Abstract
Importance
Evidence regarding corticosteroid use for severe coronavirus disease 2019 (COVID-19) is limited.Objective
To determine whether hydrocortisone improves outcome for patients with severe COVID-19.Design, setting, and participants
An ongoing adaptive platform trial testing multiple interventions within multiple therapeutic domains, for example, antiviral agents, corticosteroids, or immunoglobulin. Between March 9 and June 17, 2020, 614 adult patients with suspected or confirmed COVID-19 were enrolled and randomized within at least 1 domain following admission to an intensive care unit (ICU) for respiratory or cardiovascular organ support at 121 sites in 8 countries. Of these, 403 were randomized to open-label interventions within the corticosteroid domain. The domain was halted after results from another trial were released. Follow-up ended August 12, 2020.Interventions
The corticosteroid domain randomized participants to a fixed 7-day course of intravenous hydrocortisone (50 mg or 100 mg every 6 hours) (n = 143), a shock-dependent course (50 mg every 6 hours when shock was clinically evident) (n = 152), or no hydrocortisone (n = 108).Main outcomes and measures
The primary end point was organ support-free days (days alive and free of ICU-based respiratory or cardiovascular support) within 21 days, where patients who died were assigned -1 day. The primary analysis was a bayesian cumulative logistic model that included all patients enrolled with severe COVID-19, adjusting for age, sex, site, region, time, assignment to interventions within other domains, and domain and intervention eligibility. Superiority was defined as the posterior probability of an odds ratio greater than 1 (threshold for trial conclusion of superiority >99%).Results
After excluding 19 participants who withdrew consent, there were 384 patients (mean age, 60 years; 29% female) randomized to the fixed-dose (n = 137), shock-dependent (n = 146), and no (n = 101) hydrocortisone groups; 379 (99%) completed the study and were included in the analysis. The mean age for the 3 groups ranged between 59.5 and 60.4 years; most patients were male (range, 70.6%-71.5%); mean body mass index ranged between 29.7 and 30.9; and patients receiving mechanical ventilation ranged between 50.0% and 63.5%. For the fixed-dose, shock-dependent, and no hydrocortisone groups, respectively, the median organ support-free days were 0 (IQR, -1 to 15), 0 (IQR, -1 to 13), and 0 (-1 to 11) days (composed of 30%, 26%, and 33% mortality rates and 11.5, 9.5, and 6 median organ support-free days among survivors). The median adjusted odds ratio and bayesian probability of superiority were 1.43 (95% credible interval, 0.91-2.27) and 93% for fixed-dose hydrocortisone, respectively, and were 1.22 (95% credible interval, 0.76-1.94) and 80% for shock-dependent hydrocortisone compared with no hydrocortisone. Serious adverse events were reported in 4 (3%), 5 (3%), and 1 (1%) patients in the fixed-dose, shock-dependent, and no hydrocortisone groups, respectively.Conclusions and relevance
Among patients with severe COVID-19, treatment with a 7-day fixed-dose course of hydrocortisone or shock-dependent dosing of hydrocortisone, compared with no hydrocortisone, resulted in 93% and 80% probabilities of superiority with regard to the odds of improvement in organ support-free days within 21 days. However, the trial was stopped early and no treatment strategy met prespecified criteria for statistical superiority, precluding definitive conclusions.Trial registration
ClinicalTrials.gov Identifier: NCT02735707.Free full text

Effect of Hydrocortisone on Mortality and Organ Support in Patients With Severe COVID-19
Key Points
Question
Does intravenous hydrocortisone, administered either as a 7-day fixed-dose course or restricted to when shock is clinically evident, improve 21-day organ support–free days (a composite end point of in-hospital mortality and the duration of intensive care unit–based respiratory or cardiovascular support) in patients with severe coronavirus disease 2019 (COVID-19)?
Findings
In this bayesian randomized clinical trial that included 403 patients and was stopped early after results from another trial were released, treatment with a 7-day fixed-dose course of hydrocortisone or shock-dependent dosing of hydrocortisone, compared with no hydrocortisone, resulted in 93% and 80% probabilities of superiority, respectively, with regard to the odds of improvement in organ support–free days within 21 days.
Meaning
Although suggestive of benefit for hydrocortisone in patients with severe COVID-19, the trial was stopped early and no treatment strategy met prespecified criteria for statistical superiority, precluding definitive conclusions.
Abstract
Importance
Evidence regarding corticosteroid use for severe coronavirus disease 2019 (COVID-19) is limited.
Objective
To determine whether hydrocortisone improves outcome for patients with severe COVID-19.
Design, Setting, and Participants
An ongoing adaptive platform trial testing multiple interventions within multiple therapeutic domains, for example, antiviral agents, corticosteroids, or immunoglobulin. Between March 9 and June 17, 2020, 614 adult patients with suspected or confirmed COVID-19 were enrolled and randomized within at least 1 domain following admission to an intensive care unit (ICU) for respiratory or cardiovascular organ support at 121 sites in 8 countries. Of these, 403 were randomized to open-label interventions within the corticosteroid domain. The domain was halted after results from another trial were released. Follow-up ended August 12, 2020.
Interventions
The corticosteroid domain randomized participants to a fixed 7-day course of intravenous hydrocortisone (50 mg or 100 mg every 6 hours) (n =
= 143), a shock-dependent course (50 mg every 6 hours when shock was clinically evident) (n
143), a shock-dependent course (50 mg every 6 hours when shock was clinically evident) (n =
= 152), or no hydrocortisone (n
152), or no hydrocortisone (n =
= 108).
108).
Main Outcomes and Measures
The primary end point was organ support–free days (days alive and free of ICU-based respiratory or cardiovascular support) within 21 days, where patients who died were assigned –1 day. The primary analysis was a bayesian cumulative logistic model that included all patients enrolled with severe COVID-19, adjusting for age, sex, site, region, time, assignment to interventions within other domains, and domain and intervention eligibility. Superiority was defined as the posterior probability of an odds ratio greater than 1 (threshold for trial conclusion of superiority >99%).
Results
After excluding 19 participants who withdrew consent, there were 384 patients (mean age, 60 years; 29% female) randomized to the fixed-dose (n =
= 137), shock-dependent (n
137), shock-dependent (n =
= 146), and no (n
146), and no (n =
= 101) hydrocortisone groups; 379 (99%) completed the study and were included in the analysis. The mean age for the 3 groups ranged between 59.5 and 60.4 years; most patients were male (range, 70.6%-71.5%); mean body mass index ranged between 29.7 and 30.9; and patients receiving mechanical ventilation ranged between 50.0% and 63.5%. For the fixed-dose, shock-dependent, and no hydrocortisone groups, respectively, the median organ support–free days were 0 (IQR, –1 to 15), 0 (IQR, –1 to 13), and 0 (–1 to 11) days (composed of 30%, 26%, and 33% mortality rates and 11.5, 9.5, and 6 median organ support–free days among survivors). The median adjusted odds ratio and bayesian probability of superiority were 1.43 (95% credible interval, 0.91-2.27) and 93% for fixed-dose hydrocortisone, respectively, and were 1.22 (95% credible interval, 0.76-1.94) and 80% for shock-dependent hydrocortisone compared with no hydrocortisone. Serious adverse events were reported in 4 (3%), 5 (3%), and 1 (1%) patients in the fixed-dose, shock-dependent, and no hydrocortisone groups, respectively.
101) hydrocortisone groups; 379 (99%) completed the study and were included in the analysis. The mean age for the 3 groups ranged between 59.5 and 60.4 years; most patients were male (range, 70.6%-71.5%); mean body mass index ranged between 29.7 and 30.9; and patients receiving mechanical ventilation ranged between 50.0% and 63.5%. For the fixed-dose, shock-dependent, and no hydrocortisone groups, respectively, the median organ support–free days were 0 (IQR, –1 to 15), 0 (IQR, –1 to 13), and 0 (–1 to 11) days (composed of 30%, 26%, and 33% mortality rates and 11.5, 9.5, and 6 median organ support–free days among survivors). The median adjusted odds ratio and bayesian probability of superiority were 1.43 (95% credible interval, 0.91-2.27) and 93% for fixed-dose hydrocortisone, respectively, and were 1.22 (95% credible interval, 0.76-1.94) and 80% for shock-dependent hydrocortisone compared with no hydrocortisone. Serious adverse events were reported in 4 (3%), 5 (3%), and 1 (1%) patients in the fixed-dose, shock-dependent, and no hydrocortisone groups, respectively.
