Abstract
Free full text

Macrophage responses associated with COVID-19: A pharmacological perspective
Abstract
COVID-19 has caused worldwide death and economic destruction. The pandemic is the result of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which has demonstrated high rates of infectivity leading to great morbidity and mortality in vulnerable populations. At present, scientists are exploring various approaches to curb this pandemic and alleviate its health consequences, while racing to develop a vaccine. A particularly insidious aspect of COVID-19 is the delayed overactivation of the body's immune system that is manifested as the cytokine storm. This unbridled production of pro-inflammatory cytokines and chemokines can directly or indirectly cause massive organ damage and failure. Systemic vascular endothelial inflammation and thrombocytopenia are potential consequences as well. In the case of COVID-19, the cytokine storm often fits the pattern of the macrophage activation syndrome with lymphocytopenia. The basis for the imbalance between the innate and adaptive immune systems is not clearly defined, but highlights the effect of SARS-CoV-2 on macrophages. Here we discuss the potential underlying basis for the impact of SARS-CoV-2 on macrophages, both direct and indirect, and potential therapeutic targets. These include granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin 6 (IL-6), interferons, and CXCL10 (IP-10). Various biopharmaceuticals are being repurposed to target the cytokine storm in COVID-19 patients. In addition, we discuss the rationale for activating the macrophage alpha 7 nicotinic receptors as a therapeutic target. A better understanding of the molecular consequences of SARS-CoV-2 infection of macrophages could lead to novel and more effective treatments for COVID-19.
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in Wuhan, China in December of 2019 and quickly wreaked havoc around the world in the form of the pandemic COVID-19, causing death, undermining economies, overwhelming medical professionals, and challenging the scientific community (Liu et al., 2020a). This positive-sense single-stranded RNA virus has proven to be highly contagious, being spread by symptomatic and likely asymptomatic individuals (Furukawa et al., 2020; Huff and Singh, 2020; Oran and Topol, 2020). As the name suggests, the primary target of SARS-CoV-2 is the lungs, but other organs such as blood vessels, heart, and brain are susceptible as well. All age groups are vulnerable to infection, but generally exhibit different degrees or classes of symptoms, with those over 60, male, and with underlying medical conditions more likely to exhibit severe symptoms and succumb to viral toxicity (Conti and Younes, 2020; Team, 2020). Some 81% exhibit mild, moderate, or no symptoms; 14% show severe symptoms; and 5% experience critical disease with high mortality (Wu and McGoogan, 2020). An especially alarming complication of COVID-19 is the cytokine storm that develops after a week or two of delay in severely infected individuals.
SARS-CoV-2 has 4 structural proteins, namely the E (envelope), S (spike), M (membrane), and N (nucleocapsid) proteins (Guo et al., 2020). The N protein holds the RNA genome, while the S, E, and M proteins form the viral envelope. The virus primarily gains entry into a human cell by binding to the exopeptidase angiotensin converting enzyme 2 (ACE2). This protein is located on the membrane surface of several cell types including alveolar type II and endothelial cells. Proteins other than ACE2 may function as receptors for entry as well (Guo et al., 2020). Cell entry is facilitated by cleavage of the spike protein by the serine protease TMPRSS2 or a furin-like proprotein convertase, thereby exposing the fusion peptide. Besides inducing cell death, viral infection can initiate an inflammatory response, which with SARS-CoV-2 is thought to manifest among other things as widespread vascular endothelial dysfunction (Teuwen et al., 2020). Beyond this, however, increasing evidence supports the conclusion that SARS-CoV-2 may exert some of its lethal effects by insidiously compromising the body's immune response. Here we summarize evidence for macrophages as targets of SARS-CoV-2 and the implication that has for immunomodulatory treatments of COVID-19 (Fig. 1 ).
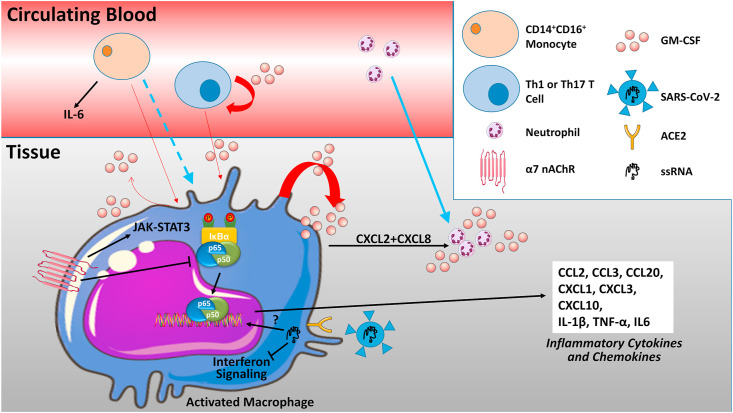
– Macrophages at the center of the cytokine storm. With inflammation, macrophages, T cells, endothelial cells and a number of other immune and mesenchymal cells, produce the monomeric glycoprotein granulocyte-macrophage colony-stimulating factor (GM-CSF) (red arrows). Besides stimulating the production of granulocytes and monocytes, GM-CSF can serve as a chemoattractant for the migration of monocytes and neutrophils into the tissue (blue arrows), and can alter neutrophil receptors. GM-CSF signaling promotes a pro-inflammatory M1 macrophage phenotype and the production of a number of inflammatory cytokines and chemokines by monocyte-derived or tissue macrophages (black arrows). Macrophages themselves are direct targets of the SARS-CoV-2 via expression of the receptor for viral binding ACE2, as well as TMPRSS2 or a furin-like proprotein convertase. The effect of SARS-CoV-2 on macrophage phenotype is not defined, although inhibition of protective interferon signaling is reported. Lung macrophages also express the G protein-coupled alpha 7 nicotinic receptors (nAChRs α7) that signal through JAK-STAT3 and oppose inflammatory signaling by blocking the translocation of p65/p50 NF-κB into the nucleus upon IκBα (inhibitor of NF-κB) degradation. See text for additional details. Some of the content is adapted from Servier Medical Art (https://smart.servier.com/).
2. Cytokine storm
Progression of COVID-19 in more severe cases is marked by the delayed occurrence of a cytokine storm or cytokine release syndrome, due to overactivation of the immune system. Although not definitively established, this phenomenon is thought to contribute to the acute respiratory distress syndrome (ARDS) and widespread organ damage that foretells death. Nor is it clear what relationship there is between the cytokine storm and thrombocytopenia, which is common in patients with COVID-19 and may ultimately contribute to adverse outcome, although both enhanced platelet activation/consumption and destruction are likely outcomes of the cytokine storm. Multi-organ (micro-) thrombosis seems to characterize severe COVID-19 cases (McFadyen et al., 2020; Prieto-Pérez et al., 2020), and likely reflects in part the production of pro-inflammatory cytokines, such as IL-1β and TNF-α, by macrophages (Conti et al., 2020a).
Notably, excessive activation or proliferation of macrophages is a contributing factor to hemophagocytic histiocytosis (HH) also known as secondary hemophagocytic lymphosistiocytosis (Xu et al., 2020). HH has been identified as a deregulation of the immune system, characterized by hemophagocytosis by macrophages, overactivation of cytotoxic T cells, and pro-inflammatory cytokine massive release (Ramos-Casals et al., 2014). HH is the histological counterpart of the macrophage activation syndrome. A clinical study performed on post-mortem bone marrow samples taken from patients who died from COVID- 19 showed findings highly consistent with the diagnosis of HH (Prieto-Pérez et al., 2020). Elevated blood ferritin has also been shown to be associated with poor outcome in a retrospective study of 150 COVID-19 patients (Mehta et al., 2020a).
