Abstract
Free full text

Benefits and adverse effects of hydroxychloroquine, methotrexate and colchicine: searching for repurposable drug candidates
Abstract
Repurposing of antirheumatic drugs has garnered global attention. The aim of this article is to overview available evidence on the use of widely used antirheumatic drugs hydroxychloroquine, methotrexate and colchicine for additional indications. Hydroxychloroquine has endothelial stabilizing and anti-thrombotic effects. Its use has been explored as an adjunctive therapy in refractory thrombosis in antiphospholipid syndrome. It may also prevent recurrent pregnancy losses in the absence of antiphospholipid antibodies. Hydroxychloroquine favourably modulates atherogenic lipid and glycaemic profiles. Methotrexate has been tried for modulation of cardiovascular events in non-rheumatic clinical conditions, although a large clinical trial failed to demonstrate a benefit. Colchicine has been shown to successfully reduce the risk of recurrent cardiovascular events in a large multicentric trial. Potential antifibrotic effects of colchicine require further exploration. Hydroxychloroquine, methotrexate and colchicine are also being tried at different stages of the ongoing Coronavirus Disease 19 (COVID-19) pandemic for prophylaxis and treatment. While the use of these agents is being diversified, their adverse effects should be timely diagnosed and prevented. Hydroxychloroquine can cause retinopathy and rarely cardiac and auditory toxicity, retinopathy being dose and time dependent. Methotrexate can cause transaminitis, cytopenias and renal failure, particularly in acute overdoses. Colchicine can rarely cause myopathies, cardiomyopathy, cytopenias and transaminitis. Strong evidence is warranted to keep balance between benefits of repurposing these old antirheumatic drugs and risk of their adverse effects.
Introduction
Rheumatologists are at the forefront of research and practice of using various anti-inflammatory and immunomodulatory drugs. Their decades-long clinical experience can be valuable at the time of drug repurposing and attempting to use widely tested antirheumatic drugs for newer indications [1]. Numerous immunomodulatory drugs have been employed to induce remission of autoimmune disease and prevent adverse effects of concomitant high-dose corticosteroid and other synthetic disease-modifying anti-rheumatic drug (DMARD) therapies, particularly in elderly patients and those with comorbidities [2]. Although precise mechanisms of action of most old antirheumatic drugs remain unknown, these are exemplified for their relative safety and utility, allowing corticosteroid dose tapering and long-term disease control.
A Scopus search conducted on 10th August 2020 of the number of publications mentioning these drugs to this day revealed an ongoing interest in these drugs in the published literature. Overall, 163,999 documents are retrievable for methotrexate, 38,713 for colchicine, and 22,852 for hydroxychloroquine (Fig. 1).
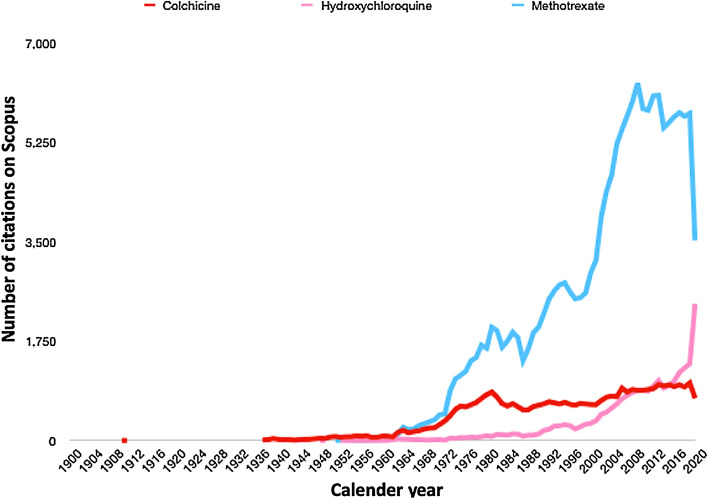
Annual publication activity in Scopus as of August 10, 2020 with the keywords “methotrexate”, “colchicine” and “hydroxychloroquine” for articles published up to 2020. Graph might appear to dip for some entries as the year 2020 is still ongoing. The graph seems to suggest that there is still ongoing interest in these older disease-modifying antirheumatic drugs
The repurposing of traditionally used DMARDs has garnered significant attention recently with the advent of the Coronavirus disease 19 (COVID-19) pandemic [3]. This opinion article aims to sensitize readers towards the potential utility of these drugs in cardiovascular disease and fibrosis (both of which have been reported as sequelae of COVID-19) [4–6] as well as serve as a reminder of their adverse effect profile. Such potential adverse effects need to be kept in mind by specialists who do not routinely use these drugs when they are explored for other indications. In this article we overview some of the effects of hydroxychloroquine, methotrexate and colchicine and reflect on their positive and negative sides to guide repurposing of drug therapies. The choice of articles for review reflects the authors’ understanding and viewpoints regarding the potential therapeutic roles of these drugs.
Hydroxychloroquine
Hydroxychloroquine (HCQ), a relatively safe derivative of chloroquine, is effectively used for the treatment of systemic lupus erythematosus and other inflammatory rheumatic diseases. It acts by accumulating in lysosomes, where its basic pH modifies the normally acidic milieu. This interferes with the loading and presentation of antigens by class II major histocompatibility complex (MHC) protein. Also, it interferes with the activation of toll-like receptors (TLR) by deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) moieties [7].