Conclusions and Relevance
Among patients with severe COVID-19, treatment with a 7-day fixed-dose course of hydrocortisone or shock-dependent dosing of hydrocortisone, compared with no hydrocortisone, resulted in 93% and 80% probabilities of superiority with regard to the odds of improvement in organ support–free days within 21 days. However, the trial was stopped early and no treatment strategy met prespecified criteria for statistical superiority, precluding definitive conclusions.
Trial Registration
ClinicalTrials.gov Identifier: NCT02735707
Introduction
Coronavirus disease 2019 (COVID-19) is an acute respiratory illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). First identified in Wuhan, China, in December 2019, more than 20 million COVID-19 cases and 750 000 deaths had been reported worldwide by August 2020.1 Though many therapies are being evaluated, strong evidence of benefit is lacking.2 One class of agents that has received considerable attention is corticosteroids. Corticosteroids were reported to be beneficial in several conditions analogous to COVID-19, including sepsis, pneumonia, and acute respiratory distress syndrome (ARDS).3,4,5 However, other trials in these conditions, as well as in influenza and coronavirus respiratory syndromes, showed no benefit or possible harm.3,6,7 Consequently, advice for COVID-19 has been mixed.8 The China National Health Commission suggested hydrocortisone is appropriate9; the Surviving Sepsis Campaign recommended against corticosteroid use in the absence of ARDS, but suggested possible benefit in those with ARDS10; while the World Health Organization (WHO) initially recommended no corticosteroid treatment.11 In practice, corticosteroids have been given variably to patients with COVID-19, and observational studies suggest both benefit and harm.12,13,14 To reduce this uncertainty, several research groups launched randomized clinical trials (RCTs).
000 deaths had been reported worldwide by August 2020.1 Though many therapies are being evaluated, strong evidence of benefit is lacking.2 One class of agents that has received considerable attention is corticosteroids. Corticosteroids were reported to be beneficial in several conditions analogous to COVID-19, including sepsis, pneumonia, and acute respiratory distress syndrome (ARDS).3,4,5 However, other trials in these conditions, as well as in influenza and coronavirus respiratory syndromes, showed no benefit or possible harm.3,6,7 Consequently, advice for COVID-19 has been mixed.8 The China National Health Commission suggested hydrocortisone is appropriate9; the Surviving Sepsis Campaign recommended against corticosteroid use in the absence of ARDS, but suggested possible benefit in those with ARDS10; while the World Health Organization (WHO) initially recommended no corticosteroid treatment.11 In practice, corticosteroids have been given variably to patients with COVID-19, and observational studies suggest both benefit and harm.12,13,14 To reduce this uncertainty, several research groups launched randomized clinical trials (RCTs).
In March 2020, investigators for the REMAP-CAP (Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia) Study began randomizing patients with COVID-19 to alternative dosing strategies of the corticosteroid, hydrocortisone. Enrollment was halted on June 17, following the announcement by the RECOVERY Collaborative Group that dexamethasone reduced mortality compared with standard of care in patients with COVID-19 receiving either invasive mechanical ventilation or supplemental oxygen.15 This report describes the effects of hydrocortisone, in doses of similar glucocorticoid equivalency to that used in RECOVERY, in severely ill patients with COVID-19 enrolled in REMAP-CAP.
Methods
Study Design
REMAP-CAP is an ongoing, international, multicenter, open-label trial that combines features of an adaptive platform trial with a pragmatic point-of-care trial to determine best treatment strategies for patients with severe pneumonia in both pandemic and nonpandemic settings. A detailed description of the trial design is provided elsewhere.16 The trial uses a novel design, a randomized embedded multifactorial adaptive platform (REMAP).17 The design has 5 key features: randomization, allowing causal inference; embedding of study procedures into routine care processes, facilitating enrollment, trial efficiency, and generalizability; a multifactorial statistical model comparing multiple interventions across multiple patient subgroups; response-adaptive randomization with preferential assignment to those interventions that appear most favorable after interim analyses; and a platform structured to permit continuous, potentially perpetual, enrollment.
The trial randomizes patients to multiple interventions within multiple domains, evaluating effectiveness within different patient strata. The term domain refers to a common therapeutic area (eg, antiviral therapy or immunoglobulin therapy) within which several interventions or intervention dosing strategies can be randomly assigned (including a control, such as no antiviral, as appropriate). All trial procedures are governed by a master, or “core,” protocol and a series of appendices that describe aspects specific to each therapeutic domain, to adaptations during a pandemic, and to region-specific trial governance and conduct. The trial’s core protocol, relevant protocol appendices, and statistical analysis plans (SAPs) are provided in Supplement 1. The trial is overseen by an international trial steering committee (ITSC), which is blinded to treatment assignment and outcome, and an unblinded independent data and safety monitoring board (Supplement 1). The study was approved by the relevant ethics committees at all participating sites and is conducted in accordance with Good Clinical Practice guidelines and the principles described in the Declaration of Helsinki.
The REMAP-CAP investigators introduced several design adaptations for COVID-19 (see Pandemic Appendix, January 31, 2020, and subsequent updates, in Supplement 1). Specifically, all patients hospitalized with suspected or proven COVID-19 were assigned to the COVID-19 patient stratum. They were further classified as clinically moderately or severely ill, and, depending on their moderate or severe state, were eligible for randomized assignment to alternative interventions within several COVID-19–specific domains, including antiviral, corticosteroid, targeted immune modulation, immunoglobulin, and therapeutic anticoagulation domains. The corticosteroid domain was eligible only to patients in the severe state. During the study period, the trial enrolled participants with severe COVID-19 at 121 clinical sites in Australia, Canada, France, Ireland, the Netherlands, New Zealand, the United Kingdom, and the United States. Written or verbal informed consent, in accordance with local legislation, was obtained for all patients or from their surrogates.
Achieving a racially and ethnically diverse sample was a goal of the trial because of evidence of disparities in outcome and treatment effectiveness in pandemic and nonpandemic pneumonia. Participants (or their surrogates) self-reported their race/ethnicity via fixed categories appropriate to their region.
Participants
Patients aged 18 years or older with presumed or confirmed SARS-CoV-2 infection who were admitted to an intensive care unit (ICU) for provision of respiratory or cardiovascular organ support were classified as severe and eligible for enrollment in the COVID-19 corticosteroid domain. An ICU could include an area of the hospital repurposed to function as an ICU for surge capacity management. Respiratory organ support was defined as invasive or noninvasive mechanical ventilation or high-flow nasal cannula if the flow rate was 30 L/min or greater and fraction of inspired oxygenof 0.4 or greater. Cardiovascular organ support was defined as the intravenous infusion of any vasopressor or inotrope. Exclusion criteria included presumption that death is imminent with lack of commitment to full support and participation in the trial in the prior 90 days. Additional exclusion criteria for the corticosteroid domain included known hypersensitivity to hydrocortisone, systemic corticosteroid use, and more than 36 hours elapsed since ICU admission. Further details regarding eligibility are listed in the corticosteroid domain–specific appendix in Supplement 1 and in eAppendix 1 in Supplement 2.
Treatment Allocation
The COVID-19 corticosteroid domain contained fixed-dose and shock-dependent hydrocortisone interventions and a standard of care with no hydrocortisone (or other corticosteroid) use. Investigators at each participating site selected a priori 2 or more study group assignments to which patients could be randomized, based on local equipoise (see eAppendix 2 in Supplement 2 for the breakout by site of which sites selected which combinations). Participants were randomized to each locally available group using balanced assignment. Participants were randomly assigned via a computer software program to each locally available group using proportional assignment (eg, 1:1 if 2 groups available and 1:1:1 if 3 groups available).
