, HDL and LDL cholesterol, and triglycerides; comorbidities; glaucoma; intraocular pressure; spherical equivalent refractive error; axial length; and large drusen or age-related macular degeneration based on graded fundus photographs. Health care access/utilization factors included having: health and vision insurance coverage; a particular doctor or clinic; and access to care.
Statistical Analyses
The association between prevalent risk factors and lens opacity type (e.g., no opacity, cortical-only, nuclear-only, mixed-type) was assessed in univariate analysis by Chi-square and t-tests. Multivariate logistic regression estimated the odds ratios (OR) and 95% confidence intervals (CIs) between sociodemographic, lifestyle, biological, and health care access/utilization factors and each opacity type (dichotomous outcome for each lens opacity type; control group representing an absence of lens opacity). Candidate covariates were explored within each of 4 exposure categories; variables with a p <0.10 were evaluated in the combined multivariate model and included in the final model using a threshold of p <0.05 to identify the final independent association of significant exposure for each opacity type. PSC-only outcomes were not assessed due to limited numbers (n = 7). Mixed opacities were analyzed to assess exposure-outcome relationships in people that had more than one type of opacity present between both eyes. Models were evaluated as sex-combined and sex-specific, but reported as sex-combined given the similar results and lack of statistical power to assess risk factors by sex. Possible confounding was assessed by noting the change in beta-estimate (>10%) with addition of the risk factor to the main effect model. Effect modification of the association between lens opacity and exposure was tested by incorporating interaction terms in final regression models. To further assess the relationship between exposure and outcomes, local regression methods adjusting for final logistic regression model covariates were used to generate LOWESS (locally weighted smoothing regression) plots. All analyses were conducted assuming a two-sided 0.05 significance level using SAS v. 9.4 (SAS Institute).
RESULTS
Among 5782 eligible individuals, 4582 (79.2%) participants completed a CHES clinical examination. CHES participants were similar in age (mean age 61 vs 63), more likely female (63% vs 52%), and more educated (67% vs 58% completed ≥ 12 years of school) compared to Chinese living in the US.4,9 Among the 4234 out of the 4582 (92.4%) CHES participants with LOCS II grading, 927 (21.9%) had nuclear-only, 386 (9.1%) had cortical-only, 7 (0.2%) had PSC-only, 531 (12.5%) had mixed-type, and 2383 (56.3%) had no lens opacities; only 42 (0.9%) had missing LOCS II data. Of the 454 participants with cataract extraction, 303 (66.7%) had bilateral cataract extraction, 3 (0.1%) were monocular, and only 148 (3.2%) had previous cataract surgery in one eye with LOCS II grading in the other eye. Only 348 participants did not have any LOCS II grading (303 with bilateral cataract extractions, 3 who were monocular with cataract extraction), and only 42 (0.9%) had missing data due to advanced cataracts, poor dilation, or refusal of dilation.4,10 LOCS II inter-grader agreement was moderate-to-excellent for all opacity types (cortical opacities, weighted κ = 0.86 [0.72–1.00; 95% CI]; nuclear opacities, weighted κ = 1.0; PSC opacities, weighted κ = 0.94 [0.71–1.0]),4,10 consistent with other assessments.11,14,19
Table 1 shows the distribution of potential exposures by opacity type. Mean age was younger in participants without opacities: 57.4 (SD ± 5.7) years for no opacities, 62.9 (SD ± 7.8) years for cortical-only, 64.4 (SD ± 8.7) years for nuclear-only, and 68.1 (SD ± 8.8) years for mixed-type opacities (Table 1). The distribution of additional covariates (e.g., sociodemographic, lifestyle, biological, and health care access/utilization factors) demonstrates general similarities across various opacity types. Variables significant with outcomes at the univariate level (p <0.1) were included in the final model for each opacity type (dichotomous outcome for each lens opacity type; control group representing an absence of lens opacity).
Table 1.
Distribution of Characteristics Stratified by Lens Opacity Type in Participants of the Chinese American Eye Study (CHES)
| Variables | No Opacity | Mixed Type | Cortical Only | Nuclear Only | |
|---|---|---|---|---|---|
| n = 2363 | n = 531 | n = 386 | n = 927 | ||
| SOCIODEMOGRAPHIC FACTORS | |||||
| Mean age | 57.4 ± 5.7 | 68.1 ± 8.8 | 62.9 ± 7.8 | 64.4 ± 8.7 | |
| Female gender | 1498 (63.4%) | 323 (60.8%) | 270 (70%) | 572 (61.7%) | |
| Low acculturation score (< 1.9) | 917 (38.9%) | 218 (41.1%) | 162 (42.1%) | 343 (37.1%) | |
| Marital status | |||||
| Never married | 174 (7.5%) | 21 (4%) | 15 (3.9%) | 36 (4%) | |
| Married | 1842 (79%) | 389 (74%) | 293 (76.3%) | 704 (77%) | |
| Divorced/separated | 317 (13.6%) | 116 (22.1%) | 76 (19.8%) | 178 (19.4%) | |
| Income < $20,000 | 1120 (51.3%) | 308 (63.4%) | 221 (62.1%) | 532 (60.5%) | |
| Education | |||||
| Less than high school | 736 (31.4%) | 170 (32.4%) | 114 (29.6%) | 311 (33.8%) | |
| High school and college | 1605 (68.6%) | 355 (67.6%) | 271 (43.6%) | 610 (66.2%) | |
| Language | |||||
| Chinese only | 951 (40.4%) | 231 (43.6%) | 140 (36.3%) | 398 (43%) | |
| Most Chinese some English | 1074 (45.6%) | 221 (41.7%) | 186 (48.2%) | 411 (44.4%) | |
| Equally Chinese and English or more English | 331 (14.1%) | 78 (14.7%) | 60 (15.5%) | 115 (12.4%) | |
| LIFESTYLE FACTORS | |||||
| Smoking status | |||||
| Never | 2005 (85.1%) | 445 (84.3%) | 347 (90.1%) | 783 (85%) | |
| Ex-smoker | 167 (7.1%) | 59 (11.2%) | 24 (6.2%) | 72 (7.8%) | |
| Current smoker | 183 (7.8%) | 24 (4.6%) | 14 (3.6%) | 71 (7.7%) | |
| Alcohol | |||||
| Never | 1991 (84.6%) | 453 (86%) | 337 (87.3%) | 809 (87.4%) | |
| Ex-drinker | 62 (2.6%) | 25 (4.7%) | 10 (2.6%) | 33 (3.6%) | |
| Current drinker | 301 (12.8%) | 49 (9.3%) | 39 (10.1%) | 84 (9.1%) | |
| Treated for diabetesa | 24 (13.4%) | 15 (13.8%) | 8 (16.3%) | 16 (12.3%) | |