The use of HCQ reduces the risk of thrombosis and diminishes vascular inflammation and endothelial dysfunction in a murine model of antiphospholipid syndrome (APS) [8]. In 22 patients with antiphospholipid antibodies (aPL) treated with HCQ, levels of soluble tissue factor reduced after three months of HCQ therapy. However, other markers of thrombogenic potential, such as annexin 5 activity and anti-domain 1 immunoglobulin G activity, and complement activation did not differ [9]. In 189 patients with systemic lupus erythematosus (SLE) from Italy (43 positive for aPL) followed up over a median of 13 years, there was a markedly reduced risk of vascular events in those treated with HCQ for five years (hazard ratio [HR] 0.04, 95% confidence interval [CI] 0.004–0.48) [10]. Another retrospective study analysed insurance databases in Taiwan (1946 patients with SLE on HCQ during first year compared with a group of SLE patients not on HCQ during the same period) [11]. Over a mean period of 7.4 years, HCQ therapy was associated with a small but non-significant reduction in the risk of vascular events (HR 0.91, 95% CI 0.71–1.15). The data was limited due to the lack of data about aPL for these patients [11].
A multicentric randomized controlled trial (RCT) reported 20 patients with aPL positivity in the absence of any systemic autoimmune disease who received either HCQ or placebo. Over a mean of 1.7 years of follow-up, neither group developed thrombotic events, resulting in premature termination of the trial and a subsequent inability to draw meaningful conclusions [12]. Another recent open-label study in Greece with primary APS treated with standard anticoagulation regimen (with or without antiplatelets) randomized 50 patients to receive either HCQ in addition to standard care or standard care only. Over mean 2.6 years follow-up, those on HCQ had a lower risk of incident thrombosis, which did not remain significant (HR 0.09, 95% CI 0.01–1.26) after adjusting for confounders. Possibly, a larger sample size might have attained results with statistical significance [13]. The European League against Rheumatism (EULAR) recommends HCQ therapy (based on expert opinion and anecdotal evidence) for patients with APS and recurrent pregnancy loss despite optimal doses of anticoagulation and antiplatelet agents during pregnancy [14].
Various ongoing clinical trials are evaluating the role of add-on HCQ in APS. The HIBISCUS multicentre multinational trial is evaluating the effect of HCQ (400 mg daily for the duration of pregnancy) compared to placebo, in addition to standard therapy (i.e. preventative dose of low molecular weight heparin with aspirin), on live births in women with primary APS. A related study, the HIBISCUS-T trial, is evaluating the preventative role for recurrent thrombosis in patients with thrombotic APS treated with HCQ or placebo in addition to oral anticoagulation with vitamin K antagonists for 24 months [15]. The HYPATIA trial is another multicentric trial spanning multiple European nations assessing the effect of HCQ in a placebo-controlled RCT involving patients with either APS or persistent aPL positivity. This trial intends to assess the impact of HCQ, started before pregnancy and continued for 9 months, on pregnancy morbidity associated with aPL, i.e. a composite of early pregnancy losses, pre-term delivery (< 34 weeks) or placental insufficiency [16]. Another French multicentric trial, the HYDROSAPL trial, is also assessing the effect of HCQ on pregnancy outcomes in primary APS [17]. Apart from APS, HCQ is also being explored for its potential utility in patients with recurrent pregnancy loss. In this French RCT (the BBQ trial), patients with recurrent pregnancy loss shall be treated with HCQ 400 mg daily or placebo starting pre-conception until the 10th week of pregnancy, with the objective of assessing whether treatment improves pregnancy outcomes in such patients [18].
34 weeks) or placental insufficiency [16]. Another French multicentric trial, the HYDROSAPL trial, is also assessing the effect of HCQ on pregnancy outcomes in primary APS [17]. Apart from APS, HCQ is also being explored for its potential utility in patients with recurrent pregnancy loss. In this French RCT (the BBQ trial), patients with recurrent pregnancy loss shall be treated with HCQ 400 mg daily or placebo starting pre-conception until the 10th week of pregnancy, with the objective of assessing whether treatment improves pregnancy outcomes in such patients [18].