Procedures
The study used an open-label design, in which the clinical team was provided instructions for hydrocortisone prescriptions. Hydrocortisone was supplied by each site’s pharmacy. Other aspects of care were provided as per each site’s standard of care. Data were collected on baseline characteristics, corticosteroid use, adverse events, and outcomes by site investigators via a combination of interactive web-based response technology and electronic health record abstraction with built-in validation and logic checks. Although clinical staff were aware of their individual patient’s treatment assignment, neither they nor the ITSC were provided any information about aggregate patient outcomes.
Interventions
Participants were randomized to receive a fixed dose of intravenous hydrocortisone, 50 mg, every 6 hours for 7 days; intravenous hydrocortisone, 50 mg, every 6 hours while in shock for up to 28 days; or no hydrocortisone. A second fixed-dose regimen of 100 mg every 6 hours for 7 days was being incorporated across sites when the study was halted, such that only 2 patients were assigned to that group. The rationale underlying the shock-dependent dosing strategy was that restricting hydrocortisone to the period when the patient had overt shock would maximize the risk-benefit ratio. Shock was defined as the requirement for intravenous vasopressor infusion for the treatment of shock presumed due to COVID-19 and not due to untreated hypovolemia or secondary consequences of other therapies (eg, sedation agents). Hydrocortisone was discontinued in the shock-dependent group once shock was considered to have resolved or vasopressors had been discontinued for 24 hours. In all groups, systemic corticosteroid therapy was permitted if a new clinical indication developed for which corticosteroids are an established treatment such as postextubation stridor, bronchospasm, or anaphylaxis.
In addition to assignment to interventions in the corticosteroid domain, participants could be randomly assigned to other interventions within other therapeutic domains, depending on whether the site was active for that domain, patient eligibility, and consent (see Supplement 1 and https://www.remapcap.org for more details).
Outcomes
The primary outcome was respiratory and cardiovascular organ support–free days up to day 21, an ordinal end point with death within the hospital as the worst outcome (labeled –1), then the length of time free of both respiratory and cardiovascular organ support, such that the best outcome would be 21 organ support–free days. Organ support was defined using the same criteria as those for study entry. This outcome was used in a recent registration trial in septic shock approved by the Food and Drug Administration (although up to 28 days), with a 1.5-day difference (7.5%-15% relative difference) considered to be the minimal clinically important difference.18
Secondary outcomes were in-hospital mortality, ICU and hospital length of stay, respiratory support–free days, cardiovascular organ support–free days, a composite outcome of progression to invasive mechanical ventilation, extracorporeal membrane oxygenation (ECMO) or death among those not ventilated at baseline, and the WHO ordinal scale (range, 0-8, where 0 =
= no illness, 1-7
no illness, 1-7 =
= increasing level of care, and 8
increasing level of care, and 8 =
= death) assessed at day 14.19,20 This scale was used in a recent COVID-19 RCT of remdesivir, where an odds ratio of 1.32 was considered clinically important, although few data support that assumption.20
death) assessed at day 14.19,20 This scale was used in a recent COVID-19 RCT of remdesivir, where an odds ratio of 1.32 was considered clinically important, although few data support that assumption.20
Study Power and Sample Size
The trial was designed with no maximum sample size, given the uncertainty of the pandemic. Sample size calculations for the primary outcome were calculated using trial simulations of the adaptive design rules. If both hydrocortisone groups had effect sizes (odds ratios) of 1.75 compared with the no hydrocortisone group, there would be 90% power to determine whether either group was superior to the no hydrocortisone group with a sample size of 500 patients. If the effect was 1.5, there would be 90% power with a sample size of 1000 patients.
Statistical Analysis
The SAP for the COVID-19 corticosteroid domain was written by blinded steering committee members, posted online (https://www.remapcap.org/) before data lock and analysis, and appears in Supplement 1). The primary analysis was generated from a bayesian cumulative logistic model, which estimated posterior probability distributions of the 21-day organ support–free days (primary outcome) based on the evidence accumulated in the trial in terms of the observed primary outcome and assumed prior knowledge in the form of a prior distribution. Data from the United Kingdom national clinical audit on all COVID-19 ICU admissions (provided by Intensive Care National Audit & Research Centre, London, United Kingdom) were used to inform prior distributions, necessary for bayesian analyses, including initial estimates of the effect of age on outcome. Prior distributions for treatment effects were neutral.
The primary model adjusted for location (site, nested within country), age (categorized into 6 groups), sex, and time period (2-week epochs). The model estimated treatment effects for each intervention within each domain and prespecified treatment-by-treatment interactions across domains. The primary analysis was conducted on all randomized patients who met severe COVID-19 criteria as of June 17, 2020, and not just those randomized within the corticosteroid domain. This approach allowed maximal incorporation of all information, providing the most robust estimation of the coefficients of all included covariates. Not all patients were eligible for all domains nor for all interventions within each domain (depending on site participation, baseline entry criteria, and patient or surrogate preference). Therefore, the model included covariate terms reflecting each patient’s intervention and domain eligibility, such that the estimate of an intervention’s effectiveness relative to any other intervention within that domain was generated from those patients who might have been randomized to either.
Because the primary model included information about assignment to interventions within domains whose evaluation is ongoing, it was run by the fully unblinded statistical analysis committee (Supplement 1), which conducts all protocol-specified trial update analyses and reports those results to the data and safety monitoring board. For the primary analysis, the 2 fixed-dose hydrocortisone groups were combined, such that there were 3 groups: fixed-dose, shock-dependent, and no hydrocortisone. The cumulative log odds for the primary end point was modeled such that a parameter greater than 0 reflects an increase in the cumulative odds for the organ support–free day outcome, which implies benefit. The model assumed proportional effects across the ordinal organ support–free days scale. This assumption was assessed by inspection of the distribution for clinically important deviations. Patients missing the primary end point (n =
= 5) were ignored; there was no imputation of missing primary (or secondary) end point values. A patient who survived to hospital discharge was assumed to be free of organ support through 21 days (last status carried forward).
5) were ignored; there was no imputation of missing primary (or secondary) end point values. A patient who survived to hospital discharge was assumed to be free of organ support through 21 days (last status carried forward).
The model was fit using a Markov Chain Monte Carlo algorithm that drew iteratively (10 000 draws) from the joint posterior distribution, allowing calculation of odds ratios with their 95% credible intervals (CrIs) and the probability that each corticosteroid domain intervention (including the no hydrocortisone group) was optimal, that either hydrocortisone group was superior to no hydrocortisone, and that the fixed-dose and shock-dependent hydrocortisone groups were equivalent. An odds ratio greater than 1 represents more survival and more days free from ICU organ support. Although this analysis was conducted in response to the disclosure of the RECOVERY trial results, it was also the first interim analysis of the COVID-19 patient cohort, which had preexisting internal statistical triggers for trial conclusions and disclosure of results (99% probability of superiority or inferiority, defined as odds ratio >1 and <1, respectively, and 90% probability for equivalence, defined as an odds ratio between 1/1.2 and 1.2).
000 draws) from the joint posterior distribution, allowing calculation of odds ratios with their 95% credible intervals (CrIs) and the probability that each corticosteroid domain intervention (including the no hydrocortisone group) was optimal, that either hydrocortisone group was superior to no hydrocortisone, and that the fixed-dose and shock-dependent hydrocortisone groups were equivalent. An odds ratio greater than 1 represents more survival and more days free from ICU organ support. Although this analysis was conducted in response to the disclosure of the RECOVERY trial results, it was also the first interim analysis of the COVID-19 patient cohort, which had preexisting internal statistical triggers for trial conclusions and disclosure of results (99% probability of superiority or inferiority, defined as odds ratio >1 and <1, respectively, and 90% probability for equivalence, defined as an odds ratio between 1/1.2 and 1.2).
Analysis of the primary outcome was then repeated in a second model using only data from those patients enrolled in the corticosteroid domain with no adjustment for assignment to interventions in other domains. Although using less information, this analysis is more typical for an RCT. Further secondary analyses explored the effects of excluding patients who were ruled out for COVID-19 (defined as documented negative test results for SARS-CoV-2 infection and no positive test results), of excluding adjustment for site and time epoch, and of combining the fixed-dose and shock-dependent hydrocortisone groups.