From multiple observations, both CD4+ and especially CD8+ (or cytotoxic) T-cells appear to be over-activated early-on in COVID-19 resulting in the excessive production of granulocyte-macrophage colony-stimulating factor (GM-CSF), which in turn stimulates monocytes/macrophages to produce interleukin-6 (IL-6) and other inflammatory factors. With time, there is a significant decrease in peripheral CD4+ and CD8+ T lymphocytes, as well as natural killer (NK cells) in COVID-19 patients, perhaps secondarily to their sustained activation by macrophage-derived interferon gamma-induced protein 10 (IP-10), also known as CXCL10. With disease progression, neutrophilia may occur, especially in those with severe critical pulmonary conditions (Liu et al., 2020b).
3. Macrophage (monocytes)
3.1. Inflammatory signature
Human monocytes and macrophages express ACE2, as well as TMPRSS2 and furin, and would seem to be a widespread target for SARS-CoV-2 infection (Abassi et al., 2020; Wang et al., 2020b). Evidence was reported in COVID-19 patients for the infection of macrophages of the spleen and lymph nodes with SARS-CoV-2, which was associated with severe lymphocyte apoptosis (Wang et al., 2020b). Moreover, infected macrophages were shown to produce IL-6, a pro-inflammatory cytokine that directly promotes lymphocyte necrosis and would explain in part the common characteristic of lymphocytopenia in COVID-19 patients. Based on their morphology and ability to produce IL-6, TNF-α, and IL-10, as well as surface expression of CD11b, CD14, CD16, CD68, CD80, CD163, and CD206, circulating monocytes have an activated or pro-inflammatory phenotype. The expression of CD163 and CD206 suggests a bias towards the intermediate or regulatory phenotype, with CD163 expression being a feature of activated monocytes/macrophages in hemophagocytic lymphosistiocytosis syndrome (Wang et al., 2020b). An increase in the pool size of the intermediate subtype of monocytes may be characteristic of severe COVID-19 (Merad and Martin, 2020). The activated plasma blood monocyte phenotype and lymphocytopenia would seem to persist into the recovery stage as well (Wen et al., 2020).
Multiple studies have demonstrated that the lungs are a target of macrophages in COVID-19 (Chua et al., 2020; Wang et al., 2020b). Inflammatory macrophages are increased with increased levels of nonresident macrophages, which in the upper respiratory tract have a highly inflammatory phenotype with the expression of a number of chemokines and pro-inflammatory cytokines IL-1B, IL-8, IL-18, and TNF-α (Chua et al., 2020; Liao et al., 2020). Macrophages in the lower airways were found to have an even stronger inflammatory signature and overall there was a strong correlation between activation status of non-resident macrophages and COVID-19 disease severity (Chua et al., 2020). Other immune cells, such as mast cells, likely act synergistically with macrophages to cause lung damage (Kritas et al., 2020).
3.2. Interferon suppression
Although CXCL10, as well as CCL2, are interferon (IFN)-induced genes, there is evidence for impaired or delayed Type 1 IFN signaling in SARS-CoV-2-infected cells. One ex vivo experiment with lung tissue showed that SARS-CoV-2 induced less IFNs and pro-inflammatory mediators than SARS-CoV (Chu et al., 2020). Single-cell RNA sequencing analysis of bronchoalveolar lavage samples from severe and mild COVID-19 patients revealed that SARS-CoV-2 mainly infects the epithelial and recruited inflammatory macrophage subsets (Bost et al., 2020). In the latter, a disease severity-associated downregulation of type I IFN genes was noted. Notably, IFN is known to exhibit multiple biological functions such as antiviral, antiproliferative, and immunomodulatory effects (Nile et al., 2020; Wang et al., 2019). How SARS-CoV-2 thwarts intrinsic innate immune responses in monocyte-macrophages is not defined, although in monocyte-derived dendritic cells (but not macrophages) viral antagonism of STAT1 phosphorylation was reported (Yang et al., 2020). In contrast, work in Vero cells, indicates that SARS-CoV-2-infected cells are still responsive to type I IFN treatment unlike SARS-CoV-infected cells (Lokugamage et al., 2020). Of note, ACE2 was shown to be an interferon-stimulated gene in human lung cells, which is also upregulated by smoking and viral infections (Smith et al., 2020). A discussion of possible means by which SARS-CoV-2 attenuates the interferon response can be found elsewhere (Paces et al., 2020). Recently, it was reported that the SARS-CoV-2 viral ORF6, ORF8 and N proteins were potential inhibitors of the type I interferon signaling pathway (Li et al., 2020).
In light of these observations and urgent need to identify new therapies to control COVID-19 severity, IFN approved drugs have emerged as a potential treatment for COVID-19 patients. For instance, it has been demonstrated that the administration of recombinant IFNs to SARS-CoV and SARS-CoV-2 patients decreased viral protein synthesis and replication (Falzarano et al., 2013; Li et al., 2019; Zumla et al., 2016). In agreement, a recent published study on MERS-CoV patients reported that a combination of remdisevir and IFN beta showed a superior antiviral effect when compared with lopinavir/ritonavir combination (Sheahan et al., 2020). Therefore, testing the efficacy and safety of recombinant IFNs may be a worthwhile promising approach in the setting of COVID-19. Triple antiviral therapy with lopinavir-ritonavir, ribavirin and interferon beta-1b was reported to be safe and superior to lopinavir-alone in improving symptoms and reducing viral shedding and hospitalization in those with mild to moderate COVID-19 (Hung et al., 2020). On the other hand, there is evidence that IFN might be playing an important role in COVID-19 hyper-inflammation, suggesting that timing is a consideration (Conti et al., 2020c; Lee et al., 2020). Analysis of monocytes by single-cell RNA-seq from patients with severe COVID-19 exhibited signs of a type I IFN response along with TNF/IL-1β-driven inflammation.
3.3. Possible contribution of nicotine and nicotinic acetylcholine receptors
Although multiple investigations report a detrimental impact of nicotine on COVID-19 patients through up-regulating ACE2 receptors in the lungs (Farsalinos et al., 2020; Leung et al., 2020; Russo et al., 2020), recently published epidemiological studies reveal that smokers are either asymptomatic or show less severe respiratory symptoms compared with non-smokers (Covid et al., 2020; Farsalinos et al., 2020b; Kloc et al., 2020; Miyara et al., 2020; Petrilli et al., 2020). A disruption of the cholinergic anti-inflammatory pathway in COVID-19 patients has been noted (Farsalinos et al., 2020a, 2020c). It has been reported that over-responsiveness of the immune system, otherwise known as the cytokine storm, highly correlates with enhanced severity of COVID-19 infection, substantially increasing the mortality rate (Wang et al., 2020a; Ye et al., 2020). In the human lungs, the inflammatory response is mainly mediated by lung macrophages with two main types: the alveolar and interstitial macrophages (Kloc et al., 2020). Under physiological conditions, the alveolar macrophages exhibit anti-inflammatory characteristics by dampening the adaptive immune response and suppressing pro-inflammatory cytokines release (Kloc et al., 2020). Following a viral infection such as COVID-19, the alveolar macrophages switch from the anti- to pro-inflammatory phenotype, initiating consequently an inflammatory response, then switch back during the resolution phase to the anti-inflammatory phenotype, promoting thereafter tissue repair in the site of injury (Hu and Christman, 2019; Hussell and Bell, 2014). In the context of COVID-19 infection, an accumulation of macrophages in the lungs of COVID-19 patients has been observed (Wang et al., 2020a). Besides resident macrophages, monocyte-derived and non-resident macrophages have been described in COVID-19 patients (Chua et al., 2020); however, a better understanding of their interrelationship is needed.