| Prior use of anti-inflammatory drugs | 146 (6.2%) | 63 (11.9%) | 43 (11.2%) | 75 (8.1%) | |
| Prior use of statins | 168 (7.1%) | 108 (20.4%) | 46 (12.0%) | 143 (15.5%) | |
| Prior use of contraceptive pills | 153 (10.3%) | 35 (10.9%) | 31 (11.7%) | 59 (10.4%) | |
| Prior use of female hormones | 87 (5.9%) | 35 (11.0%) | 30 (11.2%) | 49 (8.6%) | |
| BIOLOGICAL FACTORS | |||||
| Mean height (cm) | 161.3 ± 8.3 | 158.5 ± 7.9 | 158.4 ± 7.6 | 160.2 ± 8.1 | |
| Mean weight (kg) | 64 ± 11.6 | 61.8 ± 10.6 | 62.2 ± 11.3 | 63.2 ± 10.6 | |
| Mean waist-to-hip ratio | 0.87 ± 0.07 | 0.89 ± 0.07 | 0.87 ± 0.07 | 0.88 ± 0.07 | |
| Mean random blood glucose | 100.5 ± 33.4 | 107.3 ± 43.1 | 103.2 ± 3 | 103.1 ± 36.7 | |
| Mean HbA1C | 5.8 ± 0.7 | 6.2 ± 1.1* | 6.0 ± 0.8 | 6 ± 0.8 | |
| Mean spherical equivalent | −0.6 ± 3.1 | −0.6 ± 4 | −0.6 ± 3.2 | −0.5 ± 3.4 | |
| Self-reported diabetes | 184 (7.8%) | 112 (21.5%) | 50 (13.2%) | 130 (14.2%) | |
| Diabetes mellitus | 286 (12.1%) | 141 (26.6%) | 75 (19.4%) | 174 (18.8%) | |
| Hypertension | 773 (32.7%) | 313 (59%) | 178 (46.1%) | 455 (49.1%) | |
| Self-reported history of cataract | 207 (10.2%) | 265 (55.2%) | 117 (35.1%) | 257 (32.8%) | |
| Family history of cataract | 1342 (60.5%) | 263 (57.6%) | 183 (52.3%) | 498 (62.3%) | |
| Mean BMI | 24.5 ± 3.6 | 24.5 ± 3.2 | 24.7 ± 3.7 | 24.6 ± 3.4 | |
| BMIb | |||||
| Normal/underweight | 1405 (60%) | 313 (60%) | 224 (58.2%) | 513 (56.7%) | |
| Overweight | 803 (34.1%) | 186 (35.4%) | 124 (32.2%) | 338 (37.4%) | |
| Obese | 147 (6.2%) | 26 (5%) | 37 (9.6%) | 54 (6%) | |
| Any AMD | 275 (13.3%) | 91 (20%) | 58 (16.1%) | 138 (17.8%) | |
| Comorbiditiesc | |||||
| 0 | 921 (39.0%) | 109 (20.5%) | 117 (30.3%) | 281 (30.3%) | |
| 1 | 622 (26.3%) | 127 (23.9%) | 82 (21.2%) | 209 (22.6%) | |
| ≥2 | 820 (34.7%) | 295 (55.6%) | 187 (48.5%) | 437 (47.1%) | |
| Glaucoma | 313 (13.3%) | 116 (21.9%) | 66 (17.1%) | 196 (21.1%) | |
| Mean pulse pressure | 45.6 ± 12.4 | 55.3 ± 15.3 | 49.9 ± 13.2 | 51.8 ± 14.9 | |
| Mean total cholesterol | 194.3 ± 35.9 | 186.8 ± 37.3 | 194.5 ± 40.8 | 192.2 ± 35.5 | |
| Mean LDL cholesterol | 108.0 ± 31.3 | 102.0 ± 33.6 | 106.4 ± 33.3 | 105.3 ± 29.9 | |
| Mean HDL cholesterol | 49.8 ± 16.1 | 50.3 ± 15.9 | 49.8 ± 15.6 | 51.5 ± 15.4 | |
| Mean triglycerides | 195.9 ± 122.6 | 179.3 ± 101.8 | 203.3 ± 120.8 | 189.9 ± 119.0 | |
| Mean intraocular pressure (OD, mmHg) | 11.14 ± 4.99 | 10.84 ± 5.16 | 11.25 ± 5.08 | 10.91 ± 5.21 | |
| Mean axial length (OD, mm) | 23.85 ± 1.53 | 23.86 ± 1.50 | 23.87 ± 1.42 | 23.82 ± 1.39 | |
| Mean systolic blood pressure | 123.0 ± 18.1 | 130.7 ± 19.2 | 126.7 ± 17.9 | 128.6 ± 19.3 | |
| Mean diastolic blood pressure | 77.3 ± 10.4 | 75.4 ± 10.9 | 76.9 ± 9.9 | 76.7 ± 10.8 | |
| HEALTH CARE ACCESS and UTILIZATION FACTORS | |||||
| Insurance | |||||
| Not insured | 1232 (52.6%) | 147 (28%) | 156 (40.1%) | 376 (41%) | |
| Medical only | 911 (39%) | 289 (55.1%) | 180 (47.2%) | 433 (47.1%) | |
| Medical and vision insurance | 199 (8.5%) | 89 (17%) | 45 (11.8%) | 110 (12%) | |
AMD = age-related macular degeneration; SD = standard deviation; HbA1C = hemoglobin A1C level
Data are shown in column frequencies and percent (%) or mean and standard deviation (SD).
All models are reported as sex-combined given the similar sex-specific results and the lack of statistical power to assess risk factors by sex. The final model in multivariate analysis identified the independent risk factors for prevalent opacities presented in Table 2 by specific opacity type (cortical-only, nuclear-only, mixed-type). Older age was strongly associated with all opacity outcomes, with a dose-response associated with older age (test for trend, p <0.0001, nuclear-only, cortical-only, mixed-type).
Table 2.
Independent Factors Associated with Prevalent Lens Opacities (Cataracts) by Type in Chinese Americans (CHES)
| Lens Opacity Type | |||
|---|---|---|---|
| Risk Factors4,5 | Cortical Only6 | Nuclear Only7 | Mixed8,9 |
| Socio-demographic factors | |||
 Age10 Age10 | |||
  50–59 50–59 | 1.0 | 1.0 | 1.0 |
  60–69 60–69 | 2.6 (2.0, 3.4) | 2.8 (2.3–3.3) | 3.8 (2.9–5.1) |
  70–79 70–79 | 8.3 (5.4, 12.7) | 9.1 (6.5–12.8) | 9.2 (6.6–12.8) |
  80+ 80+ | 11.5 (4.9, 26.9) | 14.4 (7.0–29.7) | 9.0 (5.6–14.6) |
 Marital status Marital status | |||
  Never married Never married | -- | 1.0 | -- |
  Divorced/widowed Divorced/widowed | 1.0 (0.8–1.3) | ||
  Married/living with partner Married/living with partner | 1.5 (1.1–2.1) | ||
 Alcohol consumption Alcohol consumption | |||
  Never Never | -- | 1.0 | -- |
  Ex-drinker Ex-drinker | 1.0 (0.6–1.8) | ||
  Current drinker Current drinker | 0.6 (0.5–0.9) | ||
| Biological factors | |||
 Height (per 10 cm) Height (per 10 cm) | 0.7 (0.6–0.8) | -- | 0.8 (0.7–0.9) |
 Weight (kg) Weight (kg) | -- | 0.98 (0.97–0.99) | 0.98 (0.97–0.99) |
 Waist-to-hip ratio (0.1 unit) Waist-to-hip ratio (0.1 unit) | -- | 1.2 (1.1–1.4) | 1.3 (1.1–1.6) |
 Family history of cataract Family history of cataract | 1.5 (1.2–1.9) | -- | -- |
 Diabetes mellitus Diabetes mellitus | 1.5 (1.1–2.1) | 1.4 (1.1–1.9) | -- |
 High-density lipoprotein (per 10 mg/dl) High-density lipoprotein (per 10 mg/dl) | -- | 1.1 (1.02–1.2) | -- |
 Diastolic blood pressure (per 10 mmHg) Diastolic blood pressure (per 10 mmHg) | -- | 0.98 (0.96–0.99) | |
 HbA1C (per %) HbA1C (per %) | -- | 1.3 (1.1–1.4) | |
Cortical-only opacities had a more-than-doubling-effect with each additional decade of age compared to the referent 50–59 years age group in adjusted models1 (60–69 years, ORadj = 2.6, 95% CI = 2.0–3.4; 70–79 years, ORadj = 8.3, 95% CI = 5.4–12.7; 80+ years, ORadj = 11.5, 95% CI = 4.9–26.9). Cortical-only opacities were significantly associated with a family history of cataract (ORadj = 1.5, 95% CI = 1.2–1.9) and diabetes (ORadj = 1.5, 95% CI = 1.1–2.1), while greater height was protective (per each 10 cm increase, ORadj = 0.7, 95% CI = 0.6–0.8), (Table 2). Additional sub-analysis among women identified prior use of hormone therapy as significantly associated with cortical-only opacities (ORadj = 1.7, 95% CI = 1.0–2.7), data not shown.