Other cardioprotective effects of HCQ, apart from reduction of thrombotic events, have also been proposed. In a model of induced myocardial infarction in rats, the administration of HCQ was associated with reduced apoptosis in the infarcted myocardial tissue after 12 weeks [19]. In patients with rheumatoid arthritis (RA) and Sjogren’s syndrome, the use of HCQ has been associated with a reduced risk of developing diabetes mellitus (Table (Table1)1) [20–23]. In animal models of insulin resistance induced by a high fat diet, the addition of HCQ was associated with better survival of the insulin-producing islet of Langerhans, as well as decreased release of inflammatory adipokines and markers of endothelial stress. Thus, a beneficial effect of HCQ on mechanisms driving insulin resistance has been postulated [24]. In a small proof-of-concept study of patients with type 2 diabetes mellitus (n =
= 17) inadequately controlled with a combination of metformin and sulfonylurea, the addition of HCQ 400 mg daily was compared with pioglitazone 45 mg daily. Although pioglitazone was more effective in reducing levels of glycated haemoglobin and achieving glycaemic control, nearly two-thirds of the patients on HCQ could attain less than 7.5% level of glycated haemoglobin [25]. Another small clinical trial of 39 patients with pre-diabetes to receive HCQ (n
17) inadequately controlled with a combination of metformin and sulfonylurea, the addition of HCQ 400 mg daily was compared with pioglitazone 45 mg daily. Although pioglitazone was more effective in reducing levels of glycated haemoglobin and achieving glycaemic control, nearly two-thirds of the patients on HCQ could attain less than 7.5% level of glycated haemoglobin [25]. Another small clinical trial of 39 patients with pre-diabetes to receive HCQ (n =
= 20) or placebo (n
20) or placebo (n =
= 19) demonstrated improvements in glycaemic control over 4 months HCQ therapy [26]. These studies suggest the potential for HCQ as an add-on agent for diabetes mellitus. However, until the utility of HCQ is proven for the indications of diabetes mellitus, dyslipidemia or cardiovascular disease, its indiscriminate use for these indications should be avoided [27]. Considering the evidences for anti-platelet and anti-thrombotic actions of HCQ, as well as favourable lipid profile in patients treated with HCQ, it is possible that this drug might have an adjuvant role in the prevention of incident or recurrent cardiovascular events. This might need exploration in future clinical trials [28].
19) demonstrated improvements in glycaemic control over 4 months HCQ therapy [26]. These studies suggest the potential for HCQ as an add-on agent for diabetes mellitus. However, until the utility of HCQ is proven for the indications of diabetes mellitus, dyslipidemia or cardiovascular disease, its indiscriminate use for these indications should be avoided [27]. Considering the evidences for anti-platelet and anti-thrombotic actions of HCQ, as well as favourable lipid profile in patients treated with HCQ, it is possible that this drug might have an adjuvant role in the prevention of incident or recurrent cardiovascular events. This might need exploration in future clinical trials [28].
Table 1
Hydroxychloroquine and the risk of incident diabetes mellitus in cohort studies of patients with rheumatic diseases
| References | Country/database | Number of individuals | Salient findings |
|---|---|---|---|
| [20]/2017 | USA/National Data Bank for Rheumatic Diseases | 13,669 patients with RA | Over a median of 4.6 years of follow-up, the use of HCQ reduced the risk of developing DM (HR 0.67, 95% CI 0.57–0.8) |
| [21]/2017 | Taiwan/insurance database | 84,989 patients with AS, RA, PS, PsA | Use of HCQ reduced risk of DM (HR 0.7, 95% CI 0.63–0.78). Use of HCQ along with anti-TNF agents also reduced risk of DM (HR 0.49, 95% CI 0.36–0.66) |
| [22]/2019 | USA/Corrona dataset | 21,775 patients with RA | Use of HCQ reduced the risk of diabetes non-significantly compared to other non-biological DMARDs (adjusted HR based on a propensity score model 0.45, 95% CI 0.13–1.53) |
| [23]/2019 | Taiwan/insurance database | 7774 patients with SS | Use of HCQ decreased risk of new-onset DM (HR 0.51, 95% CI 0.28–0.96) |
AS ankylosing spondylitis, CI confidence intervals, DM diabetes mellitus, DMARD disease-modifying antirheumatic drugs, HCQ hydroxychloroquine, HR hazard ratio, RA rheumatoid arthritis, PS psoriasis, PsA psoriatic arthritis, SS Sjogren’s syndrome, TNF tumour necrosis factor alpha, USA United States of America
Methotrexate
Methotrexate is the first-line DMARD for the management of inflammatory arthritides such as rheumatoid arthritis and psoriatic arthritis. The drug’s anti-folate mechanism of action is historically implicated in the treatment of neoplastic (lymphoblastic) diseases. The immunosuppressive action of low-dose methotrexate therapy (up to 25 mg/week) primarily relies on the inhibition of the enzyme 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) transformylase (ATIC), resulting in higher levels of AICAR that inhibits adenosine monophosphate deaminase and adenosine deaminase. The latter results in higher extracellular levels of adenine, further converted to adenosine, which acts via adenosine receptors to exert anti-inflammatory actions. Further, inhibition of the enzyme dihydrofolate reductase (DHFR) also results in uncoupling of nitric oxide (NO) synthetase, by increasing levels of dihydrobiopterin and tetrahydrobiopterin, resulting in greater release of NO as opposed to reactive oxygen species. Methotrexate also exerts an effect to decrease downstream inflammatory signalling operating via nuclear factor kappa B (NFκB). While supplementation with folic acid antagonizes the anti-folate action of methotrexate (responsible for most of its side effects), it does little to inhibit other anti-inflammatory pathways [29].