Of note, lung macrophages have been shown to express ACE2 receptors, facilitating therefore the entry of SARS-CoV-2 to host cells (Tsaytler et al., 2011; Verdecchia et al., 2020). Besides ACE2 receptors, lung macrophages express alpha 7 nicotinic receptors (nAChRs α7) (Abrial et al., 2012). nAChRs α7 are potentially implicated in attenuating the cytokine storm through decreasing pro-inflammatory cytokine release (Kalamida et al., 2007; Tracey, 2002). For instance, it has been indicated that activation of nAChRs α7 located on lung macrophages by acetylcholine and/or nicotine mitigates the hyper-inflammatory response mediated disease severity (Lu et al., 2014; Tindle et al., 2020). Strong evidence reveals that the cholinergic anti-inflammatory pathway mediated by nAChRs α7 inhibits the translocation of the pro-inflammatory marker NF-κB to the nucleus and activates the JAK2-STAT3 pathway, consequently suppressing the inflammatory response and decreasing the cytokine storm in the lungs (Báez-Pagán et al., 2015; Changeux et al., 2020; Lu et al., 2014). Given the observed lower number of hospitalized COVID-19 patients among smokers, the potential role of medicinal nicotine to alleviate COVID-19 progression and development should be rapidly studied and clearly distinguished from conventional smoking that has no therapeutic effects.
3.4. Chemokine profile: a possible role for CXCL10 (IP-10)
Longitudinal profiling of 71 COVID-19 patients identified early expression of inhibitory mediators IL-10 and IL-1RA, along with the chemokine CCL5 (aka RANTES), in those with mild but not severe disease (Zhao et al., 2020). CCL5 is chemotactic for T cells, as well as eosinophils and basophil. On the other hand, the majority of cytokines associated with the cytokine storm in viral infections, including IL-6 and IFN-γ, were only increased at a late stage in severe illness, with TNF and GM-CSF not showing a difference between mild and severe cases.
Multiple studies have documented the upregulation of not only inflammatory cytokines but also chemokines in COVID-19 patients. Chemokines are low molecular weight proteins that act largely as chemoattractants for immune cell recruitment during inflammation, as well as modulators of immune cell homeostasis and angiogenesis (Coperchini et al., 2020). Compared to non-ICU patients, COVID-19 patients admitted to the ICU, exhibited higher plasma levels of IL2, IL7, IL10, GSCF, CXCL10 (IP-10), CCL2 (MCP1), CCL3 (MIP1A), and TNFα, indicating activation of T-helper 1 (Th1) cell function (Huang et al., 2020), although increased circulating levels of Th2-immune related cytokines IL-4 and IL-10 implicated in inflammation suppression are noted as well (Han et al., 2020). Transcriptomic analysis of bronchoalveolar lavage fluid of COVID-19 patients revealed an upregulation of CXCL1, CXCL2, CXCL6, CXCL8 (IL8), CXCL10 (IP-10), CCL2 (MCP-1), CCL3 (MIP-1A), and CCL4 (MIP1B) (Xiong et al., 2020). CXCL10 (IP-10) is a chemoattractant for monocytes/macrophages, dendritic cells, NK cells, and T cells; CCL2 (MCP-1) is a chemoattractant for monocytes, dendritic cells, and memory T cells. CXCL2 and CXCL8, which are secreted by monocytes/macrophage, serve as potent chemoattractants for neutrophils. Single cell RNA sequencing of nasopharyngeal and bronchial samples from COVID-19 patients identified increased inflammatory macrophages that express CCL2, CCL3 (MIP-1A), CCL20, CXCL1, CXCL3, CXCL10 (IP-10), CXCL8 (IL8), IL1B and TNF-α (Chua et al., 2020). Levels correlated with disease severity. CXCL10 (IP-10) levels were previously associated with the severe acute respiratory syndrome (SARS) disease progression and resolution due to the SARS-CoV virus (Altara et al., 2016; Jiang et al., 2005), and development of ARDS in preclinical models (Coperchini et al., 2020). The elevated nasopharyngeal levels of CXCL10 with COVID-19 may permit this chemokine to be used in widespread immunoassay testing for early detection of SARS-CoV-2-infection (Cheemarla et al., 2020).
3.5. Possible contribution of GM-CSF
Mounting evidence suggests that immunomodulatory agents, including GM-CSF, could be a promising therapy for COVID-19 (Lang et al., 2020; Mehta et al., 2020a). GM-CSF is known to be implicated in the production of granulocytes, monocytes, macrophages, and dendritic cells from progenitor cells, a process known as myelopoiesis (Egea et al., 2010; Fleetwood et al., 2007). It has been demonstrated that GM-CSF is secreted by different cell types including alveolar type II epithelial cells, playing therefore a key role in the integrity of alveolar barriers and maturation of alveolar macrophages (Cakarova et al., 2009; Rösler and Herold, 2016). Multiple investigations have considered GM-CSF as a pivotal cytokine that activates both the innate and adaptive immune response. For instance, GM-CSF can polarize myeloid cells into a pro-inflammatory phenotype, releasing subsequently reactive oxygen species and pro-inflammatory cytokines such as IL-1β, IL-6, TNF-α, and chemokines including CCL17, CCL2, and IL8, which can attract lymphocytes, monocytes, and neutrophils to the site of inflammation (Hamilton, 2020). It has also been reported that GM-CSF can prime dendritic cells to activate T cells, boosting thereafter the immune response by enhancing the recruitment of myeloid cells to the site of injury (Cao et al., 2015; Komuczki et al., 2019; Zhang et al., 2013). Since the goal of enhancing lung tissues integrity and dampening hyper-active immune response may lead to a drastic decrease in morbidity and mortality rate in COVID-19 patients, administration of GM-CSF as a promising therapy is being clinically investigated (Lang et al., 2020). Pre-clinical investigations revealed that overexpression of GM-CSF decreased apoptosis in alveolar wall cells, consequently preventing hyperoxia-induced lung damage (Baleeiro et al., 2006; Paine et al., 2003). A clinical study performed by Matute-Bello et al. reported that in ARDS patients, increased GM-CSF in bronchoalveolar lavage fluid was associated with decreased mortality rate through potentially improved alveolar macrophage survival (Matute-Bello et al., 2000). This observation was further strengthened with a clinical study completed by Herold et al. showing that administration of inhaled GM-CSF to patients with pneumonia-associated ARDS enhanced oxygenation and lung compliance (Herold et al., 2014). Currently, a clinical study is assessing the potential beneficial effect of using inhaled and intravenous GM-CSF agonist in respiratory failure COVID-19 patients (NCT04400929).