Identical analyses were conducted to estimate the effect on mortality, except the outcome was dichotomous (alive or dead at hospital discharge). There were also 7 secondary outcome analyses (all using the corticosteroid domain cohort): time to death, respiratory support–free days, cardiovascular organ support–free days, length of ICU stay, length of hospital stay, the WHO ordinal scale at 14 days, and progression to invasive mechanical ventilation, ECMO, or death in those not receiving invasive mechanical ventilation at enrollment. The time-to-death and length-of-stay outcomes were time-to-event analyses with results expressed as hazard ratios. The proportional hazards assumption was assessed by testing whether scaled Schoenfeld residuals and time were independent (P >
> .05) for each covariate. All 3 models met the assumption. The primary safety analysis compared the proportion of patients who developed 1 or more serious adverse events across groups. All analyses were prespecified and are listed in section 15 of the COVID-19 Corticosteroid Domain SAP (pp 391-431) in Supplement 1. Data management and summaries were created using R version 3.5.2, and the primary analysis was computed in R version 4.0.0 using the rstan package version 2.19.3 (R Foundation). Additional data management and analysis were performed in R, SQL 2016, SPSS version 26 (IBM), and Stata version 14.2 (StataCorp).
.05) for each covariate. All 3 models met the assumption. The primary safety analysis compared the proportion of patients who developed 1 or more serious adverse events across groups. All analyses were prespecified and are listed in section 15 of the COVID-19 Corticosteroid Domain SAP (pp 391-431) in Supplement 1. Data management and summaries were created using R version 3.5.2, and the primary analysis was computed in R version 4.0.0 using the rstan package version 2.19.3 (R Foundation). Additional data management and analysis were performed in R, SQL 2016, SPSS version 26 (IBM), and Stata version 14.2 (StataCorp).
Study Termination
Following a press release from the RECOVERY trial on June 16, 2020, and in response to discussions held across the participating sites, the blinded international trial steering committee decided on June 17, 2020, to stop enrollment of patients with COVID-19 in the corticosteroid domain due to a loss of equipoise. No data from the trial were reviewed prior to the decision.
Results
Participants
Between March 9 and June 17, of 1165 screened patients, 614 met criteria for severe COVID-19, were enrolled in REMAP-CAP, and were randomized within at least 1 therapeutic domain (Figure 1). Patients were recruited at 121 sites, of whom 113 (93%) were open for the corticosteroid domain, though 24 sites (21%) only permitted randomization to fixed-dose or shock-dependent hydrocortisone groups (eAppendix 2 in Supplement 2). Among the 614 patients with severe COVID-19, 403 were enrolled in the corticosteroid domain and randomly assigned to the fixed-dose (n =
= 143), shock-dependent (n
143), shock-dependent (n =
= 152), and no (n
152), and no (n =
= 108) hydrocortisone groups. There were 24 participants (of whom 19 were in the corticosteroid domain) for whom either they or the local ethics board requested withdrawal of all data.
108) hydrocortisone groups. There were 24 participants (of whom 19 were in the corticosteroid domain) for whom either they or the local ethics board requested withdrawal of all data.
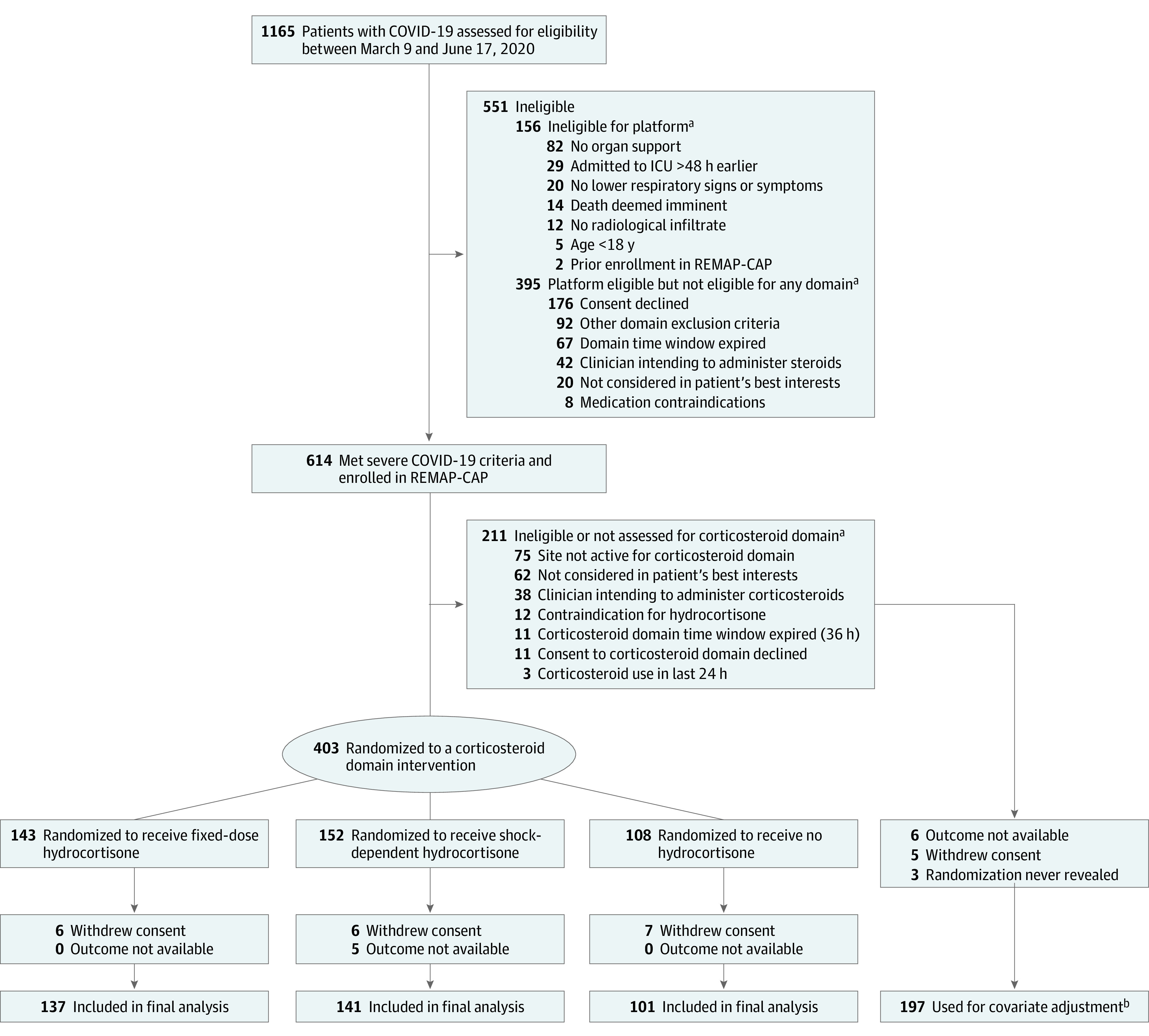
COVID-19 indicates coronavirus disease 2019; ICU, intensive care unit; and REMAP-CAP, Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia.
aPatients could meet more than 1 ineligibility criterion.
bThe primary analysis of alternative interventions within the corticosteroid domain is estimated from a model that adjusts for patient factors and for assignment to interventions in other domains. To obtain the most reliable estimation of the effect of these patient factors and of other interventions on the primary outcome, all patients enrolled in the severe COVID-19 cohort (for whom there is consent and follow-up) are included. Importantly, however, the model also factors eligibility for the corticosteroid domain and its interventions, such that the final estimate of a corticosteroid domain intervention’s effectiveness relative to any other within that domain is generated from those patients that might have been randomized to either.
The baseline characteristics of the corticosteroid study groups whose data were available (n =
= 384) were similar across groups and typical of patients requiring ICU care for COVID-19 (Table 1 and eAppendix 2 in Supplement 2). For an additional 11 patients, of whom 5 were in the corticosteroid domain, follow-up data were unavailable. Thus, the final cohort available for outcome analysis comprised 576 participants in the REMAP-CAP severe COVID-19 cohort (whose data are used for covariate adjustment in the primary analysis), of whom 379 were randomized within the corticosteroid domain (after removing 5 patients in the shock-dependent hydrocortisone group whose outcomes were not available). The mean age for the 3 groups ranged between 59.5 and 60.4 years; most patients were male (range, 70.6%-71.5%); body mass index ranged between 29.7 and 30.9; and patients receiving mechanical ventilation ranged between 50.0% and 63.5% (Table 1).