Recently accumulated evidence suggests a favourable modulation of cardiovascular risk in patients with rheumatic diseases treated with methotrexate [30, 31]. A pilot study evaluated the safety of methotrexate therapy (5 mg/week) in 16 patients with coronary artery disease undergoing percutaneous coronary intervention (PCI), started 2 weeks prior to the procedure and continued for 8 weeks after the procedure. No major safety signals were observed during this period [32]. A RCT, the TETHYS trial, evaluated the role of methotrexate in patients with ST segment elevation myocardial infarction (STEMI). Methotrexate administered at a dose of 0.05 mg/kg, repeated 6 h after PCI in 41 patients, was compared to 43 patients receiving placebo. This trial aimed to analyse area under the curve for serial measurements of creatine kinase and troponin I over the first 72 h after STEMI as a surrogate for area of infarct. No significant differences in the area of infarct measured either using creatine kinase or troponin I could be demonstrated between the two groups. A matter of concern was that the left ventricular ejection fraction (LVEF) was significantly lesser in patients treated with methotrexate compared to those receiving placebo, three months after completion of the treatment [33]. Another large multicentric RCT, the CIRT trial, evaluated the role of methotrexate in the secondary prevention of cardiovascular events. In this trial, patients who had a prior myocardial infarction or had triple vessel coronary artery disease on a background of diabetes mellitus or metabolic syndrome were randomized to receive methotrexate 15–20 mg/week (2391 patients) or placebo (2395 patients). The primary endpoint was a composite of first occurrence of a major adverse cardiovascular event (non-fatal myocardial infarction, stroke, or death due to cardiovascular cause), later modified to include hospitalization for unstable angina resulting in a revascularization procedure. The results of this trial did not favour the use of methotrexate, with a small but insignificant reduction in the risk of developing the primary end point with methotrexate (HR 0.96, 95% CI 0.79–1.16). Apart from a higher incidence of non-basal cell carcinoma in the group receiving methotrexate, no other significant safety signals were observed [34]. These studies do not seem to suggest a role for methotrexate in the prevention of cardiovascular events. An ongoing study (NCT03516903) is evaluating the targeting of left ventricular remodelling following STEMI with a nano-emulsion containing methotrexate. It remains to be seen whether such a strategy is useful to favourably modulate cardiovascular events.
Colchicine
Colchicine has been used for the management of gout, familial Mediterranean fever (FMF) and Behcet’s disease for decades [35]. It acts predominantly on neutrophils, monocytes and macrophages, decreasing their chemotaxis and release of various inflammatory cytokines. It interferes with the activation of the NLRP-3 inflammasome complex, thereby reducing the secretion of downstream cytokines such as IL-1ß and IL-18 [36, 37]. Knocking out the expression of NLRP-3 has been shown to reduce atherosclerosis as well as dampen the severity of myocardial injury following myocardial infarction in animal models [37]. In a murine model, the administration of colchicine improved survival and residual left ventricular function following myocardial infarction [38]. In a model of atherosclerosis induced by high fat diet in rabbits, the administration of colchicine reduced the uptake of 18-fluodeoxyglucose (18-FDG) in atherosclerotic plaques in the abdominal aorta. Moreover, in those rabbits with an increased cholesterol level in the peripheral blood, the burden of atherosclerotic plaques was significantly lesser along with greater circulating high-density lipoprotein (HDL) cholesterol [39]. In platelet-rich plasma from healthy volunteers, in-vitro treatment with colchicine significantly reduced platelet aggregation [40]. Refractoriness to colchicine when used in the longer term in disease states such as FMF has been associated with oxidative stress [41].
Several clinical studies have also assessed the modulation of cardiovascular risk by colchicine. In a parallel-group observational study, 40 patients with coronary artery disease (CAD) were treated with standard care and compared with another 40 patients also treated with colchicine 0.5 mg daily in addition to standard care. Multivariable analysis revealed a significant effect of colchicine on the reduction of the volume of atherosclerotic plaques [42]. In a double-blind placebo-controlled trial of patients with CAD short-term colchicine therapy significantly reduced systemic inflammation and improved endothelial function [43]. In another study, patients with acute coronary syndromes (ACS) planned for PCI were randomized to treatment with add-on colchicine (n =
= 12) or just standard treatment (n
12) or just standard treatment (n =
= 13); those on colchicine had lower levels of chemokines, suggesting a reduction in local inflammatory activity at the site of pathology [44]. In the low dose colchicine for myocardial infarction (LoDoCo-MI) study, 237 patients with acute myocardial infarction were randomized to receive colchicine or placebo in addition to standard care, and C-reactive protein (CRP) levels were compared at 1 month. There was no significant difference in the proportions of patients attaining CRP levels lesser than 2 mg/L amongst patients treated with colchicine or placebo [45]. The ongoing LoDoCo2 trial is evaluating clinical cardiovascular outcomes in 5,552 patients with stable CAD treated with add-on colchicine or placebo [46]. The COLIN trial, evaluated treatment with add-on colchicine (n