The potential benefits of administrating GM-CSF agonist in the context of COVID-19 patients, however, should be carefully studied, particularly in the late stage of COVID-19 where lung injury is thought to be driven by the cytokine storm rather than viral overload (Siddiqi and Mehra, 2020). Paradoxically, considerable interest in administrating anti-GM-CSF is gaining interest in the setting of COVID-19, given that a marked increase in GM-CSF expressing natural killer, B cells, and CD+ 4 and CD+ 8 T cells was observed in COVID-19 ICU patients when compared to mild cases (Zhou et al., 2020). However, given the role of GM-CSF in boosting the immune response to remove pathogen and enhancing lung repair, it is important to consider that the observed increase could be a result of exacerbated COVID-19 severity and related comorbidities. The rational is that during COVID-19 infection, over-activation of myeloid cells could be a critical mediator of enhanced cytokine storm, consequently aggravating tissue damage. Therefore, anti-GM-CSF therapy may decrease the detrimental immune response, and thus exert beneficial effects (Barnes et al., 2020; Mehta et al., 2020a; Merad and Martin, 2020), a hypothesis that was supported by a preclinical study of SARS-CoV infection animal model, showing that GM-CSF mediated the infiltration of inflammatory monocytes/macrophages into the lungs (Channappanavar et al., 2016). Taking together, these findings suggest that GM-CSF is a key player in regulating myeloid cell induced hyper-inflammation in many tissues including the lungs. Anti-GM-CSF approach in patients with COVID-19, however, should be well monitored, given the critical contribution of GM-CSF in alveolar macrophage function and pathogen clearance.
As of the start of May 2020, there were some 49 clinical trials underway targeting the cytokine storm in COVID-19 patients (Wang et al., 2020b). The vast majority involve biologicals. Besides those involving GM-CSF, prominent among them are a number of studies involving anti-IL-6 strategies. In addition, antagonistic antibodies directed against TNF, IL-1, IL-1R, and IL-8 are being investigated for attenuating excessive immune activation and the cytokine storm (Conti et al., 2020b). The rationale behind those targeting the actions of GM-CSF latter in COVID-19 is that this cytokine constitutes an autocrine/paracrine positive feedback loop that helps drive the cytokine storm (Mehta et al., 2020b). In a preliminary study, dexamethasone showed promise in reducing mortality of hospitalized COVID-19 patients if they were receiving respiratory support (mechanical ventilation or oxygen) (Group et al., 2020), but targeting the cytokine storm via broad-spectrum immunosuppression does raise a number of concerns (Theoharides and Conti, 2020).
3.6. Possible contribution of the renin angiotensin system
SRS-CoV-2 can gain entry into monocytes/macrophages via ACE2, although the virus is not thought to replicate in these cells. In this way, macrophages may act as a sort of “Trojan horse”, allowing for the delivery of the virus to lung and other tissue parenchyma (Abassi et al., 2020). ACE2 is a protease that forms part of the beneficial counterpoint to the renin-angiotensin system (Forrester et al., 2018). By removing the carboxy-terminus amino acid, it converts the vasoconstrictive and pro-inflammatory octapeptide angiotensin II (Ang II) to Ang (1–7), which has beneficial effects including vasodilation and anti-inflammation actions via the Mas receptor.
An additional consequence of virus-mediated ACE2 loss might be increased Ang II inflammatory effects via the Ang II type 1 (AT1) receptor or diminished protective signaling via the Mas receptor (Abassi et al., 2020). Although multiple studies reported increased ACE2 expression in COVID-19 patients who are on angiotensin converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) (Ferrario et al., 2005; Igase et al., 2008), recent emerging investigations suggested that ACEIs and ARBs could exert protective effects through up-regulating ACE2, modulating negatively therefore the severity of COVID-19 (Kuba et al., 2005) and reversing the marked increase in Ang II levels, decreasing consequently its deleterious effects on the cardiopulmonary system (Danser et al., 2020; Sommerstein et al., 2020; Zheng et al., 2020). A study done by Kuba et al. showed that the administration of exogenous ACE2 to ARDS animal model substantially decreased inflammation and enhanced oxygenation (Kuba et al., 2005). Similarly, epidemiological studies revealed that ACEIs and ARBs decreased the risk of pneumonia in general population (Liu et al., 2013; Shinohara and Origasa, 2012). Therefore, investigation aimed at testing the potential beneficial or detrimental effects of ACEIs and ARBs in the context of COVID-19 is being undertaken (Buckley et al., 2020).
4. Conclusions
Substantial evidence indicates that pro-inflammatory macrophages play a critical role in the pathological consequences of COVID-19. Additional evidence is needed concerning the phenotype of these cells. Nor is it clear what the relationship is between SARS-CoV-2 infection and monocyte/macrophage activation status, namely whether these immune cells are simply responding to the viral infection or are hijacked by the virus to act in an uncontrolled rogue manner. Emerging evidence indicates that targeting the cytokines and chemokines associated with their activation or restoring their innate immunity control may provide the means to successfully combat COVID-19.
Author agreement
Our work is original, written based on the most recent studies and discoveries, and is being submitted only to European Journal of Pharmacology, has not been published already, and is not under consideration for publication or in press elsewhere. All authors agree to the submission of the manuscript.
Dedication
This manuscript is dedicated to G. Warren and Jessie Booz, two gentle, loving, caring, and gifted individuals, and all of those wonderful and remarkable individuals who were taken from us way too soon by the COVID-19 pandemic.
CRediT authorship contribution statement
George W. Booz: Writing - review & editing, All authors contributed to the writing of this manuscript. Raffaele Altara: modified the figure and provided assistance in producing a high quality TIFF, All authors contributed to the writing of this manuscript. Fouad A. Zouein: Writing - review & editing, coordinated the author contributions, All authors contributed to the writing of this manuscript.
Acknowledgements
This work was supported by a grant to FAZ from the American University of Beirut Faculty of Medicine (MPP – 320145/320095) and by Centre National de la Recherche Scientifique (CNRS) #103507/103487/103941; Seed grant #100410; and Collaborative Research Stimulus (CRS) #103556. RA acknowledges the support of the Institute of Experimental Medical Research (IEMR, OUS). GWB acknowledges the support of the Department of Pharmacology and Toxicology (UMMC).