Nuclear-only opacities demonstrated a strong dose-response with each additional decade compared to the referent 50–59 years age group in adjusted models2 (60–69 years, ORadj = 2.8, 95% CI=2.3–3.3; 70–79 years, ORadj = 9.1, 95% CI = 6.5–12.8; 80+ years, ORadj = 14.4, 95% CI = 7.0–29.7). Additionally, nuclear opacities were associated with higher waist-to-hip ratio (per 0.1 unit increase, ORadj = 1.2, 95% CI = 1.1–1.4), higher HDL (per 10 mg/dl increase, ORadj = 1.1, 95% CI = 1.02–1.2), history of diabetes (ORadj = 1.4, 95% CI = 1.1–1.9), and being married or living with a partner (ORadj = 1.5, 95% CI = 1.1–2.1). Inversely, current alcohol consumption (ORadj = 0.6, 95% CI = 0.5–0.9) and greater weight (per kg increase, ORadj = 0.98, 95% CI = 0.97–0.99) were protective (Table 2).
Mixed-type opacities also revealed a strong relationship with older age, compared to the referent 50–59 years age group in adjusted models3 (60–69 years, ORadj = 3.8, 95% CI = 2.9–5.1; 70–79 years, ORadj = 9.2, 95% CI = 6.6–12.8; 80+ years, ORadj = 9.0, 95% CI = 5.6–14.6). Additionally, mixed-type opacities were also associated with greater waist-to-hip ratio (per 0.1 unit increase, ORadj = 1.3, 95% CI = 1.1–1.6), and higher HbA1C (per unit increase, ORadj = 1.3, 95% CI = 1.1–1.4). Both taller height (per each 10 cm increase, ORadj = 0.8, 95% CI = 0.7–0.9) and higher DBP (per 10 mmHg increase, ORadj = 0.98, 95% CI = 0.96–0.99) were protective.
Figure 1, a LOWESS plot, demonstrates the independent predicted prevalence for cortical-only and nuclear-only opacities with older age. Nuclear opacity has a higher prevalence at every age group, after adjusting for all covariates. The LOWESS plot in Figures 2–4 demonstrates the independent predicted prevalence between a) cortical-only and height (Figure 2), b) nuclear-only and HDL (Figure 3) and c) nuclear-only and waist-to-hip ratio (Figure 4).
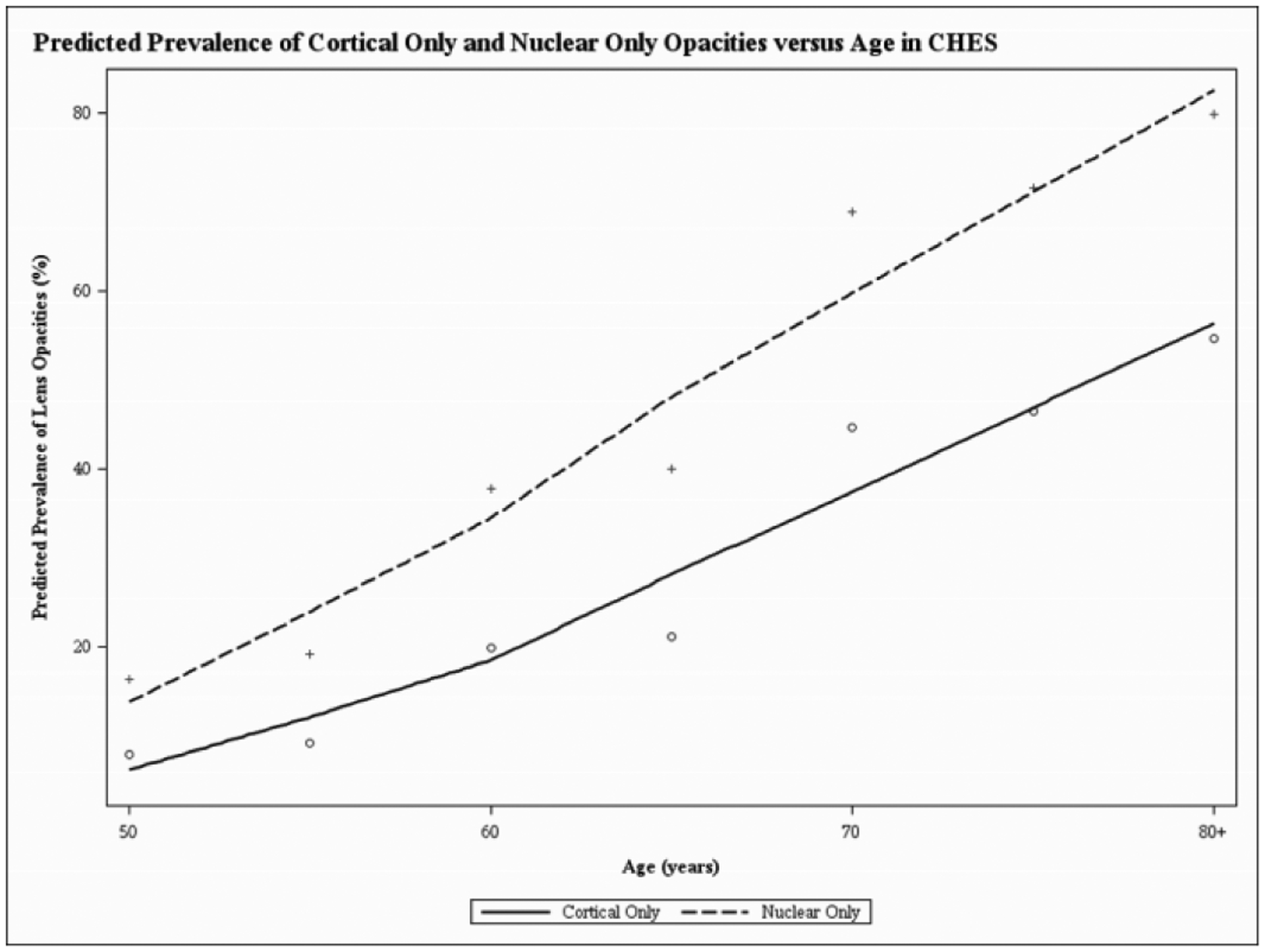
Locally weighted scatterplot smoothing regression (LOWESS) plot characterizing the independent relationship between age (in years) and predicted prevalence of cortical and nuclear opacities, after adjusting for covariates, in the Chinese American Eye Study (CHES). The final adjusted model for cortical-only opacities included the following covariates: age, sex, height, family history of cataract and diabetes mellitus. The final adjusted model for nuclear-only opacities included the following covariates: age, sex, marital status, alcohol consumption, weight, waist-to-hip ratio, diabetes mellitus and high-density lipoprotein.