13); those on colchicine had lower levels of chemokines, suggesting a reduction in local inflammatory activity at the site of pathology [44]. In the low dose colchicine for myocardial infarction (LoDoCo-MI) study, 237 patients with acute myocardial infarction were randomized to receive colchicine or placebo in addition to standard care, and C-reactive protein (CRP) levels were compared at 1 month. There was no significant difference in the proportions of patients attaining CRP levels lesser than 2 mg/L amongst patients treated with colchicine or placebo [45]. The ongoing LoDoCo2 trial is evaluating clinical cardiovascular outcomes in 5,552 patients with stable CAD treated with add-on colchicine or placebo [46]. The COLIN trial, evaluated treatment with add-on colchicine (n =
= 23) compared to placebo (n
23) compared to placebo (n =
= 21) in patients with STEMI. Neither the primary outcome of difference in peak serum CRP during hospital admission between the two groups, nor secondary outcomes for differences in other biochemical, clinical or imaging outcomes (assessed by echocardiography or magnetic resonance imaging) at 1 month were successfully met [47]. A large multicentric trial evaluated the role of add-on colchicine (n
21) in patients with STEMI. Neither the primary outcome of difference in peak serum CRP during hospital admission between the two groups, nor secondary outcomes for differences in other biochemical, clinical or imaging outcomes (assessed by echocardiography or magnetic resonance imaging) at 1 month were successfully met [47]. A large multicentric trial evaluated the role of add-on colchicine (n =
= 2366) compared to placebo (n
2366) compared to placebo (n =
= 2379) in patients following myocardial infarction. The trial assessed the composite end-point of cardiovascular death, successful resuscitation after cardiac arrest, stroke, recurrent myocardial infarction, or angina requiring an intervention for revascularization. Followed up for a median 22.6 months, treatment with colchicine was associated with a reduced risk of the composite outcome compared to placebo (HR 0.77, 95% CI 0.61–0.96). Gastrointestinal side-effects were similar, however, a greater incidence of pneumonia was observed in patients receiving colchicine (0.9% vs 0.4% on placebo) [48]. A recent systematic review also confirmed an atheroprotective effect of colchicine, with a significant reduction in future cerebrovascular ischemic events with colchicine (odds ratio 0.33, 95% CI 0.15–0.70) as observed in six clinical trials [49].
2379) in patients following myocardial infarction. The trial assessed the composite end-point of cardiovascular death, successful resuscitation after cardiac arrest, stroke, recurrent myocardial infarction, or angina requiring an intervention for revascularization. Followed up for a median 22.6 months, treatment with colchicine was associated with a reduced risk of the composite outcome compared to placebo (HR 0.77, 95% CI 0.61–0.96). Gastrointestinal side-effects were similar, however, a greater incidence of pneumonia was observed in patients receiving colchicine (0.9% vs 0.4% on placebo) [48]. A recent systematic review also confirmed an atheroprotective effect of colchicine, with a significant reduction in future cerebrovascular ischemic events with colchicine (odds ratio 0.33, 95% CI 0.15–0.70) as observed in six clinical trials [49].
Colchicine may also exert anti-fibrotic effects. Clinical trials have demonstrated the potential role of colchicine in reducing the occurrence of atrial fibrillation following catheter ablation and cardiac surgeries [50]. It has been postulated that the modulation of atrial fibrosis by colchicine is mediated by its effects on interleukin-17 and renin-related pathways [51]. In a trial of patients undergoing coronary artery bypass grafting, perioperative treatment with colchicine was associated with reduction in post-surgical constrictive pericarditis [52]. In a rat model of hypertension induced by nephrectomy, treatment with colchicine ameliorated both glomerular and interstitial fibrosis in the remaining kidney [53]. In another animal model of renal injury induced by ureteric obstruction in rats, treatment with colchicine reduced both cortical fibrosis and tubulointerstitial injury [54]. Anecdotal reports also exist of the regression of retroperitoneal fibrosis in patients treated with colchicine [55]. In ten patients with keloids of the ear, treatment with colchicine for a month prior to keloid excision (along with pressure therapy and intralesional corticosteroid therapy in some) was associated with lack of recurrence of the keloids, although long-term follow-up data were unavailable for a majority of patients [56]. A potential role of colchicine in retarding the progression of oral submucous fibrosis has also been proposed [57]. Treatment with topical colchicine was also effective in reducing induced spinal epidural fibrosis in an experimental model in rats [58]. In a clinical trial involving patients with liver cirrhosis of different aetiologies, 37 patients were treated with standard treatment alone and another 37 with add-on colchicine. Followed up over a mean of 4.4 years, those treated with colchicine had a greater proportion of survival. Biochemical tests revealed that colchicine therapy was associated with lower serum levels of pro-collagen III peptide, a marker of fibrosis [59]. Another report described the combination of colchicine with ursodeoxycholic acid (UDCA) in patients with primary biliary cirrhosis who did not respond to UDCA alone. After a follow-up of 20 years, two-thirds of 18 patients continued to survive when treated with this combination treatment regimen [60]. It is possible that the anti-fibrotic effects of colchicine are mediated by its inhibitory actions on cytotoxic T lymphocytes as well as stimulation of endogenous mechanisms to regress fibrosis such as collagenases [61]. The potential anti-fibrotic effects of colchicine merit systematic evaluation by clinical trials in patients with systemic fibrosing diseases such as systemic sclerosis, or major organ fibrosis such as interstitial lung diseases.