References
- Abassi Z., Knaney Y., Karram T., Heyman S.N. The lung macrophage in SARS-CoV-2 infection: a friend or a foe? Front. Immunol. 2020;11:1312. 10.3389/fimmu.2020.01312. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Abrial C., Delyle S.G., Buenestado A., Naline E., Papke R., Devillier P. Role of nicotinic receptors in the regulation of cytokines production by human lung macrophages. Eur. Respir. Soc. 2012;40(Suppl 56) 5094A. [Google Scholar]
- Altara R., Manca M., Brandao R.D., Zeidan A., Booz G.W., Zouein F.A. Emerging importance of chemokine receptor CXCR3 and its ligands in cardiovascular diseases. Clin. Sci. (Lond.) 2016;130:463–478. 10.1042/CS20150666. [Abstract] [CrossRef] [Google Scholar]
- Báez-Pagán C.A., Delgado-Vélez M., Lasalde-Dominicci J.A. Activation of the macrophage α7 nicotinic acetylcholine receptor and control of inflammation. J. Neuroimmune Pharmacol. 2015;10:468–476. [Europe PMC free article] [Abstract] [Google Scholar]
- Baleeiro C.E., Christensen P.J., Morris S.B., Mendez M.P., Wilcoxen S.E., Paine R., III GM-CSF and the impaired pulmonary innate immune response following hyperoxic stress. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;291:L1246–L1255. [Abstract] [Google Scholar]
- Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M., Daßler-Plenker J., Guerci P., Huynh C., Knight J.S. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J. Exp. Med. 2020;217:e20200652. 10.1084/jem.20200652. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Bost P., Giladi A., Liu Y., Bendjelal Y., Xu G., David E., Blecher-Gonen R., Cohen M., Medaglia C., Li H., Deczkowska A., Zhang S., Schwikowski B., Zhang Z., Amit I. Host-viral infection maps reveal signatures of severe COVID-19 patients. Cell. 2020;181:1475. 10.1016/j.cell.2020.05.006. 1488 e1412. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Buckley L.F., Cheng J.W.M., Desai A. Cardiovascular Pharmacology in the time of COVID-19: a focus on angiotensin-converting enzyme 2. J. Cardiovasc. Pharmacol. 2020;75:526–529. 10.1097/FJC.0000000000000840. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Cakarova L., Marsh L.M., Wilhelm J., Mayer K., Grimminger F., Seeger W., Lohmeyer J., Herold S. Macrophage tumor necrosis factor-α induces epithelial expression of granulocyte–macrophage colony-stimulating factor: impact on alveolar epithelial repair. Am. J. Respir. Crit. Care Med. 2009;180:521–532. [Abstract] [Google Scholar]
- Cao Y., Goods B.A., Raddassi K., Nepom G.T., Kwok W.W., Love J.C., Hafler D.A. Functional inflammatory profiles distinguish myelin-reactive T cells from patients with multiple sclerosis. Sci. Transl. Med. 2015;7 287ra274-287ra274. [Europe PMC free article] [Abstract] [Google Scholar]
- Changeux J.-P., Amoura Z., Rey F.A., Miyara M. A nicotinic hypothesis for Covid-19 with preventive and therapeutic implications. Comptes Rendus. Biologies. 2020;343:33–39. [Abstract] [Google Scholar]
- Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K., Perlman S. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19:181–193. [Europe PMC free article] [Abstract] [Google Scholar]
- Cheemarla N.R., Brito A.F., Fauver J.R., Alpert T., Vogels C.B.F., Omer S.B., Ko A., Grubaugh N.D., Landry M.L., Foxman E.F. Host response-based screening to identify undiagnosed cases of COVID-19 and expand testing capacity. medRxiv. 2020 10.1101/2020.06.04.20109306. 2020.06.04.20109306. [CrossRef] [Google Scholar]
- Chu H., Chan J.F., Wang Y., Yuen T.T., Chai Y., Hou Y., Shuai H., Yang D., Hu B., Huang X., Zhang X., Cai J.P., Zhou J., Yuan S., Kok K.H., To K.K., Chan I.H., Zhang A.J., Sit K.Y., Au W.K., Yuen K.Y. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin. Infect. Dis. 2020:ciaa410. 10.1093/cid/ciaa410. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Chua R.L., Lukassen S., Trump S., Hennig B.P., Wendisch D., Pott F., Debnath O., Thurmann L., Kurth F., Volker M.T., Kazmierski J., Timmermann B., Twardziok S., Schneider S., Machleidt F., Muller-Redetzky H., Maier M., Krannich A., Schmidt S., Balzer F., Liebig J., Loske J., Suttorp N., Eils J., Ishaque N., Liebert U.G., von Kalle C., Hocke A., Witzenrath M., Goffinet C., Drosten C., Laudi S., Lehmann I., Conrad C., Sander L.E., Eils R. COVID-19 severity correlates with airway epithelium-immune cell interactions identified by single-cell analysis. Nat. Biotechnol. 2020;38:970–979. 10.1038/s41587-020-0602-4. [Abstract] [CrossRef] [Google Scholar]
- Conti P., Caraffa A., Gallenga C.E., Ross R., Kritas S.K., Frydas I., Younes A., Di Emidio P., Ronconi G., Toniato E. IL-1 induces throboxane-A2 (TxA2) in COVID-19 causing inflammation and micro-thrombi: inhibitory effect of the IL-1 receptor antagonist (IL-1Ra) J. Biol. Regul. Homeost. Agents. 2020;34 10.23812/20-34-4EDIT-65. [Abstract] [CrossRef] [Google Scholar]
- Conti P., Gallenga C.E., Tete G., Caraffa A., Ronconi G., Younes A., Toniato E., Ross R., Kritas S.K. How to reduce the likelihood of coronavirus-19 (CoV-19 or SARS-CoV-2) infection and lung inflammation mediated by IL-1. J. Biol. Regul. Homeost. Agents. 2020;34:333–338. 10.23812/Editorial-Conti-2. [Abstract] [CrossRef] [Google Scholar]
- Conti P., Ronconi G., Caraffa A., Gallenga C.E., Ross R., Frydas I., Kritas S.K. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents. 2020;34:327–331. 10.23812/CONTI-E. [Abstract] [CrossRef] [Google Scholar]
- Conti P., Younes A. Coronavirus COV-19/SARS-CoV-2 affects women less than men: clinical response to viral infection. J. Biol. Regul. Homeost. Agents. 2020;34:339–343. 10.23812/Editorial-Conti-3. [Abstract] [CrossRef] [Google Scholar]
- Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. 10.1016/j.cytogfr.2020.05.003. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Covid C., Covid C., Covid C., Chow N., Fleming-Dutra K., Gierke R., Hall A., Hughes M., Pilishvili T., Ritchey M. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019—United States, February 12–March 28, 2020. MMWR (Morb. Mortal. Wkly. Rep.) 2020;69:382–386. [Europe PMC free article] [Abstract] [Google Scholar]
- Danser A.H.J., Epstein M., Batlle D. Renin-angiotensin system blockers and the COVID-19 pandemic: at present there is No evidence to abandon renin-angiotensin system blockers. Hypertension. 2020;75:1382–1385. 10.1161/HYPERTENSIONAHA.120.15082. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Egea L., Hirata Y., Kagnoff M.F. GM-CSF: a role in immune and inflammatory reactions in the intestine. Expet Rev. Gastroenterol. Hepatol. 2010;4:723–731. [Europe PMC free article] [Abstract] [Google Scholar]
- Falzarano D., de Wit E., Martellaro C., Callison J., Munster V.J., Feldmann H. Inhibition of novel beta coronavirus replication by a combination of interferon-alpha2b and ribavirin. Sci. Rep. 2013;3:1686. 10.1038/srep01686. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Farsalinos K., Angelopoulou A., Alexandris N., Poulas K. COVID-19 and the nicotinic cholinergic system. Eur. Respir. J. 2020;56:2001589. 10.1183/13993003.01589-2020. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Farsalinos K., Barbouni A., Niaura R. Systematic review of the prevalence of current smoking among hospitalized COVID-19 patients in China: could nicotine be a therapeutic option? Intern Emerg Med. 2020;15:845–852. 10.1007/s11739-020-02355-7. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Farsalinos K., Niaura R., Le Houezec J., Barbouni A., Tsatsakis A., Kouretas D., Vantarakis A., Poulas K. Editorial: Nicotine and SARS-CoV-2: COVID-19 may be a disease of the nicotinic cholinergic system. Toxicol. Rep. 2020;7:658–663. 10.1016/j.toxrep.2020.04.012. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A., Diz D.I., Gallagher P.E. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. 10.1161/CIRCULATIONAHA.104.510461. [Abstract] [CrossRef] [Google Scholar]