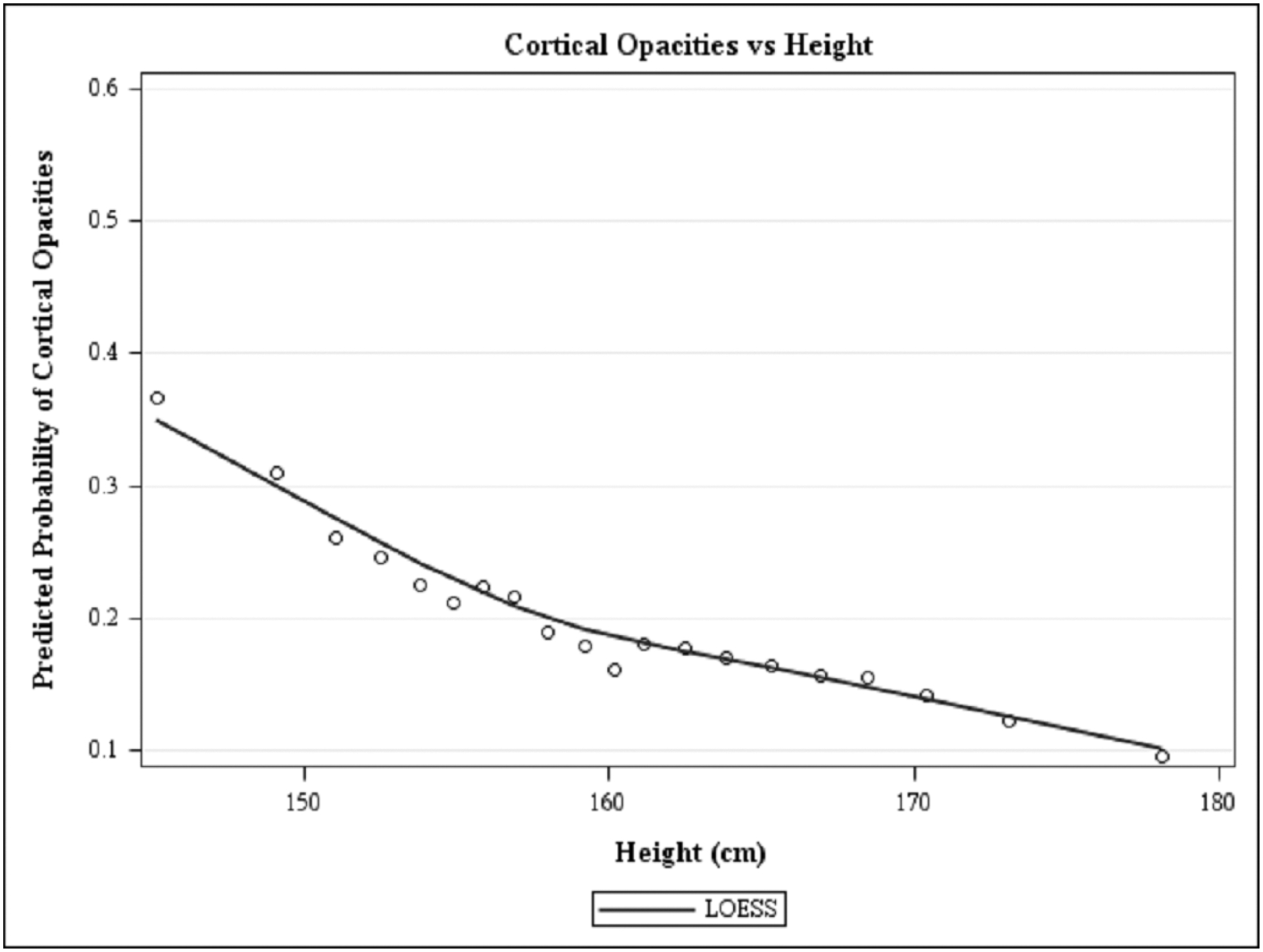
Locally weighted scatterplot smoothing regression (LOWESS) plot characterizing the independent relationship between height (in cm) and predicted prevalence of cortical, after adjusting for covariates, in the Chinese American Eye Study (CHES). The final adjusted model for cortical-only opacities included the following covariates: age, sex, height, family history of cataract and diabetes mellitus.
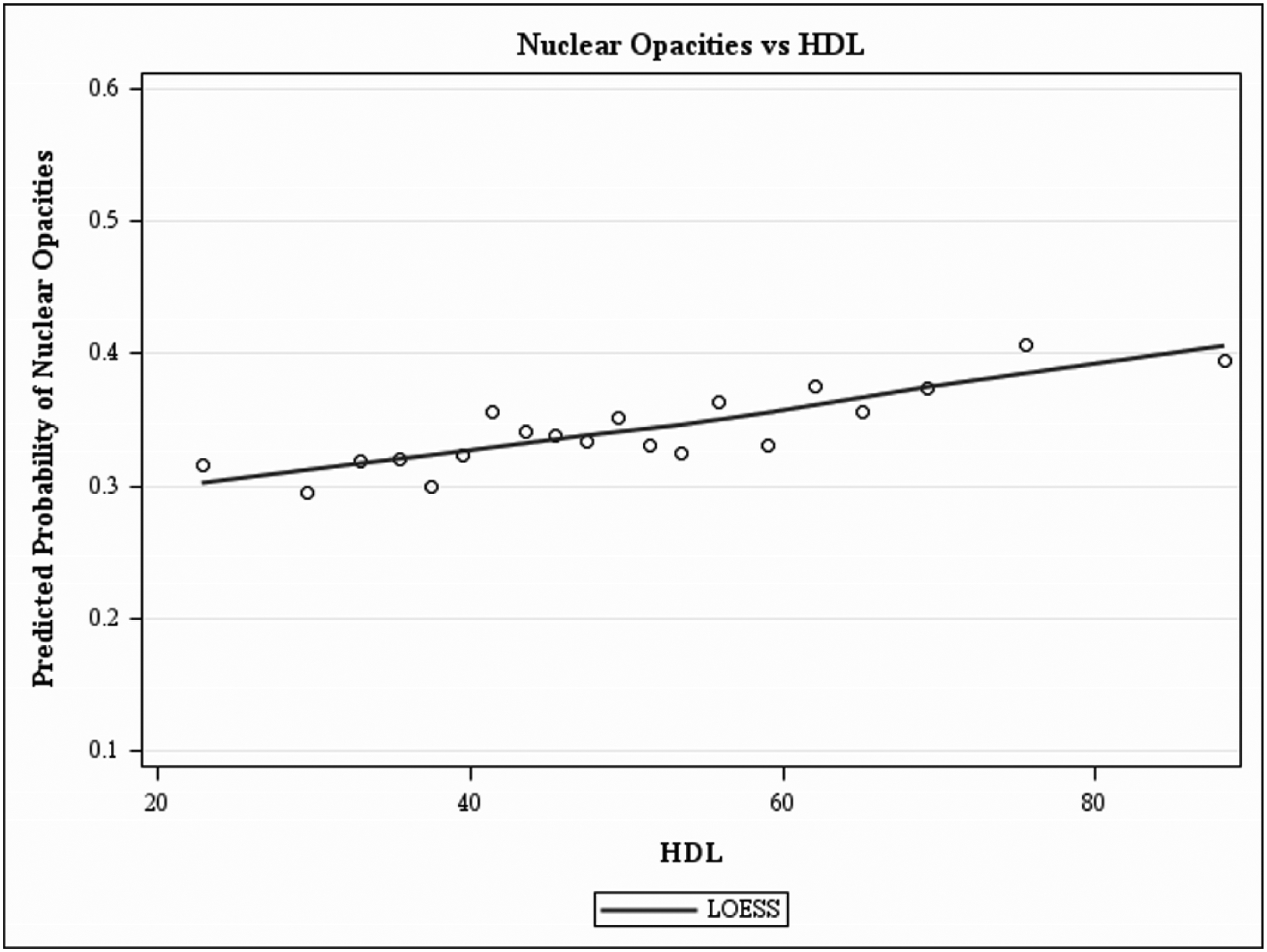
Locally weighted scatterplot smoothing regression (LOWESS) plot characterizing the independent relationship between HDL (mg/dl) and predicted prevalence of nuclear opacities after adjusting for covariates, in the Chinese American Eye Study (CHES). The final adjusted model for nuclear-only opacities included the following covariates: age, sex, marital status, alcohol consumption, weight, waist-to-hip ratio, diabetes mellitus and high-density lipoprotein.