Exploration of repurposing of hydroxychloroquine, methotrexate and colchicine for coronavirus disease 19 (COVID-19)
The COVID-19 pandemic has swept the world. Based on preliminary evidence, abnormal activation of the immune system and cytokine storm appear to be associated with disease severity. In this context, commonly used antirheumatic drugs are being explored in COVID-19 [3]. While hydroxychloroquine has been recommended for prophylaxis of high-risk contacts of COVID-19, there is little evidence at present to support this [62]. Occasional reports have even described patients on long term hydroxychloroquine therapy for rheumatic diseases who eventually developed COVID-19 [63]. Overall, the level of enthusiasm regarding the use of hydroxychloroquine in COVID-19 has dampened due to mixed, often negative preliminary results of trials [64]. It has been hypothesized that the timing (early initiation) of hydroxychloroquine might play a role in protection against severe COVID-19, and the results of trials on chemoprophylaxis of COVID-19 with hydroxychloroquine might help answer this question [65]. In this context, it is necessary to mention that hydroxychloroquine (and its analogue, chloroquine) have been previously tried in other viral infections such as Zika virus and Ebola virus infections. While in-vitro efficacy had been observed, this did not translate into clinical efficacy, possibly due to the fact that the drugs were unable to attain a concentration in the endosome necessary for their anti-viral properties to take effect [66, 67]. Anecdotal reports have suggested reduced severity of COVID-19 in patients on colchicine for other indications [68]. Another retrospective review of records of patients from an European centre compared 140 patients treated with standard of care (varying combinations of hydroxychloroquine, lopinavir/ritonavir and corticosteroids) with 122 patients treated with colchicine at 0.5–1 mg/day in addition to standard of care. Treatment with colchicine was associated with 85% reduction in hazard of death (hazard ratio 0.15, 95% confidence intervals 0.06–0.37) [69]. However, in another recent study, six out of seven children with paediatric autoinflammatory syndromes who developed COVID-19 were on colchicine [70]. Ongoing clinical trials identified on a search on clinicaltrials.gov on 19 August 2020 are summarized in Table Table2.2. The results of these trials shall help better understand the role of antirheumatic drugs in COVID-19 in the coming times.
Table 2
Ongoing clinical trials registered on clinicaltrials.gov exploring hydroxychloroquine, methotrexate and colchicine for coronavirus disease 19
| Drug | Number of trials registered as of 19th August, 2020 | Aspects being explored |
|---|---|---|
| Hydroxychloroquine | 229 | Use of this drug alone or in combination of other agents, or compared to other agents (eg. lopinavir/ritonavir, oseltamivir, favipiravir) for treatment of mild or severe COVID-19 |
| Chemoprophylaxis in contacts, health care workers | ||
| Reduction of progression to severe disease/ prevention of hospitalization in milder COVID-19 | ||
| Methotrexate | 3 | Treatment of severe COVID-19 with methotrexate-containing nanoparticles |
| Risk of COVID-19 in patients with rheumatic diseases on drugs like methotrexate | ||
| Colchicine | 19 | Treatment of mild or severe COVID-19 |
| Prevention of progression of mild COVID-19 to severe disease | ||
| Treatment of myocardial injury in the context of COVID-19 |
COVID-19 coronavirus disease 19
It is increasingly being understood that recovery from COVID-19 is prolonged. Nearly one-half of patients with COVID-19 from Italy had persistence of symptoms, including fatigue and joint pains, after 2 months of onset of clinical disease [5]. Neuro-cognitive disorders are also being described in COVID-19 survivors, at least in part driven by the endothelial injury due to the infection [71]. A significant proportion of patients with pneumonitis due to COVID-19 are likely to develop residual pulmonary fibrosis [6, 72]. COVID-19 causes endothelial injury, and this might be responsible for long-term cardiovascular consequences of the disease, including a predisposition towards future cardiovascular events [4, 73]. Therefore, age-old rheumatic drugs should be explored in such select recovered populations. Particularly, it can be hypothesized that the anti-fibrotic effects of colchicine might help in post-COVID-19 fibrosis, including lung fibrosis.
Adverse effects of hydroxychloroquine, methotrexate and colchicine
Table Table33 summarizes side effects of the examined drugs. HCQ entails the risk of toxicities in the long term [74]. Ocular toxicity occurs in the form of retinal damage. Up to 1 in 20 patients might develop retinopathy after five years of using hydroxychloroquine, and this is often silent in the earlier stages [75]. In the later stages, macular vision may be affected, resulting in maculopathy and vision loss. Fundoscopy and perimetry are insensitive for picking up earlier changes of HCQ retinopathy. Recent recommendations suggest limiting the dose of HCQ to 5 mg/kg/day, since higher doses increase the risk of retinopathy after 5 years [76]. Before initiation of HCQ, baseline fundus evaluation with the macula assessment is recommended. If this is abnormal, more sensitive techniques such as spectral domain optical coherence tomography may be employed and repeated annually [76]. Other adverse effects HCQ-related skin pigmentation due to sun exposure, myopathy, and conduction blocks [77]. Cardiac toxicity may be potentiated by other drugs resulting in prolongation of the QT interval such as azithromycin [78]. Rare instances of sensorineural hearing loss have also been noted [79].