- Fleetwood A.J., Lawrence T., Hamilton J.A., Cook A.D. Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: implications for CSF blockade in inflammation. J. Immunol. 2007;178:5245–5252. [Abstract] [Google Scholar]
- Forrester S.J., Booz G.W., Sigmund C.D., Coffman T.M., Kawai T., Rizzo V., Scalia R., Eguchi S. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol. Rev. 2018;98:1627–1738. 10.1152/physrev.00038.2017. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Furukawa N.W., Brooks J.T., Sobel J. Evidence supporting transmission of severe acute respiratory syndrome coronavirus 2 while presymptomatic or asymptomatic. Emerg. Infect. Dis. 2020;26:e201595. 10.3201/eid2607.201595. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Group R.C., Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B., Green C., Felton T., Chadwick D., Rege K., Fegan C., Chappell L.C., Faust S.N., Jaki T., Jeffery K., Montgomery A., Rowan K., Juszczak E., Baillie J.K., Haynes R., Landray M.J. Dexamethasone in hospitalized patients with covid-19 - preliminary report. N. Engl. J. Med. 2020 10.1056/NEJMoa2021436. NEJMoa2021436. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Guo G., Ye L., Pan K., Chen Y., Xing D., Yan K., Chen Z., Ding N., Li W., Huang H., Zhang L., Li X., Xue X. New insights of emerging SARS-CoV-2: epidemiology, etiology, clinical features, clinical treatment, and prevention. Front Cell Dev Biol. 2020;8:410. 10.3389/fcell.2020.00410. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Hamilton J.A. GM-CSF in inflammation. J. Exp. Med. 2020;217:e20190945. 10.1084/jem.20190945. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Han H., Ma Q., Li C., Liu R., Zhao L., Wang W., Zhang P., Liu X., Gao G., Liu F., Jiang Y., Cheng X., Zhu C., Xia Y. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microb. Infect. 2020;9:1123–1130. 10.1080/22221751.2020.1770129. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Herold S., Hoegner K., Vadász I., Gessler T., Wilhelm J., Mayer K., Morty R.E., Walmrath H.-D., Seeger W., Lohmeyer J. Inhaled granulocyte/macrophage Colony–stimulating factor as treatment of pneumonia-associated acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2014;189:609–611. [Abstract] [Google Scholar]
- Hu G., Christman J.W. Alveolar macrophages in lung inflammation and resolution. Front. Immunol. 2019;10:2275. 10.3389/fimmu.2019.02275. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. 10.1016/S0140-6736(20)30183-5. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Huff H.V., Singh A. Asymptomatic transmission during the COVID-19 pandemic and implications for public health strategies. Clin. Infect. Dis. 2020:ciaa654. 10.1093/cid/ciaa654. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Hung I.F., Lung K.C., Tso E.Y., Liu R., Chung T.W., Chu M.Y., Ng Y.Y., Lo J., Chan J., Tam A.R., Shum H.P., Chan V., Wu A.K., Sin K.M., Leung W.S., Law W.L., Lung D.C., Sin S., Yeung P., Yip C.C., Zhang R.R., Fung A.Y., Yan E.Y., Leung K.H., Ip J.D., Chu A.W., Chan W.M., Ng A.C., Lee R., Fung K., Yeung A., Wu T.C., Chan J.W., Yan W.W., Chan W.M., Chan J.F., Lie A.K., Tsang O.T., Cheng V.C., Que T.L., Lau C.S., Chan K.H., To K.K., Yuen K.Y. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–1704. 10.1016/S0140-6736(20)31042-4. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Hussell T., Bell T.J. Alveolar macrophages: plasticity in a tissue-specific context. Nat. Rev. Immunol. 2014;14:81–93. [Abstract] [Google Scholar]
- Igase M., Kohara K., Nagai T., Miki T., Ferrario C.M. Increased expression of angiotensin converting enzyme 2 in conjunction with reduction of neointima by angiotensin II type 1 receptor blockade. Hypertens. Res. 2008;31:553–559. 10.1291/hypres.31.553. [Abstract] [CrossRef] [Google Scholar]
- Jiang Y., Xu J., Zhou C., Wu Z., Zhong S., Liu J., Luo W., Chen T., Qin Q., Deng P. Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. Am. J. Respir. Crit. Care Med. 2005;171:850–857. 10.1164/rccm.200407-857OC. [Abstract] [CrossRef] [Google Scholar]
- Kalamida D., Poulas K., Avramopoulou V., Fostieri E., Lagoumintzis G., Lazaridis K., Sideri A., Zouridakis M., Tzartos S.J. Muscle and neuronal nicotinic acetylcholine receptors. FEBS J. 2007;274:3799–3845. [Abstract] [Google Scholar]
- Kloc M., Ghobrial R.M., Kubiak J.Z. How nicotine can inhibit cytokine storm in the lungs and prevent or lessen the severity of COVID-19 infection? Immunol Lett. 2020;224:28–29. 10.1016/j.imlet.2020.06.002. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Komuczki J., Tuzlak S., Friebel E., Hartwig T., Spath S., Rosenstiel P., Waisman A., Opitz L., Oukka M., Schreiner B. Fate-mapping of GM-CSF expression identifies a discrete subset of inflammation-driving T helper cells regulated by cytokines IL-23 and IL-1β Immunity. 2019;50:1289–1304. e1286. [Abstract] [Google Scholar]
- Kritas S.K., Ronconi G., Caraffa A., Gallenga C.E., Ross R., Conti P. Mast cells contribute to coronavirus-induced inflammation: new anti-inflammatory strategy. J. Biol. Regul. Homeost. Agents. 2020;34:9–14. 10.23812/20-Editorial-Kritas. [Abstract] [CrossRef] [Google Scholar]
- Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. 10.1038/nm1267. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Lang F.M., Lee K.M.-C., Teijaro J.R., Becher B., Hamilton J.A. GM-CSF-based treatments in COVID-19: reconciling opposing therapeutic approaches. Nat. Rev. Immunol. 2020;20:507–514. 10.1038/s41577-020-0357-7. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Lee J.S., Park S., Jeong H.W., Ahn J.Y., Choi S.J., Lee H., Choi B., Nam S.K., Sa M., Kwon J.S., Jeong S.J., Lee H.K., Park S.H., Park S.H., Choi J.Y., Kim S.H., Jung I., Shin E.C. Immunophenotyping of COVID-19 and influenza highlights the role of type I interferons in development of severe COVID-19. Sci Immunol. 2020;5:eabd1554. 10.1126/sciimmunol.abd1554. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Leung J.M., Yang C.X., Sin D.D. COVID-19 and nicotine as a mediator of ACE-2. Eur. Respir. J. 2020;55:2001261. 10.1183/13993003.01261-2020. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Li C.C., Wang X.J., Wang H.R. Repurposing host-based therapeutics to control coronavirus and influenza virus. Drug Discov. Today. 2019;24:726–736. 10.1016/j.drudis.2019.01.018. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Li J.Y., Liao C.H., Wang Q., Tan Y.J., Luo R., Qiu Y., Ge X.Y. The ORF6, ORF8 and nucleocapsid proteins of SARS-CoV-2 inhibit type I interferon signaling pathway. Virus Res. 2020;286 10.1016/j.virusres.2020.198074. 198074. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., Cheng L., Li J., Wang X., Wang F., Liu L., Amit I., Zhang S., Zhang Z. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020;26:842–844. 10.1038/s41591-020-0901-9. [Abstract] [CrossRef] [Google Scholar]