384) were similar across groups and typical of patients requiring ICU care for COVID-19 (Table 1 and eAppendix 2 in Supplement 2). For an additional 11 patients, of whom 5 were in the corticosteroid domain, follow-up data were unavailable. Thus, the final cohort available for outcome analysis comprised 576 participants in the REMAP-CAP severe COVID-19 cohort (whose data are used for covariate adjustment in the primary analysis), of whom 379 were randomized within the corticosteroid domain (after removing 5 patients in the shock-dependent hydrocortisone group whose outcomes were not available). The mean age for the 3 groups ranged between 59.5 and 60.4 years; most patients were male (range, 70.6%-71.5%); body mass index ranged between 29.7 and 30.9; and patients receiving mechanical ventilation ranged between 50.0% and 63.5% (Table 1).
Table 1.
| Characteristic | No./total No. (%) of participantsa | ||
|---|---|---|---|
Fixed-dose hydrocortisone (n = = 137)b 137)b | Shock-dependent hydrocortisone (n = = 146) 146) | No hydrocortisone (n = = 101) 101) | |
| Age, mean (SD), y | 60.4 (11.6) | 59.5 (12.7) | 59.9 (14.6) |
| Sex | |||
| Male | 98 (71.5) | 103 (70.6) | 72 (71.3) |
| Female | 39 (28.5) | 43 (29.5) | 29 (28.7) |
| Body mass indexc | |||
| No. | 135 | 141 | 100 |
| Mean (SD) | 30.9 (7.3) | 30.7 (7.4) | 29.7 (7.5) |
| Race/ethnicityd | |||
| White | 79/111 (71.2) | 80/105 (76.2) | 45/79 (57.0) |
| Asian | 18/111 (16.2) | 11/105 (10.5) | 22/79 (27.9) |
| Black | 4/111 (3.6) | 7/105 (6.7) | 4/79 (5.1) |
| Mixed | 4/111 (3.6) | 0/105 | 2/79 (2.5) |
| Otherd | 6/111 (5.4) | 7/105 (6.7) | 6/79 (7.6) |
| Confirmed SARS-CoV-2 infectione | 109/134 (81.3) | 87/125 (69.6) | 79/100 (79.0) |
| Preexisting conditions | |||
| Diabetes | 50/129 (38.8) | 39/144 (27.1) | 30/98 (30.6) |
| Respiratory disease | 27/127 (21.3) | 28/144 (19.4) | 20/98 (20.4) |
| Asthma/COPD | 21/137 (15.3) | 25/144 (17.4) | 16/100 (16.0) |
| Other | 7/127 (5.5) | 4/144 (2.8) | 4/95 (4.2) |
| Kidney disease | 13/128 (10.2) | 11/127 (8.7) | 8/92 (8.7) |
| Severe cardiovascular disease | 9/136 (6.6) | 13/140 (9.3) | 6/99 (6.1) |
| Immunosuppressive disease | 7/127 (5.5) | 9/144 (6.3) | 2/95 (2.1) |
| Chronic immunosuppressive therapy | 8/137 (5.8) | 7/142 (4.9) | 6/100 (6.0) |
| Time to enrollment, median (IQR) | |||
| From hospital admission, d | 1.2 (0.8-2.6) | 1.0 (0.7-2.8) | 1.1 (0.7-2.0) |
| From ICU admission, h | 15.1 (7.5-19.8) | 12.3 (5.4-18.8) | 13.5 (8.1-17.5) |
| Acute respiratory support | |||
| None/supplemental oxygen only | 0 | 1 (0.7) | 0 |
| High-flow nasal cannula | 17 (12.4) | 23 (15.8) | 16 (15.8) |
| Noninvasive ventilation only | 33 (24.1) | 49 (33.6) | 32 (31.7) |
| Invasive mechanical ventilation | 87 (63.5) | 73 (50.0) | 53 (52.5) |
| ECMO | 1/137 (0.7) | 0/143 | 2/99 (2.0) |
| Vasopressor support | 56 (40.9) | 47 (32.2) | 30 (29.7) |
| APACHE II score, median (IQR)f | |||
| No. | 123 | 130 | 94 |
| Median (IQR) | 18 (10-23) | 17 (12-24) | 15 (12-21) |
| Glasgow Coma Scale score, mean (SD)g | |||
| No. | 131 | 133 | 98 |
| Mean (SD) | 13 (4) | 13 (4) | 14 (3) |
| Acute physiology and laboratory valuesh | |||
| Pao2/Fio2 | |||
| No. | 130 | 142 | 96 |
| Mean (SD) | 149 (83) | 137 (74) | 138 (78) |
| Creatinine, mg/dL | |||
| No. | 136 | 143 | 98 |
| Median (IQR) | 0.9 (0.7-1.2) | 0.9 (0.7-1.3) | 0.8 (0.6-1.2) |
| Lactate, mmol/L | |||
| No. | 124 | 124 | 88 |
| Median (IQR) | 1.2 (0.9-1.5) | 1.1 (0.9-1.6) | 1.1 (0.8-1.5) |
| Platelet count, ×109/L | |||
| No. | 135 | 143 | 98 |
| Mean (SD) | 254 (117) | 259 (112) | 259 (112) |
| Bilirubin, mg/dL | |||
| No. | 129 | 134 | 93 |
| Median (IQR) | 0.1 (0.1-0.2) | 0.1 (0.1-0.2) | 0.1 (0.1-0.2) |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; COPD, chronic obstructive pulmonary disease; ECMO, extracorporeal membrane oxygenation; Fio2, fraction of inspired oxygen; ICU, intensive care unit; IQR, interquartile range; Pao2, partial pressure of arterial oxygen; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
SI conversion factors: To convert bilirubin to μmol/L, multiply by 17.104; creatinine to μmol/L, multiply by 88.4; lactate to mg/dL, divide by 0.111.
 =
= 135) or 100 mg (n
135) or 100 mg (n =
= 2) intravenous hydrocortisone every 6 hours for 7 days.
2) intravenous hydrocortisone every 6 hours for 7 days.Intervention Fidelity
Information on corticosteroid dosing during the first week (defined as study day 1 through day 8) was available for 376 participants (99%) in the corticosteroid domain. Among those assigned to the fixed-dose hydrocortisone group, 97% (n =
= 130/134) received at least 1 dose of hydrocortisone, an additional 1.5% (2/134) received an alternative systemic corticosteroid, and only 2 (1.5%) received no corticosteroid. The first dose of hydrocortisone was given before midnight of the first study day in 95% of patients (124/130) and the median duration of hydrocortisone therapy was 7 days (interquartile range [IQR], 6-8). Among those assigned to shock-dependent dosing, 43% (62/143) received at least 1 dose of hydrocortisone (and 49% [70/143] received any systemic corticosteroid, including hydrocortisone). Among those treated, the median study day on which hydrocortisone was commenced was study day 1 (IQR, 1-4), and the median duration was 3 days (IQR, 1-4) of hydrocortisone and 3 days (IQR, 2-4) of any systemic corticosteroid. Among those assigned to the no hydrocortisone group, 15% (15/99) received a systemic corticosteroid (6 of whom received hydrocortisone). For those receiving a corticosteroid, the median duration was 2 days (IQR, 2-6).
130/134) received at least 1 dose of hydrocortisone, an additional 1.5% (2/134) received an alternative systemic corticosteroid, and only 2 (1.5%) received no corticosteroid. The first dose of hydrocortisone was given before midnight of the first study day in 95% of patients (124/130) and the median duration of hydrocortisone therapy was 7 days (interquartile range [IQR], 6-8). Among those assigned to shock-dependent dosing, 43% (62/143) received at least 1 dose of hydrocortisone (and 49% [70/143] received any systemic corticosteroid, including hydrocortisone). Among those treated, the median study day on which hydrocortisone was commenced was study day 1 (IQR, 1-4), and the median duration was 3 days (IQR, 1-4) of hydrocortisone and 3 days (IQR, 2-4) of any systemic corticosteroid. Among those assigned to the no hydrocortisone group, 15% (15/99) received a systemic corticosteroid (6 of whom received hydrocortisone). For those receiving a corticosteroid, the median duration was 2 days (IQR, 2-6).