Table 3
Adverse effect profile
| Drug | Major adverse effects |
|---|---|
| Hydroxychloroquine | Retinopathya Cutaneous pigmentation Myopathy Cardiomyopathy Cardiac conduction defectsb |
| Methotrexate | Transaminitis Cytopenias (leucopenia, neutropenia, thrombocytopenia)c Acute kidney injury Mucositisc |
| Colchicine | Diarrhoea Myopathy Neuropathy |
aRisk of retinopathy generally occurs after years of use, and is related to cumulative doses. Those with renal failure or on concomitant tamoxifen therapy are at greater risk
bPer se, cardiac toxicity is very rare when hydroxychloroquine is used in the context of rheumatic diseases
cGenerally seen with methotrexate toxicity, such as in overdose (daily rather than weekly administration), or with concomitant underlying renal impairment
In the context of the ongoing COVID-19 pandemic, potential cardiac toxicity of HCQ has received widespread attention. However, speaking from personal experience as well as the published literature in rheumatic diseases [80], cardiac toxicity with HCQ (including QT prolongation) is very rare. Pharmacological interaction with azithromycin can potentially prolong QT interval when these two drugs are taken together, as has been tried in the management of COVID-19. Hence, due caution and pre-treatment with follow-up ECGs to seek any developing cardiac arrhythmias might be suggested if and when HCQ is combined with azithromycin or any other drug that can cause QT prolongation [81].
The adverse effect profile of methotrexate is mainly due to its antifolate actions [29]. Methotrexate toxicity can result in mucositis with oral ulcers, crusting of lips and diarrhoea, skin rashes, pancytopenia, transaminitis, and acute kidney injury [82]. The risk of toxicities is greater in the presence of renal failure. In a series of 120 patients with rheumatoid arthritis with concomitant renal impairment treated with methotrexate, nearly 30% developed features of methotrexate toxicity such as leucopenia, transaminitis and renal failure. Interestingly, a protective effect towards methotrexate toxicity in the presence of renal failure was observed in those individuals also taking hydroxychloroquine [83]. Acute methotrexate toxicity requires discontinuation of the drug and administration of folinic acid. Varying dose regimens of folinic acid are used, although often 15 mg 6 hourly for 10 doses is administered. Supportive treatment in the form of granulocyte colony stimulating factor, blood component transfusions, treatment of coexistent infections and nutritional care are also essential. Untreated methotrexate toxicity is associated with significant risk of mortality [82, 84]. Rarely, methotrexate may result in acute interstitial pneumonitis [85].
Colchicine therapy can result in diarrhoea and other gastrointestinal adverse effects. Long-term colchicine therapy is rarely associated with myopathy, polyneuropathy, transaminitis, and rhabdomyolysis [86]. The risk of toxicity is increased in the presence of renal failure. Also, co-administration with drugs inhibiting the enzyme cytochrome P450 oxidase 3A4 (CYP 3A4, such as clarithromycin) or the drug efflux protein p-glycoprotein portends a greater risk of adverse effects [87]. Occasional reports exist regarding the use of intravenous colchicine for prolonged periods for difficult to treat conditions such as familial Mediterranean fever. Despite intravenous use for longer term, little toxicity was observed in most patients, other than gastrointestinal side effects [88, 89]. Drug withdrawal is recommended if such toxicities develop [86, 90].
Rarely, these drugs can have cardiovascular side effects also, as holds true for most anti-rheumatic drugs: methotrexate has been associated with pericardial and myocardial injury, colchicine rarely causes myocardial injury, and HCQ therapy occasionally results in conduction system abnormalities [91]. Clinicians should be aware for the risk of cardiac adverse events when these drugs are considered for repurposing and combined therapies in different patient cohorts.
Future perspectives
Keeping in mind the diverse impacts on different body systems seen with these drugs, it is imperative to generate high-quality evidence for the use of hydroxychloroquine, methotrexate and colchicine for other indications. They are already being tried in patients with cardiovascular disease for secondary prevention. Clinical trials might also attempt to decipher the potential utility of hydroxychloroquine, methotrexate or colchicine for primary prevention of cardiovascular events in a high risk population, such as those with metabolic syndrome and in individuals with inflammatory arthritides which predispose to greater cardiovascular risk. Furthermore, the potential anti-fibrotic effects of colchicine need to be systematically studied in local fibrosing diseases (such as idiopathic interstitial lung diseases) or systemic fibrosing diseases (such as systemic sclerosis) by suitably powered clinical trials. Although recent promising developments in vaccines for COVID-19 hold promise for community level prevention, these are still preliminary and it is likely that millions of individuals shall be infected before such vaccines come into regular clinical use [92]. Therefore, the role of drugs with anti-fibrotic potential such as colchicine in preventing or managing long-term sequelae of COVID-19 should also be explored.
Conclusion
Repurposing of old antirheumatic drugs is being attempted for numerous indications today. HCQ, methotrexate and colchicine are primarily used for favourable modulation of cardiovascular risk. Clinicians must be aware of the propensity of these drugs to cause side effects, such as cytopenias with methotrexate, retinopathy with HCQ, and gastrointestinal adverse effects with colchicine. In view of the risk of adverse events, it is essential to carefully consider the risk–benefit ratio before prescribing these drugs for newer, unlicensed indications, taking particular care to identify high-risk populations such as those with underlying renal impairment and avoid drug interactions. The role of these drugs in COVID-19 shall be clarified in ongoing trials. It may also require evaluation whether these drugs have a role in managing sequelae of COVID-19.