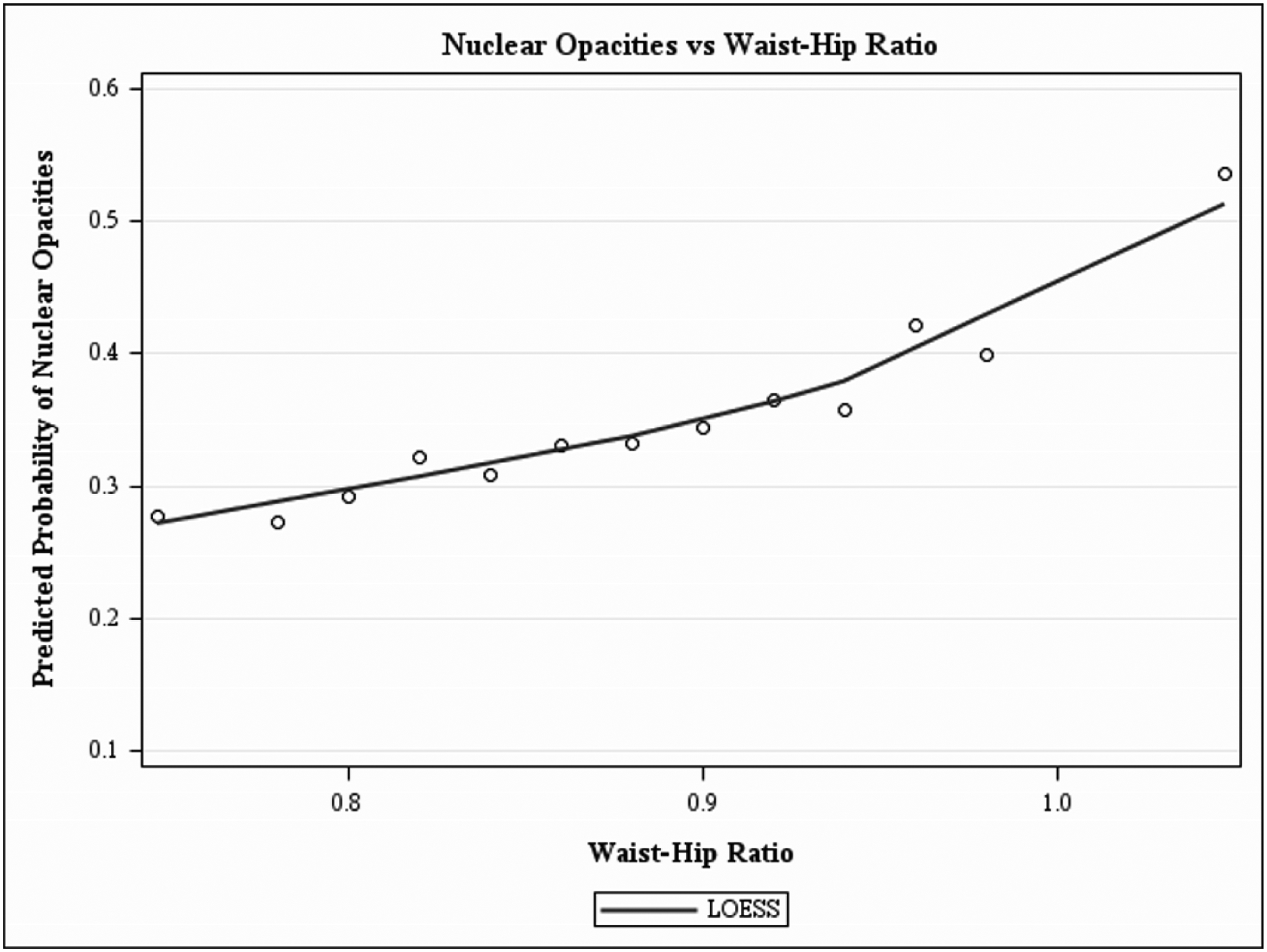
Locally weighted scatterplot smoothing regression (LOWESS) plot characterizing the independent relationship between waist-hip ratio and predicted prevalence of nuclear only opacities, after adjusting for covariates, in the Chinese American Eye Study (CHES). The final adjusted model for nuclear-only opacities included the following covariates: age, sex, marital status, alcohol consumption, weight, waist-to-hip ratio, diabetes mellitus and high-density lipoprotein.
DISCUSSION
CHES results identify sociodemographic and clinical factors significantly associated with prevalent lens opacities in a population-based analysis representative of Chinese Americans.10 In this analysis, age was strongly associated with cortical-only, nuclear-only, and mixed-type prevalent lens opacities, as were the following: (1) family history of cataract and diabetes with prevalent cortical-only opacities; (2) waist-to-hip ratio, HDL levels, and diabetes with prevalent nuclear-only opacities; (3) waist-to-hip-ratio, and HbA1C, with prevalent mixed-type opacities. Analyses also identified several factors with an inverse association, including increased height with cortical-only; current alcohol consumption and weight with nuclear-only; and greater height, weight, and diastolic hypertension with mixed-type opacities.
While CHES findings are similar to other population-based studies,5,7,18,20–23 variation across racial/ethnic groups in the burden of behavioral, biological, or environmental exposures may contribute to differences in the age-specific prevalence of lens opacities. In systemic disease outcomes (e.g., cardiovascular disease), there is increasing attention to the fact that differences in disease prevalence across racial/ethnic groups may result from a disproportionate burden across racial/ethnic groups in the exposures to various behavioral, biological, or environmental risk factors (e.g., smoking, alcohol consumption, hypertension, diabetes).24 Similarly with eye disease, it is worthwhile to consider contrasts in population-based study results and how differences in risk factor burden by racial/ethnic group may impact disease prevalence, especially among genetically similar population-based cohorts.