- Liu Y.C., Kuo R.L., Shih S.R. COVID-19: the first documented coronavirus pandemic in history. Biomed. J. 2020 10.1016/j.bj.2020.04.007. S2319-4170–5. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Liu C.L., Shau W.Y., Chang C.H., Wu C.S., Lai M.S. Pneumonia risk and use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers. J. Epidemiol. 2013;23:344–350. 10.2188/jea.je20120112. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Liu X., Zhang R., He G. Hematological findings in coronavirus disease 2019: indications of progression of disease. Ann. Hematol. 2020;99:1421–1428. 10.1007/s00277-020-04103-5. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Lokugamage K.G., Hage A., Schindewolf C., Rajsbaum R., Menachery V.D. SARS-CoV-2 is sensitive to type I interferon pretreatment. bioRxiv. 2020 10.1101/2020.03.07.982264. 2020.03.07.982264. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Lu B., Kwan K., Levine Y.A., Olofsson P.S., Yang H., Li J., Joshi S., Wang H., Andersson U., Chavan S.S., Tracey K.J. α7 nicotinic acetylcholine receptor signaling inhibits inflammasome activation by preventing mitochondrial DNA release. Mol. Med. 2014;20:350–358. 10.2119/molmed.2013.00117. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Matute-Bello G., Liles C.W., Frank Radella I., Steinberg K.P., Ruzinski J.T., Hudson L.D., Martin T.R. Modulation of neutrophil apoptosis by granulocyte colony-stimulating factor and granulocyte/macrophage colony-stimulating factor during the course of acute respiratory distress syndrome. Crit. Care Med. 2000;28:1–7. [Abstract] [Google Scholar]
- McFadyen J.D., Stevens H., Peter K. The emerging threat of (Micro)Thrombosis in COVID-19 and its therapeutic implications. Circ. Res. 2020;127:571–587. 10.1161/CIRCRESAHA.120.317447. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., Hlh across Speciality Collaboration, U.K. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. 10.1016/S0140-6736(20)30628-0. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Mehta P., Porter J.C., Manson J.J., Isaacs J.D., Openshaw P.J.M., McInnes I.B., Summers C., Chambers R.C. Therapeutic blockade of granulocyte macrophage colony-stimulating factor in COVID-19-associated hyperinflammation: challenges and opportunities. Lancet Respir Med. 2020;8:822–830. 10.1016/S2213-2600(20)30267-8. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20:355–362. 10.1038/s41577-020-0331-4. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Miyara M., Tubach F., Pourcher V., Morelot-Panzini C., Pernet J., Haroche J. Low rate of daily active tobacco smoking in patients with symptomatic COVID-19. Qeios. 2020 10.32388/WPP19W.4. [CrossRef] [Google Scholar]
- Nile S.H., Nile A., Qiu J., Li L., Jia X., Kai G. COVID-19: pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev. 2020;53:66–70. 10.1016/j.cytogfr.2020.05.002. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Oran D.P., Topol E.J. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann. Intern. Med. 2020;173:362–367. 10.7326/M20-3012. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Paces J., Strizova Z., Smrz D., Cerny J. COVID-19 and the immune system. Physiol. Res. 2020;69:379–388. 10.33549/physiolres.934492. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Paine R., III, Wilcoxen S.E., Morris S.B., Sartori C., Baleeiro C.E., Matthay M.A., Christensen P.J. Transgenic overexpression of granulocyte macrophage-colony stimulating factor in the lung prevents hyperoxic lung injury. Am. J. Pathol. 2003;163:2397–2406. [Europe PMC free article] [Abstract] [Google Scholar]
- Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O'Donnell L.F., Chernyak Y., Tobin K., Cerfolio R.J., Francois F., Horwitz L.I. 2020. Factors Associated with Hospitalization and Critical Illness Among 4,103 Patients with COVID-19 Disease in New York City. MedRxiv. [Europe PMC free article] [Abstract] [Google Scholar]
- Prieto-Pérez L., Fortes J., Soto C., Vidal-González Á., Alonso-Riaño M., Lafarga M., Cortti M.J., Lazaro-Garcia A., Pérez-Tanoira R., Trascasa Á., Antonio A., Córdoba R., Rodríguez-Pinilla S.M., Cedeño O., Peces-Barba G., Fernández-Ormaechea I., Díez Medrano M.J., López de Las Heras M., Cabello A., Petkova E., Álvarez B., Carrillo I., Silva A.M., Castellanos M., Calpena S., Valverde-Monge M., Fresneda D., Rubio-Martín R., Cornejo I., Astilleros Blanco de Cordova L., de la Fuente S., Recuero S., Górgolas M., Piris M.A. Histiocytic hyperplasia with hemophagocytosis and acute alveolar damage in COVID-19 infection. Mod. Pathol. 2020:1–8. 10.1038/s41379-020-0613-1. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Ramos-Casals M., Brito-Zeron P., Lopez-Guillermo A., Khamashta M.A., Bosch X. Adult haemophagocytic syndrome. Lancet. 2014;383:1503–1516. 10.1016/S0140-6736(13)61048-X. [Abstract] [CrossRef] [Google Scholar]
- Rösler B., Herold S. Lung epithelial GM-CSF improves host defense function and epithelial repair in influenza virus pneumonia—a new therapeutic strategy? Mol. Cell. Pediatr. 2016;3:29. 10.1186/s40348-016-0055-5. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Russo P., Bonassi S., Giacconi R., Malavolta M., Tomino C., Maggi F. COVID-19 and smoking: is nicotine the hidden link? Eur. Respir. J. 2020;55:2001116. 10.1183/13993003.01116-2020. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Sheahan T.P., Sims A.C., Leist S.R., Schafer A., Won J., Brown A.J., Montgomery S.A., Hogg A., Babusis D., Clarke M.O., Spahn J.E., Bauer L., Sellers S., Porter D., Feng J.Y., Cihlar T., Jordan R., Denison M.R., Baric R.S. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020;11:222. 10.1038/s41467-019-13940-6. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Shinohara Y., Origasa H. Post-stroke pneumonia prevention by angiotensin-converting enzyme inhibitors: results of a meta-analysis of five studies in Asians. Adv. Ther. 2012;29:900–912. 10.1007/s12325-012-0049-1. [Abstract] [CrossRef] [Google Scholar]
- Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical–therapeutic staging proposal. J. Heart Lung Transplant. 2020;39:405–407. 10.1016/j.healun.2020.03.012. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Smith J.C., Sausville E.L., Girish V., Yuan M.L., Vasudevan A., John K.M., Sheltzer J.M. Cigarette smoke exposure and inflammatory signaling increase the expression of the SARS-CoV-2 receptor ACE2 in the respiratory tract. Dev. Cell. 2020;53:514–529. 10.1016/j.devcel.2020.05.012. e513. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Sommerstein R., Kochen M.M., Messerli F.H., Grani C. Coronavirus disease 2019 (COVID-19): do angiotensin-converting enzyme inhibitors/angiotensin receptor blockers have a biphasic effect? J. Am. Heart Assoc. 2020;9:e016509. 10.1161/JAHA.120.016509. e016509. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Team C.C.-R. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 - United States, february 12-march 28, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:382–386. 10.15585/mmwr.mm6913e2. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Teuwen L.A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat. Rev. Immunol. 2020;20:389–391. 10.1038/s41577-020-0343-0. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Theoharides T.C., Conti P. Dexamethasone for COVID-19? Not so fast. J. Biol. Regul. Homeost. Agents. 2020;34 10.23812/20-EDITORIAL_1-5. [Abstract] [CrossRef] [Google Scholar]