Primary Outcome
Primary outcomes are presented in Table 2 and Figure 2. The median organ support–free days were 0 (IQR, –1 to 15), 0 (IQR, –1 to 13), and 0 (IQR, –1 to 11) for the fixed-dose, shock-dependent, and no hydrocortisone groups. Relative to the no hydrocortisone group, the median adjusted odds ratios from the primary model were 1.43 (95% CrI, 0.91-2.27) and 1.22 (95% CrI, 0.76-1.94) for the fixed-dose and shock-dependent groups, respectively, yielding 93% and 80% probabilities of superiority. There were no clinically relevant deviations from the assumption of proportional effects across the organ support–free days scale, with the 2 treatment groups having observed benefit across the entire range (Figure 2B). In the prespecified secondary analysis of the primary outcome using only data from participants in the corticosteroid domain and not adjusting for intervention assignment in other domains, the median adjusted odds ratios were 1.45 (95% CrI, 0.93-2.30) and 1.24 (95% CrI, 0.80-1.95) for the fixed-dose and shock-dependent groups, respectively, yielding 95% and 83% probabilities of superiority. Estimates when excluding those who were ruled out for COVID-19, when dropping site and time from the model, and when combining the fixed-dose and shock-dependent groups are shown in eTables 1 and 2 in Supplement 2.
Table 2.
| Outcome/analysisa | Fixed-dose hydrocortisone (n = = 137) 137) | Shock-dependent hydrocortisone (n = = 141) 141) | No hydrocortisone (n = = 101) 101) |
|---|---|---|---|
| Primary outcome, organ support–free days | |||
| Median (IQR) | 0 (–1 to 15) | 0 (–1 to 13) | 0 (–1 to 11) |
| Subcomponents of organ support–free days | |||
| In-hospital deaths, No. (%) | 41 (30) | 37 (26) | 33 (33) |
| Organ support–free days among survivors, median (IQR) | 11.5 (0 to 17) | 9.5 (0 to 16) | 6 (0 to 12) |
Primary analysis of the primary outcome, using covariate data from all severe-state participants with COVID-19 (n = = 576)b 576)b | |||
| Adjusted odds ratio | |||
| Mean (SD) | 1.47 (0.35) | 1.26 (0.31) | 1 [Reference] |
| Median (95% CrI) | 1.43 (0.91 to 2.27) | 1.22 (0.76 to 1.94) | 1 [Reference] |
| Probability of superiority to no hydrocortisone, % | 93 | 80 | |
Secondary analysis of the primary outcome, restricted to corticosteroid domain participants (n = = 379) with no adjustment for intervention assignment in other domainsc 379) with no adjustment for intervention assignment in other domainsc | |||
| Adjusted odds ratio | |||
| Mean (SD) | 1.49 (0.35) | 1.28 (0.30) | 1 [Reference] |
| Median (95% CrI) | 1.45 (0.93 to 2.30) | 1.24 (0.80 to 1.95) | 1 [Reference] |
| Probability of superiority to no hydrocortisone, % | 95 | 83 | |
Abbreviations: COVID-19, coronavirus disease 2019; IQR, interquartile range; CrI, credible interval.
 =
= 576), adjusting for age, sex, time period, site, region, domain and intervention eligibility, and intervention assignment (see COVID-19 Corticosteroid Domain statistical analysis plan in Supplement 1 and full report from the statistical analysis committee in eAppendix 3 in Supplement 2).
576), adjusting for age, sex, time period, site, region, domain and intervention eligibility, and intervention assignment (see COVID-19 Corticosteroid Domain statistical analysis plan in Supplement 1 and full report from the statistical analysis committee in eAppendix 3 in Supplement 2). =
= 379) and did not include information on assignment to interventions other than hydrocortisone.
379) and did not include information on assignment to interventions other than hydrocortisone.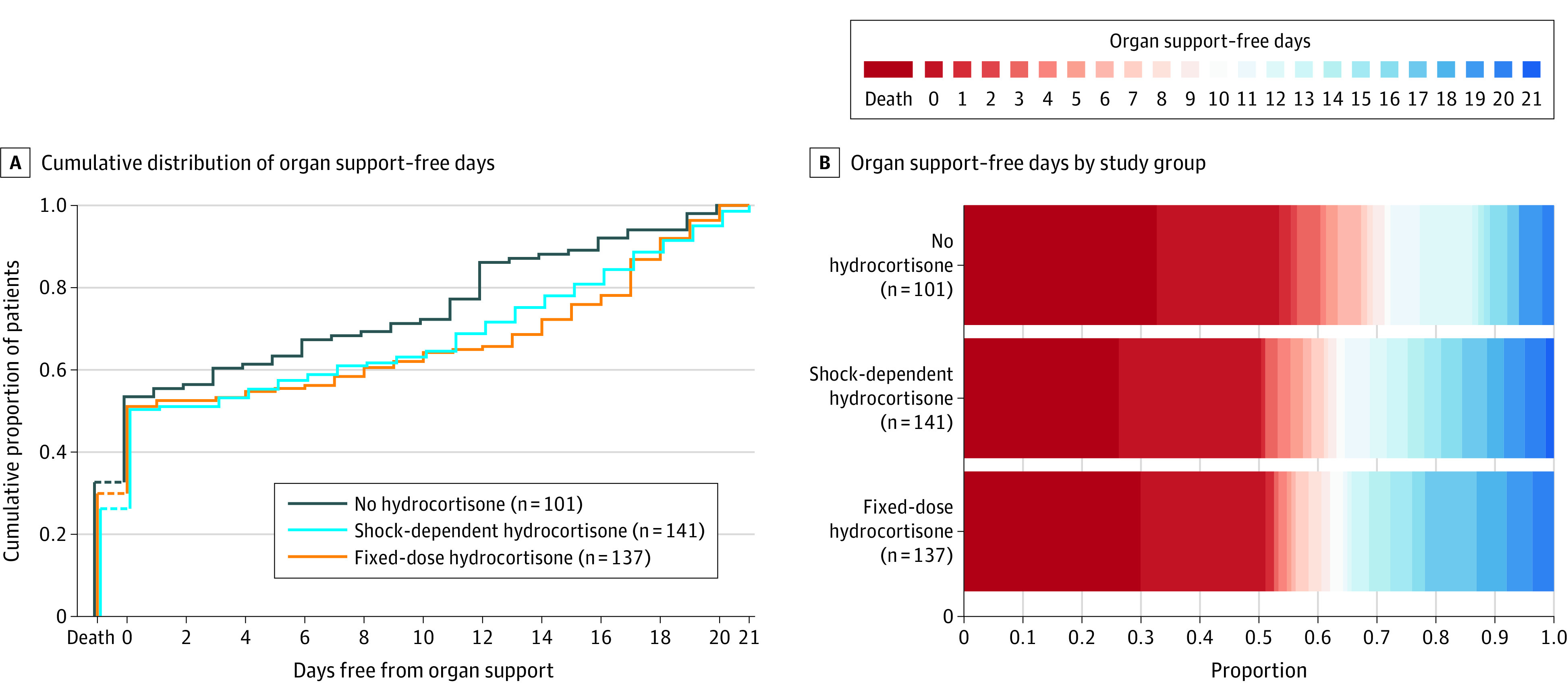
A, Distributions of organ support–free days (see the Methods section for definition) by study group as the cumulative proportion (y-axis) for each study group by day (x-axis), with death listed first. Curves that rise more slowly are more favorable. B, Organ support–free days as horizontally stacked proportions by study group. Red represents worse values and blue represents better values. The median adjusted odds ratios from the primary analysis, using a bayesian cumulative logistic model, were 1.43 (95% credible interval, 0.91-2.27) and 1.22 (95% credible interval, 0.76-1.94) for the fixed-dose and shock-dependent hydrocortisone groups compared with the no hydrocortisone group, yielding 93% and 80% probabilities of superiority over the no hydrocortisone group, respectively.