Cataract prevalence and their associated exposures have been assessed in population-based studies with different grading systems as technology has matured. In this risk factor association study, any effect of misclassification related to cataract prevalence and variation in grading systems on risk factors is likely minimal, as non-differential misclassification in a dichotomous disease outcome is commonly accepted to bias results toward the null.25 Table 3 provides a summary of association results in population-based studies, and identifies that among the five different lens-grading systems used,11,14,26–28 the LOCS II (used here), LOCS III and the Wisconsin Grading System are equally popular. Regardless of grading system, the larger picture contrasting differences in exposures identified with association studies remains informative, as dissimilarities in disease prevalence may result from a disproportionate burden across racial/ethnic groups of the exposure in question.24
Table 3.
Population-based studies assessing prevalent lens opacity outcomes, listed by lens opacity type: lens grading method, lens opacity definition, identified risk associatons and treatment of contralateral pseudophakic eye.
| CORTICAL | Ethnicity | Grading Method | Definition | Risk Associations |
|---|---|---|---|---|
| Chinese American Eye Study, 2010 – 2013 | Chinese (Urban Americans) | LOCS II 11 | LOCS II grading of ≥2 * | Gender-combined: age, height (inversely related), family history of cataract, diabetes mellitus (sex-specific provided similar results) |
| Barbados Eye Study, 1987–1992 | Afro-Caribbean | LOCS II11 | LOCS II grading of ≥2* | Gender-combined: age, lower SES status, female gender, nutritional supplement use (inversely related)22 |
| Beaver Dam Eye Study, 1988–1990 | Caucasian (Rural Wisconsin) | Wisconsin Cataract Grading System25 | Lens involvement ≥5%‡ | Gender-combined: age, sex, high density lipoprotein (women only, inversely related)56 heavy drinking, wine (inversely related), beer55 |
| Blue Mountains Eye Study, 1992–1994 | Caucasians (Urban Australia) | Wisconsin Cataract Grading System25 | Lens involvement ≥5%‡ | Gender-combined: alcohol (inversely related),42 pinguecula,43 high myopia,44 polyunsaturated fats (inversely related);62 current HRT use if ≥65 yrs (inversely related)45 |
| Salisbury Eye Evaluation, 1993–1995 | Caucasian and African American | Wilmer grading scheme28 | Graded ≥1/8* | Gender-combined: race;61 BMI;40 current HRT use (inversely related)63 |
| Tanjong Pagar, 1997–1998 | Chinese (Urban Singapore) | LOCS III14 | LOCS III score of ≥4 for NO or ≥4 for NC* | Gender-combined: age, diabetes, BMI (inversely related)21 |
| Shihpai Eye Study, 1999–2000 | Chinese (Urban Taiwan) | LOCS III14 | LOCS III score of >2* (justified as LOCS III score of >2 is close to a LOCS II score of ≥2) | Gender-combined: age, female gender, systolic blood pressure, former smoker, diabetes mellitus;20 BMI41 |
| Los Angeles Latinos Eye Study, 2000–2003 | Latinos (Urban Latino Americans) | LOCS II11 | LOCS II grading of ≥2* | Gender-combined: age, HbA1c, diabetes mellitus18 |
| Beijing Eye Study, 2001 | Chinese (Urban/Rural Chinese) | modified AREDS grading score29 | AREDS standard amount of ≥0.05† | Gender-combined: age, self-reported diagnosis diabetes mellitus;7 not alcohol48 |
| The Liwan Eye Study, 2003–2004 | Chinese (Urban Chinese) | -- | No papers identified analyzing risk associations with lens opacities (prevalent or incident cases) | |
| Singapore Malay Eye Study, 2004–2006 | Malay (Urban Muslim) | Wisconsin Cataract Grading System25 | Lens involvement ≥5%§* | Gender-combined: age, male gender (protective), current smoker;23 diabetes, hypertension, high density lipoprotein (inversely related), BMI, metabolic syndrome, increasing number of metabolic syndrome components36 |
| Handan Eye Study, 2006–2007 | Chinese (Rural Chinese) | LOCS III14 | LOCS III grading of ≥2* | Gender-combined: myopia5 |
| China Nine-Province Survey, 2006, 2014 | Chinese (Rural Chinese) | -- | Not analyzed | No papers identified analyzing risk associations with lens opacities (prevalent or incident cases) |
| Singapore Epidemiology of Eye Diseases Study | Malay (2004–2006), Indian (2007–2009), and Chinese (2009–2011) | Wisconsin Cataract Grading System25 | Lens involvement ≥5%* | Gender-combined: ACE inhibitors, fibrates, alpha-glucosidase inhibitors, insulin64 |
| NUCLEAR | Ethnicity | Grading Method | Definition | Risk Associations |
| Chinese American Eye Study, 2010 – 2013 | Chinese (Urban Americans) | LOCS II 11 | LOCS II grading of ≥2* | Gender-combined: age, marital status, alcohol consumption (inversely related), weight (inversely related), waist-to-hip ratio, diabetes mellitus, high-density lipoprotein (sex-specific provided similar results) |
| Barbados Eye Study, 1987–1992 | Afro-Caribbean | LOCS II11 | LOCS II grading of ≥2* | Gender-combined: age, lower SES status22 |
| Beaver Dam Eye Study, 1988–1990 | Caucasian (Rural Wisconsin) | Wisconsin Cataract Grading System25 | Graded at ≥4‡ | Gender-combined: age, sex, higher glycated hemoglobin (women only);56 heavy drinking, moderate liquor (inversely related), wine (inversely related)55 |
| Blue Mountains Eye Study, 1992–1994 | Caucasians (Urban Australia) | Wisconsin Cataract Grading System25 | Graded ≥3‡ | Gender-combined: ever smokers (pipe > cigarette smoking), heavy alcohol consumption in current smokers,42 pinguecula (inversely related),43 high myopia;44 higher intake of protein, vitamin A, niacin, thiamin, and riboflavin (inversely related),62 inhaled corticosteroids,57 dark brown irides60 |
| Salisbury Eye Evaluation, 1993–1995 | Caucasian and African American | Wilmer grading scheme28 | Graded ≥2* | Gender-combined: race;61 taller stature;40 BMI (inversely related), current HRT use, number of births (dose-response, inversely related)63 myopia46 |
| Tanjong Pagar, 1997–1998 | Chinese (Urban Singapore) | LOCS III14 | LOCS III score of ≥4* | Gender-combined: age, current smoker, occupation (production, laborers, clerks, home makers increased risk vs. professionals)21 |
| Shihpai Eye Study, 1999–2000 | Chinese (Urban Taiwan) | LOCS III14 | LOCS III score of >2* (justified as LOCS III score of >2 is close to a LOCS II score of ≥2) | Gender-combined: age, female gender, current smoker;20 BMI (inversely related);41 use of HRT (inversely related)20 |
| Los Angeles Latinos Eye Study, 2000–2003 | Latinos (Urban Latino Americans) | LOCS II11 | LOCS II grading of ≥2* | Gender-combined: age, spherical equivalent (inversely related), current smoker18 |
| Beijing Eye Study, 2001 | Chinese (Urban/Rural Chinese) | modified AREDS grading score29 | AREDS standard grade of ≥5† | Gender-combined: age, myopic refractive error;7 not alcohol48 |