- Tindle H.A., Newhouse P.A., Freiberg M.S. Beyond smoking cessation: Investigating medicinal nicotine to prevent and treat COVID-19. Nicotine Tob. Res. 2020;22:1669–1670. 10.1093/ntr/ntaa077. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Tracey K.J. The inflammatory reflex. Nature. 2002;420:853–859. [Abstract] [Google Scholar]
- Tsaytler P., Harding H.P., Ron D., Bertolotti A. Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science. 2011;332:91–94. [Abstract] [Google Scholar]
- Verdecchia P., Cavallini C., Spanevello A., Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Intern. Med. 2020;76:14–20. 10.1016/j.ejim.2020.04.037. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Wang C., Xie J., Zhao L., Fei X., Zhang H., Tan Y., Zhou L., Liu Z., Ren Y., Yuan L., Zhang Y., Zhang J., Liang L., Chen X., Liu X., Wang P., Han X., Weng X., Chen Y., Yu T., Zhang X., Cai J., Chen R., Shi Z., Bian X. Aveolar Macrophage Activation and Cytokine Storm in the Pathogenesis of Severe COVID-19. 2020. PPR: PPR129332. [Europe PMC free article] [Abstract] [CrossRef]
- Wang J., Jiang M., Chen X., Montaner L.J. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J. Leukoc. Biol. 2020 10.1002/JLB.3COVR0520-272R. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Wang Y., Ding Q., Lu Y.C., Cao S.Y., Liu Q.X., Zhang L. Interferon-stimulated gene 15 enters posttranslational modifications of p53. J. Cell. Physiol. 2019;234:5507–5518. 10.1002/jcp.27347. [Abstract] [CrossRef] [Google Scholar]
- Wen W., Su W., Tang H., Le W., Zhang X., Zheng Y., Liu X., Xie L., Li J., Ye J., Dong L., Cui X., Miao Y., Wang D., Dong J., Xiao C., Chen W., Wang H. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov. 2020;6:31. 10.1038/s41421-020-0168-9. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. J. Am. Med. Assoc. 2020;323:1239. 10.1001/jama.2020.2648. [Abstract] [CrossRef] [Google Scholar]
- Xiong Y., Liu Y., Cao L., Wang D., Guo M., Jiang A., Guo D., Hu W., Yang J., Tang Z., Wu H., Lin Y., Zhang M., Zhang Q., Shi M., Liu Y., Zhou Y., Lan K., Chen Y. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg. Microb. Infect. 2020;9:761–770. 10.1080/22221751.2020.1747363. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Xu P., Zhou Q., Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann. Hematol. 2020;99:1205–1208. 10.1007/s00277-020-04019-0. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Yang D., Chu H., Hou Y., Chai Y., Shuai H., Lee A.C., Zhang X., Wang Y., Hu B., Huang X., Yuen T.T., Cai J.P., Zhou J., Yuan S., Zhang A.J., Chan J.F., Yuen K.Y. Attenuated interferon and pro-inflammatory response in SARS-CoV-2-infected human dendritic cells is associated with viral antagonism of STAT1 phosphorylation. J. Infect. Dis. 2020;222:734–745. 10.1093/infdis/jiaa356. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Ye Q., Wang B., Mao J. The pathogenesis and treatment of the 'Cytokine Storm’ in COVID-19. J. Infect. 2020;80(613):607. 10.1016/j.jinf.2020.03.037. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zhang J., Roberts A., Liu C., Ren G., Xu G., Zhang L., Devadas S., Shi Y. A novel subset of helper T cells promotes immune responses by secreting GM-CSF. Cell Death Differ. 2013;20:1731–1741. [Europe PMC free article] [Abstract] [Google Scholar]
- Zhao Y., Qin L., Zhang P., Li K., Liang L., Sun J., Xu B., Dai Y., Li X., Zhang C., Peng Y., Feng Y., Li A., Hu Z., Xiang H., Ogg G., Ho L.P., McMichael A.J., Jin R., Knight J.C., Dong T., Zhang Y. Longitudinal COVID-19 profiling associates IL-1Ra and IL-10 with disease severity and RANTES with mild disease. JCI Insight. 2020;5:e139834. 10.1172/jci.insight.139834. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020;17:259–260. 10.1038/s41569-020-0360-5. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zhou Y., Fu B., Zheng X., Wang D., Zhao C., qi Y., Sun R., Tian Z., Xu X., Wei H. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl. Sci. Rev. 2020 10.1093/nsr/nwaa041. Mar 13:nwaa041. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zumla A., Chan J.F., Azhar E.I., Hui D.S., Yuen K.Y. Coronaviruses - drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016;15:327–347. 10.1038/nrd.2015.37. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.ejphar.2020.173547
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7483085
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.ejphar.2020.173547
Article citations
Macrophage‑driven pathogenesis in acute lung injury/acute respiratory disease syndrome: Harnessing natural products for therapeutic interventions (Review).
Mol Med Rep, 31(1):16, 08 Nov 2024
Cited by: 0 articles | PMID: 39513609 | PMCID: PMC11551695
Review Free full text in Europe PMC
Immunohistochemical and Morphometric Analysis of Lung Tissue in Fatal COVID-19.
Diagnostics (Basel), 14(9):914, 27 Apr 2024
Cited by: 0 articles | PMID: 38732328 | PMCID: PMC11082993
The Role of Macrophages in Lung Fibrosis and the Signaling Pathway.
Cell Biochem Biophys, 82(2):479-488, 27 Mar 2024
Cited by: 3 articles | PMID: 38536578
Review
Carbon dioxide and MAPK signalling: towards therapy for inflammation.
Cell Commun Signal, 21(1):280, 10 Oct 2023
Cited by: 1 article | PMID: 37817178 | PMCID: PMC10566067
Review Free full text in Europe PMC
In silico designing and immunoinformatics analysis of a novel peptide vaccine against metallo-beta-lactamase (VIM and IMP) variants.
PLoS One, 18(7):e0275237, 20 Jul 2023
Cited by: 7 articles | PMID: 37471423 | PMCID: PMC10358925
Go to all (21) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
COVID-19, Mast Cells, Cytokine Storm, Psychological Stress, and Neuroinflammation.
Neuroscientist, 26(5-6):402-414, 18 Jul 2020
Cited by: 149 articles | PMID: 32684080
Review
Contribution of monocytes and macrophages to the local tissue inflammation and cytokine storm in COVID-19: Lessons from SARS and MERS, and potential therapeutic interventions.
Life Sci, 257:118102, 18 Jul 2020
Cited by: 190 articles | PMID: 32687918 | PMCID: PMC7367812
Review Free full text in Europe PMC
Hazards of the Cytokine Storm and Cytokine-Targeted Therapy in Patients With COVID-19: Review.
J Med Internet Res, 22(8):e20193, 13 Aug 2020
Cited by: 29 articles | PMID: 32707537 | PMCID: PMC7428145
Review Free full text in Europe PMC
Harnessing the immune system to overcome cytokine storm and reduce viral load in COVID-19: a review of the phases of illness and therapeutic agents.
Virol J, 17(1):154, 15 Oct 2020
Cited by: 53 articles | PMID: 33059711 | PMCID: PMC7558250
Review Free full text in Europe PMC
Funding
Funders who supported this work.
American University of Beirut
CSF (1)
Grant ID: 320145
Centre National de la Recherche Scientifique (3)
Grant ID: 103507
Grant ID: 103487
Grant ID: 103941
Collaborative Research Stimulus (1)
Grant ID: 103556
Faculty of Medicine
Seed grant (1)
Grant ID: 100410