In-Hospital Mortality and Other Secondary Outcomes
The mortality analyses and secondary outcomes are presented in Table 3. The in-hospital mortality rates were 30% (n =
= 41/137), 26% (n
41/137), 26% (n =
= 37/141), and 33% (n
37/141), and 33% (n =
= 33/99) in the fixed-dose, shock-dependent, and no hydrocortisone groups, respectively. Relative to the no hydrocortisone group, the median adjusted odds ratios from the primary model were 1.03 (95% CrI, 0.53-1.95) and 1.10 (95% CrI, 0.58-2.11) (where a value >1 represents benefit) for the fixed-dose and shock-dependent hydrocortisone groups, respectively, yielding 54% and 62% bayesian posterior probabilities of superiority. Results from secondary analyses of in-hospital mortality using only data from the corticosteroid domain are presented in eTables 2 and 3 in Supplement 2. Other secondary outcome analyses are presented in Table 3. Full model results of all outcome analyses are provided in eAppendices 3 and 4 in Supplement 2.
33/99) in the fixed-dose, shock-dependent, and no hydrocortisone groups, respectively. Relative to the no hydrocortisone group, the median adjusted odds ratios from the primary model were 1.03 (95% CrI, 0.53-1.95) and 1.10 (95% CrI, 0.58-2.11) (where a value >1 represents benefit) for the fixed-dose and shock-dependent hydrocortisone groups, respectively, yielding 54% and 62% bayesian posterior probabilities of superiority. Results from secondary analyses of in-hospital mortality using only data from the corticosteroid domain are presented in eTables 2 and 3 in Supplement 2. Other secondary outcome analyses are presented in Table 3. Full model results of all outcome analyses are provided in eAppendices 3 and 4 in Supplement 2.
Table 3.
| Outcome/analysisa | Fixed-dose hydrocortisone (n = = 137) 137) | Shock-dependent hydrocortisone (n = = 141) 141) | No hydrocortisone (n = = 101) 101) |
|---|---|---|---|
Primary in-hospital mortality model, using covariate data from all severe state participants with COVID-19 (n = = 576)b 576)b | |||
| Adjusted odds ratio | |||
| Mean (SD) | 1.08 (0.37) | 1.16 (0.40) | 1 [Reference] |
| Median (95% CrI) | 1.03 (0.53-1.95) | 1.10 (0.58-2.11) | 1 [Reference] |
| Probability of superiority to no hydrocortisone, % | 54 | 62 | |
Other secondary outcomes, restricted to corticosteroid domain participants (n = = 379) with no adjustment for intervention assignment in other domainsc 379) with no adjustment for intervention assignment in other domainsc | |||
| Time to death | |||
| Adjusted hazard ratio | |||
| Mean (SD) | 0.97 (0.22) | 1.01 (0.23) | 1 [Reference] |
| Median (95% CrI) | 0.94 (0.61-1.46) | 0.98 (0.63-1.54) | 1 [Reference] |
| Probability of superiority to no hydrocortisone, % | 40 | 47 | |
| Respiratory support–free days | |||
| Adjusted odds ratio | |||
| Mean (SD) | 1.45 (0.34) | 1.31 (0.30) | 1 [Reference] |
| Median (95% CrI) | 1.42 (0.90-2.24) | 1.28 (0.81-2.00) | 1 [Reference] |
| Probability of superiority to no hydrocortisone, % | 94 | 85 | |
| Cardiovascular organ support–free days | |||
| Adjusted odds ratio | |||
| Mean (SD) | 1.68 (0.40) | 1.32 (0.31) | 1 [Reference] |
| Median (95% CrI) | 1.63 (1.03-2.59) | 1.29 (0.81-2.02) | 1 [Reference] |
| Probability of superiority to no hydrocortisone, % | 98 | 86 | |
| Length of ICU stay | |||
| Adjusted hazard ratio | |||
| Mean (SD) | 0.93 (0.14) | 0.86 (0.13) | 1 [Reference] |
| Median (95% CrI) | 0.92 (0.68-1.24) | 0.85 (0.62-1.15) | 1 [Reference] |
| Probability of superiority to no hydrocortisone, % | 29 | 14 | |
| Length of hospital stay | |||
| Adjusted hazard ratio | |||
| Mean (SD) | 0.99 (0.16) | 0.94 (0.15) | 1 [Reference] |
| Median (95% CrI) | 0.97 (0.72-1.32) | 0.93 (0.69-1.26) | 1 [Reference] |
| Probability of superiority to no hydrocortisone, % | 43 | 31 | |
| WHO scale at day 14d | |||
| Adjusted odds ratio | |||
| Mean (SD) | 1.33 (0.32) | 1.06 (0.26) | 1 [Reference] |
| Median (95% CrI) | 1.29 (0.83-2.05) | 1.03 (0.65-1.65) | 1 [Reference] |
| Probability of superiority to no hydrocortisone, % | 87 | 55 | |
Progression to invasive mechanical ventilation, ECMO, or death, restricted to those not intubated at baseline (n = = 168) 168) | |||
| Free of invasive mechanical ventilation at baseline, No. | 50 | 70 | 48 |
| Progression to intubation, ECMO, or death, No. (%) | 23 (46) | 42 (60) | 37 (77) |
| Adjusted odds ratio | |||
| Mean (SD) | 3.02 (1.40) | 1.36 (0.59) | 1 [Reference] |
| Median (95% CrI) | 2.74 (1.18-6.56) | 1.24 (0.56-2.82) | 1 [Reference] |
| Probability of superiority to no hydrocortisone, % | 99 | 70 | |
| Serious adverse events | |||
| Patients with >1 serious adverse event, No. (%) | 4 (3) | 5 (4) | 1 (1) |
Abbreviations: COVID-19, coronavirus disease 2019; CrI, credible interval; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; WHO, World Health Organization.
 =
= 576), adjusting for age, sex, time period, site, region, domain and intervention eligibility, and intervention assignment (see COVID-19 Corticosteroid Domain statistical analysis plan in Supplement 1 and full report from the statistical analysis committee in eAppendix 3 in Supplement 2).
576), adjusting for age, sex, time period, site, region, domain and intervention eligibility, and intervention assignment (see COVID-19 Corticosteroid Domain statistical analysis plan in Supplement 1 and full report from the statistical analysis committee in eAppendix 3 in Supplement 2). =
= 379) and did not include information on assignment to interventions other than hydrocortisone. Other sensitivity analyses are described in the Results section and provided in eTables 2 and 3 and eAppendices 3 and 4 in Supplement 2.
379) and did not include information on assignment to interventions other than hydrocortisone. Other sensitivity analyses are described in the Results section and provided in eTables 2 and 3 and eAppendices 3 and 4 in Supplement 2.Adverse Events
Serious adverse event rates are presented in Table 3 and eAppendix 4 in Supplement 2. There were 10 patients (2.6%) who incurred a serious adverse event (none incurred >1), 9 of whom were in the fixed-dose (n =
= 4) and shock-dependent (n
4) and shock-dependent (n =
= 5) hydrocortisone groups. Two events (severe neuromyopathy and fungemia) occurred in the fixed-dose hydrocortisone group and were considered by the site investigator as possibly related to study group assignment. The other events, none of which were considered related, were single cases of pneumonia, pulmonary embolism, elevated serum troponin, postoperative hemorrhage, intracranial hemorrhage, thrombocytopenia, ventricular tachycardia, and hypoglycemia.
5) hydrocortisone groups. Two events (severe neuromyopathy and fungemia) occurred in the fixed-dose hydrocortisone group and were considered by the site investigator as possibly related to study group assignment. The other events, none of which were considered related, were single cases of pneumonia, pulmonary embolism, elevated serum troponin, postoperative hemorrhage, intracranial hemorrhage, thrombocytopenia, ventricular tachycardia, and hypoglycemia.
Discussion
The principal findings from this study were a 93% probability of benefit of a fixed-duration dosing of hydrocortisone and an 80% probability of benefit of a shock-dependent dosing of hydrocortisone, compared with no hydrocortisone, with regard to the odds of improvement in organ support–free days within 21 days. However, the study was stopped early, the probability of benefit with hydrocortisone did not meet the prespecified statistical trigger for a trial conclusion of superiority, and no strategy was determined to be optimal.