| The Liwan Eye Study, 2003–2004 | Chinese (Urban Chinese) | -- | No papers identified analyzing risk associations with lens opacities (prevalent or incident cases) | |
| Singapore Malay Eye Study, 2004–2006 | Malay (Urban Muslim) | Wisconsin Cataract Grading System25 | Graded ≥3§* | Gender-combined: age, male gender (protective), current smoker, primary or lower education, low monthly income;23 hypertension, BMI, increasing number of metabolic syndrome components36 |
| Handan Eye Study, 2006–2007 | Chinese (Rural Chinese) | LOCS III14 | LOCS III score of ≥4* | Gender-combined: myopia, cholesterol, high-density and low-density lipoprotein (both inversely related)5 |
| China Nine-Province Survey, 2006, 2014 | Chinese (Rural Chinese) | -- | Not analyzed | No papers identified analyzing risk associations with lens opacities (prevalent or incident cases) |
| PSC | Ethnicity | Grading Method | Definition | Risk Associations |
| Chinese American Eye Study, 2010 – 2013 | Chinese (Urban Americans) | LOCS II 11 | LOCS II grading of ≥2* | Not analyzed due to small number |
| Barbados Eye Study, 1987–1992 | Afro-Caribbean | LOCS II11 | LOCS II grading of ≥2* | Not analyzed due to small number22 |
| Beaver Dam Eye Study, 1988–1990 | Caucasian (Rural Wisconsin) | Wisconsin Cataract Grading System25 | Lens involvement ≥5%‡ | Gender-combined: age, sex, higher ratios of total to high-density lipoprotein cholesterol (men only)56 heavy drinking55 |
| Blue Mountains Eye Study, 1992–1994 | Caucasians (Urban Australia) | Wisconsin Cataract Grading System25 | Any graded PSC opacity‡ | Gender-combined: ever smokers,42 ptyergium,43 myopia (dose-dependent response), high myopia, early-onset myopia,44 inhaled corticosteroids,57 dark brown irides60 increased sodium intake (dose-dependent response),58 diabetes,59
Women: (separate analysis): current use of hormone replacement therapy in women with non-surgical menopause45 |
| Salisbury Eye Evaluation, 1993–1995 | Caucasian and African American | Wilmer grading scheme28 | Any PSC* | Gender-combined: race;61 taller stature (borderline);40 past and current HRT use (inversely related);63 myopia46 |
| Tanjong Pagar, 1997–1998 | Chinese (Urban Singapore) | LOCS III14 | LOCS III score of ≥2* | Gender-combined: age, diabetes, housing type21 |
| Shihpai Eye Study, 1999–2000 | Chinese (Urban Taiwan) | LOCS III14 | LOCS III score of >2* (justified as LOCS III score of >2 is close to a LOCS II score of ≥2) | Gender-combined: higher systolic blood pressure, interaction age (≥75)*gender20 |
| Los Angeles Latinos Eye Study, 2000–2003 | Latinos (Urban Latino Americans) | LOCS II11 | LOCS II grading of ≥2* | Gender-combined: systolic blood pressure, diabetes mellitus18 |
| Beijing Eye Study, 2001 | Chinese (Urban/Rural Chinese) | modified AREDS grading score29 | AREDS standard amount of ≥0.01† | Gender-combined: age;7 not alcohol48 |
| The Liwan Eye Study, 2003–2004 | Chinese (Urban Chinese) | -- | -- | No papers identified analyzing risk associations with lens opacities (prevalent or incident cases) |
| Singapore Malay Eye Study, 2004–2006 | Malay (Urban Muslim) | Wisconsin Cataract Grading System25 | Any graded PSC opacity§ | Gender-combined: age, current smoker, public housing (SES variable);23 diabetes, hypertension36 |
| Handan Eye Study, 2006–2007 | Chinese (Rural Chinese) | LOCS III14 | LOCS III score of ≥2* | Gender-combined: myopia, hyperopia (inversely related), fasting glucose, diabetes5 |
| China Nine-Province Survey, 2006, 2014 | Chinese (Rural Chinese) | -- | Not analyzed | No papers identified analyzing risk associations with lens opacities (prevalent or incident cases) |
| ANY LENS OPACITY | Ethnicity | Grading Method | Definition | Risk Associations |
| Chinese American Eye Study, 2010 – 2013 | Chinese (Urban Americans) | LOCS II 11 | Any nuclear, cortical or PSC as specified * | Gender-combined: age, height, weight (inversely related), waist-to-hip ratio, diastolic blood pressure (inversely related), HbA1C (sex-specific provided similar results) |
| Barbados Eye Study, 1987–1992 | Afro-Caribbean | LOCS II11 | Any nuclear, cortical or PSC as specified* | Gender-combined: age, lower SES status, female gender, nutritional supplement use (inversely related)22 |
| Beaver Dam Eye Study, 1988–1990 | Caucasian (Rural Wisconsin) | Wisconsin Cataract Grading System25 | Not analyzed separately | Not analyzed separately55,56 |
| Blue Mountains Eye Study, 1992–1994; | Caucasians (Urban Australia) | Wisconsin Cataract Grading System25 | Not analyzed separately | Not analyzed separately42–45,57–60 |
| Salisbury Eye Evaluation (SEE) project, 1993–1995 | Caucasian and African American | Wilmer grading scheme28 | Any nuclear, cortical or PSC as specified* | Not analyzed separately40,61 |
| Tanjong Pagar, 1997–1998 | Chinese (Urban Singapore) | LOCS III14 | LOCS III score of ≥4 for NO or ≥4 for NC or ≥2.0 for C or ≥2.0 for P* | Gender-combined: age, occupation (production increased risk vs. professionals), current smoking; diabetes marginally significant21 |
| Shihpai Eye Study, 1999–2000 | Chinese (Urban Taiwan) | LOCS III14 | Not analyzed separately | Not analyzed separately20,41 |
| Los Angeles Latinos Eye Study, 2000–2003 | Latinos (Urban Latino Americans) | LOCS II11 | Any nuclear, cortical or PSC as specified* | Gender-combined: age, female gender, diabetes mellitus, systolic blood pressure, spherical equivalent (inversely related), large drusen18 |
| Beijing Eye Study, 2001 | Chinese (Urban/Rural Chinese) | modified AREDS grading score29 | Not analyzed separately | Gender-combined: not analyzed separately7 |
| The Liwan Eye Study, 2003–2004 | Chinese (Urban Chinese) | -- | -- | No papers identified analyzing risk associations with lens opacities (prevalent or incident cases) |
| Singapore Malay Eye Study, 2004–2006 | Malay (Urban Muslim) | Wisconsin Cataract Grading System25 | Any nuclear, cortical or PSC as specified§ | Gender-combined: age, male gender (protective), current smoker23 |
| Handan Eye Study, 2006–2007 | Chinese (Rural Chinese) | LOCS III14 | Any nuclear, cortical or PSC as specified* | Gender-combined (outcome is any lens opacity or cataract surgery): myopia5 |
| China Nine-Province Survey, 2006, 2014 | Chinese (Rural Chinese) | -- | Not analyzed |
As such, we present a review of the literature of prevalent lens opacities, as it is informative for general comparison of exposures and associated risk factors in other population-based studies. Risk factors are presented by lens opacity type, with a focus first to other population-based studies of Chinese descent, and then studies with participants of other race/ethnicity.