Abbreviations
| 18-FDG | 18-Fluorodeoxyglucose |
| ACS | Acute coronary syndromes |
| AICAR | Enzyme 5-aminoimidazole-4-carboxamide ribonucleoside |
| aPL | Antiphospholipid antibodies |
| ATIC | AICAR transformylase |
| APS | Antiphospholipid antibody syndrome |
| CAD | Coronary artery disease |
| CI | Confidence interval |
| COVID-19 | Coronavirus disease 19 |
| CRP | C-reactive protein |
| DHFR | Dihydrofolate reductase |
| DMARD | Disease-modifying anti-rheumatic drug |
| DNA | Deoxyribonucleic acid |
| EULAR | European League against Rheumatism |
| FMF | Familial Mediterranean fever |
| HCQ | Hydroxychloroquine |
| HDL | High-density lipoprotein |
| HR | Hazard ratio |
| LVEF | Left ventricular ejection fraction |
| MHC | Major histocompatibility complex |
| NFκB | Nuclear factor kappa B |
| NO | Nitric oxide |
| PCI | Percutaneous coronary intervention |
| RA | Rheumatoid arthritis |
| RCT | Randomized controlled trial |
| RNA | Ribonucleic acid |
| SLE | Systemic lupus erythematosus |
| STEMI | ST segment elevation myocardial infarction |
| UDCA | Ursodeoxycholic acid |
Author contributions
(1) Conception, design, collection of data, analysis and interpretation of data—DPM, AYG, OZ. (2) Writing the original draft—DPM; Critical revision—AYG, OZ. (3) Final approval of the version to be published—DPM, AYG, OZ. (4) Agreement to be accountable for all aspects of the manuscript in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved—DPM, AYG, OZ.
Funding
None.
Compliance with ethical standards
Durga Prasanna Misra declares that he has no conflict of interest, including no relationship with pharmaceutical companies. Armen Yuri Gasparyan declares that he has no conflict of interest, including no relationship with pharmaceutical companies. Olena Zimba declares that she has no conflict of interest, including no relationship with pharmaceutical companies.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Durga Prasanna Misra, Email: moc.liamg@arsimpagrud.
Armen Yuri Gasparyan, Email: [email protected].
Olena Zimba, Email: moc.liamg@aneloabmiz.
References
Full text links
Read article at publisher's site: https://doi.org/10.1007/s00296-020-04694-2
Read article for free, from open access legal sources, via Unpaywall:
https://link.springer.com/content/pdf/10.1007/s00296-020-04694-2.pdf
Citations & impact
Impact metrics
Article citations
DaiTongXiao improves gout nephropathy by inhibiting inflammatory response through the TLR4/MyD88/NF-κB pathway.
Front Pharmacol, 15:1447241, 07 Aug 2024
Cited by: 0 articles | PMID: 39170709 | PMCID: PMC11336418
Hydroxychloroquine and the associated risk of arrhythmias.
Glob Cardiol Sci Pract, 2024(2):e202417, 03 Mar 2024
Cited by: 0 articles | PMID: 38746066 | PMCID: PMC11090172
Review Free full text in Europe PMC
Real-world pharmacological treatment of pregnant patients with rheumatic diseases from China: a retrospective analysis from 2016 to 2021.
Front Pharmacol, 15:1353293, 17 Apr 2024
Cited by: 0 articles | PMID: 38694907 | PMCID: PMC11061436
Alkalinization Using Sodium Bicarbonate for COVID-19 Treatment: A Systematic Review and Meta-Analysis.
J Evid Based Integr Med, 29:2515690X241258403, 01 Jan 2024
Cited by: 0 articles | PMID: 38826036 | PMCID: PMC11145993
Review Free full text in Europe PMC
The Efficacy of Colchicine as an Adjunct Therapy in Non-hospitalized COVID-19 Patients: A Randomized Placebo-Controlled Trial.
Recent Adv Antiinfect Drug Discov, 19(3):254-263, 01 Jan 2024
Cited by: 0 articles | PMID: 37711106
Go to all (33) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Acute generalized exanthematous pustulosis induced by hydroxychloroquine prescribed for COVID-19.
J Allergy Clin Immunol Pract, 8(8):2777-2779.e1, 07 Jun 2020
Cited by: 16 articles | PMID: 32525093 | PMCID: PMC7276124
COVID-19: an unexpected indication for anti-rheumatic therapies?
Rheumatology (Oxford), 59(6):1200-1203, 01 Jun 2020
Cited by: 9 articles | PMID: 32374874 | PMCID: PMC7239095
Challenges and cares to promote rational use of chloroquine and hydroxychloroquine in the management of coronavirus disease 2019 (COVID-19) pandemic: a timely review.
J Toxicol Environ Health B Crit Rev, 23(4):177-181, 12 Apr 2020
Cited by: 24 articles | PMID: 32281481 | PMCID: PMC7157945
Review Free full text in Europe PMC
How to manage rheumatic patients during the coronavirus pandemic.
Panminerva Med, 62(3):176-177, 28 Apr 2020
Cited by: 1 article | PMID: 32343511

 1
1 