REMAP-CAP is designed to test numerous interventions for pandemic and nonpandemic pneumonia over time. The design has internal statistical triggers for stopping particular study questions, but external factors, such as lack of equipoise following new evidence, can also trigger termination of a portion of the trial. This analysis was prompted by the loss of equipoise following announcement that dexamethasone reduced mortality in the RECOVERY trial.18 Coincidentally, this analysis was also the first interim analysis of the severe COVID-19 cohort: had any internal threshold been triggered, the results would have been released regardless of RECOVERY. However, had RECOVERY not prompted cessation, the internal action would simply be to generate updated randomization proportions and continue enrollment.
Given the findings from contemporaneous trials, the findings might generally be considered supportive of corticosteroid use in this patient population.15,21 For example, the benefit reported in RECOVERY was in patients similar to those enrolled in this trial using a corticosteroid, dexamethasone, with a similar glucocorticoid effect to that of the fixed-dose hydrocortisone course in this trial. As such, it seems reasonable that either dexamethasone or hydrocortisone might be beneficial. In turn, it is plausible that the primary benefit is exerted through glucocorticoid, rather than mineralocorticoid effects, given dexamethasone’s lack of mineralocorticoid activity. Systemic corticosteroids have well-described adverse effects. In this open-label trial, serious adverse events were rare, precluding statistical inference. However, they were reported more commonly in the 2 hydrocortisone groups.
The findings regarding the shock-dependent hydrocortisone group are less clear, with an 80% probability of benefit. In this group, physicians only administered hydrocortisone when the patient was in shock. Thus, if corticosteroids are beneficial for COVID-19 through mechanisms other than mitigation of shock, this group was effectively undertreated, and one would anticipate less average benefit. In contrast, if the benefits of corticosteroids largely accrue to those in shock, avoidance of unnecessary corticosteroid therapy in those not in shock might improve the safety profile of corticosteroid therapy. This question remains unresolved.
Strengths of the study include the pragmatic and international design, rendering findings likely generalizable at least to other resource-rich settings around the world. In addition, all analyses were specified prior to unblinding results, results were robust to sensitivity analyses, and findings of multiple secondary outcomes demonstrated similar probabilities of benefit of hydrocortisone. An advantage of using a bayesian approach is that any data, including data following unplanned cessation in enrollment, can be analyzed and quantified as posterior probabilities, which is arguably more useful and is more quantitative than a frequentist finding of failure to reject a null hypothesis possibly because of lack of power.22,23 The platform trial design allows efficient enrollment into multiple therapeutic domains simultaneously. One concern could have been potential confounding because of treatment-by-treatment interactions. However, the results were similar with and without adjustment for other treatment assignments.
Limitations
The study has several limitations. First, the results are presented before reaching any prespecified internal trigger. Nonetheless, to our knowledge, this trial represents the largest randomized data on hydrocortisone in this patient population. Second, the study used an open-label design, although clinician and patient awareness of study assignment likely had minimal effect on the primary outcome. Third, 15% of the no hydrocortisone group received systemic corticosteroids, although typically only for a short period. This usage is similar to that in RECOVERY18 and may often have been unavoidable (eg, to treat postextubation stridor). Nonetheless, it could have biased the results toward smaller effect sizes than would have been observed had corticosteroid use been lower in the no hydrocortisone group.
Conclusions
Among patients with severe COVID-19, treatment with a 7-day fixed-dose course of hydrocortisone or shock-dependent dosing of hydrocortisone, compared with no hydrocortisone, resulted in 93% and 80% probabilities of superiority with regard to the odds of improvement in organ support–free days within 21 days. However, the trial was stopped early and no treatment strategy met prespecified criteria for statistical superiority, precluding definitive conclusions.
Notes
Supplement 2.
eAppendix 1. Enrollment criteria
eAppendix 2. Site Participation in the Corticosteroid Domain
eTable 1. Secondary Analyses of Primary Outcome (Organ Support-free Days), restricted to participants enrolled in Corticosteroid Domain
eTable 2. Secondary Analyses of Primary Outcome and of Mortality with Fixed Dose and Shock-dependent Hydrocortisone Groups Combined
eTable 3. Secondary Analyses of In-hospital Mortality
eAppendix 3. Technical Report from the Statistical Analysis Committee for SAP Outcome Analyses 15.1-4
eAppendix 4. Technical Report from Berry Consultants for SAP Outcome Analyses 15.5-20
eAppendix 5. The REMAP-CAP Investigators
References
Full text links
Read article at publisher's site: https://doi.org/10.1001/jama.2020.17022
Read article for free, from open access legal sources, via Unpaywall:
https://jamanetwork.com/journals/jama/articlepdf/2770278/jama_angus_2020_oi_200103_1604520387.33452.pdf
Citations & impact
Impact metrics
Article citations
Corticosteroids for hospitalized patients with severe/critical COVID-19: a retrospective study in Chongqing, China.
Sci Rep, 14(1):24317, 16 Oct 2024
Cited by: 0 articles | PMID: 39414922 | PMCID: PMC11484943
Reply to the Comment on: "Dexamethasone treatment for COVID-19 is related to increased mortality in hematologic malignancy patients: results from the EPICOVIDEHA Registry".
Haematologica, 109(10):3457-3458, 01 Oct 2024
Cited by: 0 articles | PMID: 38841780 | PMCID: PMC11443359
Associations between corticosteroid dosage and clinical outcomes in patients with hypoxemic COVID-19 pneumonia: A retrospective cohort study.
PLoS One, 19(9):e0308069, 06 Sep 2024
Cited by: 0 articles | PMID: 39240825 | PMCID: PMC11379263
Clinical phenotyping uncovers heterogeneous associations between corticosteroid treatment and survival in critically ill COVID-19 patients.
Intensive Care Med, 50(11):1884-1896, 26 Aug 2024
Cited by: 1 article | PMID: 39186112 | PMCID: PMC11541258
Evaluating the Risk-Benefit Profile of Corticosteroid Therapy for COVID-19 Patients: A Scoping Review.
Pharmacy (Basel), 12(4):129, 22 Aug 2024
Cited by: 0 articles | PMID: 39195858 | PMCID: PMC11360832
Review Free full text in Europe PMC
Go to all (508) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT02735707
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Effect of Hydrocortisone on 21-Day Mortality or Respiratory Support Among Critically Ill Patients With COVID-19: A Randomized Clinical Trial.
JAMA, 324(13):1298-1306, 01 Oct 2020
Cited by: 299 articles | PMID: 32876689 | PMCID: PMC7489432
Effect of Convalescent Plasma on Organ Support-Free Days in Critically Ill Patients With COVID-19: A Randomized Clinical Trial.
JAMA, 326(17):1690-1702, 01 Nov 2021
Cited by: 118 articles | PMID: 34606578 | PMCID: PMC8491132
Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients With Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial.
JAMA, 324(13):1307-1316, 01 Oct 2020
Cited by: 771 articles | PMID: 32876695 | PMCID: PMC7489411
Low-Dose Corticosteroids for Critically Ill Adults With Severe Pulmonary Infections: A Review.
JAMA, 332(4):318-328, 01 Jul 2024
Cited by: 1 article | PMID: 38865154
Review
Funding
Funders who supported this work.
Health Research Board (3)
Grant ID: CTN-2014-012
Grant ID: TMRN-2017-1
Grant ID: TMRN-2014-1
NHLBI NIH HHS (1)
Grant ID: T32 HL007820
NIGMS NIH HHS (1)
Grant ID: R35 GM119519
National Institute for Health Research (NIHR) (2)
Personalised Medicine in Sepsis
Professor Anthony Gordon, Imperial College of Science, Technology and Medicine
Grant ID: RP-2015-06-018
REMAP-CAP: Randomized, Embedded, Multifactorial Adaptive Platform trial for Community- Acquired Pneumonia (COVID-19)
Professor Anthony Gordon, Imperial College of Science, Technology and Medicine
Grant ID: Covid-19 - REMAP-CAP
 1,2
1,2 