Cortical-only Lens Opacities
In addition to a strong dose-response relationship with age, CHES identified diabetes and a positive family history of cataract as significantly related to prevalent cortical-only lens opacities, while height was inversely associated. CHES findings are mostly consistent with previous population-based studies, in which age was significantly associated with cortical opacities in all7,20,21 but 1 study,5 among Chinese, and in participants of Latino, African, and Malay descent.18,22,23 Diabetes has been associated with cortical opacities in Chinese7,20,21 and Latino18 participants, and other lens opacities in cohort and cross-sectional studies.29–36 Height has been directly associated with incident37 and prevalent38 cataracts. Factors inconsistent with CHES identified in other urban Chinese population-based studies related to cortical lens opacities include SBP,20 BMI,21,39 myopia,5 occupation,21 and former smoking.20 Additional factors identified in non-Chinese populations include current smoking in Malays;23 lower socioeconomic status in African populations;22 and pinguecula,40 high myopia,41 alcohol (protective),42 and current hormone replacement therapy among older women (protective),43 in Caucasian populations. Additionally, female gender was identified in Chinese,20 Malay,23 and African populations22 as significantly associated with cortical lens opacities.
Nuclear-only Lens Opacities
In addition to a strong dose-response relationship with age, CHES identified waist-to-hip ratio, HDL levels, and diabetes as significantly related to prevalent nuclear-only lens opacities, while current alcohol consumption and weight were inversely associated. Of these, only age was associated with nuclear lens opacities in previous Chinese population-based studies,7,21,39 but it was also associated in Latino,18 African,22 and Malay populations.23 Waist-to-hip ratio, HDL levels, and diabetes identified in CHES reflect a sedentary and Westernized lifestyle contributing to metabolic syndrome. Similar sedentary lifestyle-related factors identified in Chinese populations, including current smoker,20,21 BMI,39 cholesterol5 and occupation,21 suggest the general importance of sedentary lifestyles. Differences across populations in the prevalence of Westernized lifestyle-related chronic diseases or exposures (e.g,. reduced diabetes rates China versus US) may limit study power to detect an association resulting in variation across studies, and also confound the relationship between an exposure and lens opacities. Alcohol was identified as protective against prevalent nuclear-only lens opacities in CHES but not studies of urban and rural Chinese44, which may also relate to exposure to alcohol consumption. In the Beijing study, alcohol consumption was significantly associated with sociodemographic variables, including younger age, male gender, rural region, and lower education, suggesting again, lifestyle and exposure differences (e.g., Westernized lifestyles) between genetically similar cohorts may confound the relationship between alcohol and lens opacities. Previous cohorts with similar Westernized lifestyles report mixed results between cataracts and alcohol intake, with most42,45–49 but not all studies50,51 reporting a lack of significant findings.
Mixed-type Lens Opacities
CHES identified age, height, -to-hip ratio, weight, HbA1C, and DBP as associated with prevalent mixed opacities, which are generally consistent with previous studies,5,18,21–23 although most studies have failed to provide separate analyses on mixed opacities.7,20,38–43,51–57
Strengths of CHES include its large, population-based sample (n = 4582), participation rates (79.2%), and LOCS II grading systems11 with moderate-to-excellent inter-examiner agreement (weighted κ = 0.86–1.0). Limitations include the cross-sectional nature prohibiting analysis of baseline factors and subsequent incident disease, and similar to most other studies, the lack of an estimate for sun exposure. Finally, although the distribution of CHES participants varies somewhat compared to the general distribution of Chinese in the US (e.g., 63% vs 52% female, 67% vs 58% completing ≥12 years of school), it is unlikely these minor differences compared to the larger US Chinese population would substantially alter results in this association study. In conclusion, CHES identified important factors associated with prevalent lens opacities, including a strong, dose-response association between age and all prevalent lens opacities, suggesting an increased cataract burden in the aging Chinese Americans population. Results demonstrate consistency with other Chinese populations internationally in regards to general sedentary and Westernized lifestyle exposures. Finally, results suggest improving glycemic control together with a more active lifestyle may help reduce the population-level burden of vision loss associated with lens opacities.
Acknowledgements
The authors would like to thank the Chinese American Eye Study Data Monitoring and Oversight Committee for their advice and contributions: Alfred Sommer, MD, MHS (chair); Anne Coleman, MD, PhD; Dennis Han, MD; Craig Hanis, PhD; Louise Wideroff, PhD; Terri Young, MD.
The Chinese American Eye Study Group: Rohit Varma, MD, MPH (principal investigator); Roberta McKean-Cowdin, PhD (coinvestigator); Stanley P. Azen, PhD (coinvestigator); Mina Torres, MS (project director); Chunyi Hsu, MPH (project manager); David Dinh, BA (research assistant); Ruzhang Jiang, MD (examiner); Jie Sun, MD, PhD, MPH (examiner); Dandan Wang, MD (examiner); YuPing Wang, Certified Ophthalmic Technician (examiner); Justine Wong, BA (clinical interviewer); Shuang Wu, MS (statistician); Rucha Desai, MS (programmer).
Battelle Survey Research Center, St. Louis, Missouri: Lisa V. John, PhD (recruitment director); Michelle Cheng, MS (field supervisor).
Ocular Epidemiology Grading Center, University of Wisconsin, Madison: Stacy M. Meuer (senior grader); Ronald Klein, MD, MPH, (coinvestigator).
Financial Support:
National Institutes of Health Grant U10 017337 and an unrestricted grant from the Research to Prevent Blindness, New York, New York. The sponsor or funding organization had no role in the design or conduct of this research.
Footnotes
Conflict of Interest: No conflict of interest exists for any author.
1The final adjusted model for cortical-only opacities included the following covariates: age, sex, height, family history of cataract and diabetes mellitus.
2The final adjusted model for nuclear-only opacities included the following covariates: age, sex, marital status, alcohol consumption, weight, waist-to-hip ratio, diabetes mellitus and high-density lipoprotein.
3The final adjusted model for mixed-type opacities included the following covariates: age, sex, height, weight, waist-to-hip ratio, diastolic blood pressure, and HgA1C.
REFERENCES
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Prevalence of Lens Opacities in Adult Chinese Americans: The Chinese American Eye Study (CHES).
Invest Ophthalmol Vis Sci, 57(15):6692-6699, 01 Dec 2016
Cited by: 5 articles | PMID: 27936471 | PMCID: PMC5156510
Risk factors for cortical, nuclear, posterior subcapsular, and mixed lens opacities: the Los Angeles Latino Eye Study.
Ophthalmology, 119(3):547-554, 23 Dec 2011
Cited by: 48 articles | PMID: 22197433 | PMCID: PMC3293944
Risk factors for incident cortical, nuclear, posterior subcapsular, and mixed lens opacities: the Los Angeles Latino eye study.
Ophthalmology, 119(10):2040-2047, 06 Jul 2012
Cited by: 31 articles | PMID: 22771048 | PMCID: PMC3464350
Prevalence of lens opacities in the Barbados Eye Study.
Arch Ophthalmol, 115(1):105-111, 01 Jan 1997
Cited by: 86 articles | PMID: 9006434
Funding
Funders who supported this work.
NEI NIH HHS (1)
Grant ID: U10 EY017337
National Eye Institute (1)
Grant ID: U10 EY-017337
Research to Prevent Blindness (1)
Grant ID: Unrestricted



