Abstract
Free full text

From the Cover
Condensing water vapor to droplets generates hydrogen peroxide
Significance
Water molecules in bulk liquid are stable and inert under ambient conditions. In sharp contrast, we show that the condensation of water vapor in air to form microdroplets on cold surfaces causes the formation of hydrogen peroxide (H2O2) which we suggest is promoted by the large, intrinsic electric field at the air–water interface. This finding provides an alternative pathway for the production of atmospheric H2O2, for example, in raindrops as well as fogs and mists. This easy means of naturally producing H2O2 suggests many interesting possibilities, from the use of condensed steam for disinfection to how water microdroplets might have promoted formation of the building blocks of life in the prebiotic era.
Abstract
It was previously shown [J. K. Lee et al., Proc. Natl. Acad. Sci. U.S.A., 116, 19294–19298 (2019)] that hydrogen peroxide (H2O2) is spontaneously produced in micrometer-sized water droplets (microdroplets), which are generated by atomizing bulk water using nebulization without the application of an external electric field. Here we report that H2O2 is spontaneously produced in water microdroplets formed by dropwise condensation of water vapor on low-temperature substrates. Because peroxide formation is induced by a strong electric field formed at the water–air interface of microdroplets, no catalysts or external electrical bias, as well as precursor chemicals, are necessary. Time-course observations of the H2O2 production in condensate microdroplets showed that H2O2 was generated from microdroplets with sizes typically less than ~10 µm. The spontaneous production of H2O2 was commonly observed on various different substrates, including silicon, plastic, glass, and metal. Studies with substrates with different surface conditions showed that the nucleation and the growth processes of condensate water microdroplets govern H2O2 generation. We also found that the H2O2 production yield strongly depends on environmental conditions, including relative humidity and substrate temperature. These results show that the production of H2O2 occurs in water microdroplets formed by not only atomizing bulk water but also condensing water vapor, suggesting that spontaneous water oxidation to form H2O2 from water microdroplets is a general phenomenon. These findings provide innovative opportunities for green chemistry at heterogeneous interfaces, self-cleaning of surfaces, and safe and effective disinfection. They also may have important implications for prebiotic chemistry.
Water molecules in liquid water are considered stable and inert. We and other investigators have reported that water molecules become electrochemically active and catalytic for various reactions when bulk water is formed into micrometer-sized droplets (microdroplets). Reaction rates for various chemical reactions are accelerated in microdroplets by factors of 102 or more compared to bulk solution (1). The microdroplet environment provides conditions for a lowered entropic barrier, which allows thermodynamically unfavorable reactions to proceed in microdroplets at room temperature (2, 3). We also have shown that water microdroplets induce spontaneous charge exchanges between solutes and water molecules to induce the spontaneous reduction of organic molecules and metal ions as well as the formation of nanostructures without any added reducing agent or template (4, 5). Moreover, we have reported that water molecules undergo spontaneous oxidation to form reactive oxygen species, including hydroxyl radicals (OH) and hydrogen peroxide (H2O2) (6–8). Recent investigations attributed the origin of these unique physicochemical properties observed in microdroplets to the enrichment of reactants at the interface (9–11), restricted molecular rotations (12), partial solvation at the water surface (1, 13), and a strong interfacial electric field at the surface of the water microdroplet (14).
Microdroplets can be formed either by atomizing bulk water (top down) with various methods such as high-pressure gas nebulization (15), ultrasonic nebulization (16), vibrating micromesh nebulization (17), and piezoelectric nebulization (18), or by condensing vapor-phase molecules (bottom up) (19). A question may be asked whether those unique properties of microdroplets arise only in microdroplets formed by atomization of bulk water. In addition, it may be wondered whether the spontaneous oxidation of water to form H2O2 in microdroplets (6) was caused by the atomizing process involving friction or vibration. These questions motivated us to investigate whether H2O2 becomes spontaneously generated in water microdroplets formed by the condensation of water vapor in air on cold surfaces, and how universal might this process be. We have paid special attention to the influence of different surface properties, including hydrophilicity and surface roughness, as well as environmental factors, including relative humidity and surface temperature.
Results
To test whether H2O2 can be generated in water microdroplets formed by condensation of water vapor in air on cold surfaces, we fabricated an environment control chamber that enables the control of relative humidity and temperature (Fig. 1). The relative humidity in the chamber was maintained by a feedback control loop consisting of a humidifier and a dehumidifier. The substrate temperature was manipulated with a Peltier cooler and an electric resistive heater, which were controlled by a feedback control loop. The chamber was also equipped with an optical microscope that enabled real-time imaging of microdroplet condensation. Because of the ease in collecting and assessing the size distribution of condensed microdroplets on solid surfaces compared to suspended microdroplets formed by the condensation of vapor nuclei, we studied microdroplet condensates formed on a cold surface rather than suspended microdroplets. We have chosen silicon (Si) as a model substrate because undoped single-crystalline Si offers low electric conductivity and, consequently, minimum interference to the formation of H2O2 (20). The Si wafer also has a reasonably high thermal conductivity that promotes the condensation of water vapor (21).
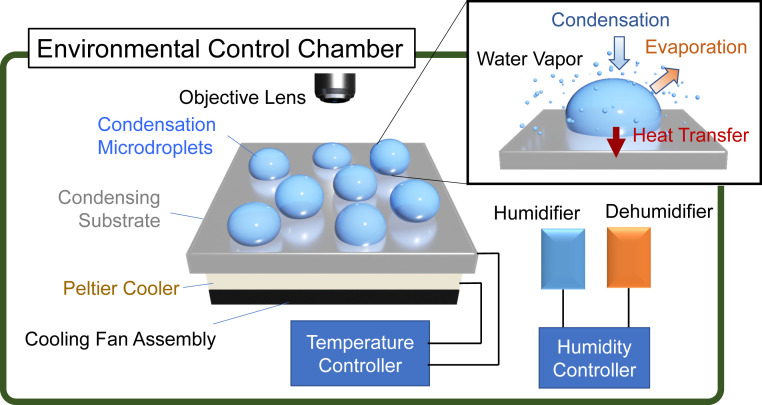
Schematic of the experiment setup. An environmental control chamber was equipped with an automatic humidity control loop. The temperature of the substrate on which water vapor was condensed to form water microdroplets was controlled using a Peltier cooler and a resistive electric heater that were controlled by a feedback temperature controller. (Inset) Processes involved in the condensation of a microdroplet.
Fig. 2A shows a bright-field image of water microdroplet condensates formed on a precleaned Si wafer after 1-min cooling at 3.5 °C. The relative humidity in the environment control chamber was maintained at 55%. The average diameter of a water microdroplet was ~4.6 ± 3.2 µm. To confirm that H2O2 is generated in condensed water microdroplets, we reacted collected water microdroplets on Si wafer surfaces with 4-carboxyphenylboronic acid. Fig. 2B presents the reaction scheme between 4-carboxyphenylboronic acid 1 and H2O2 to yield 4-hydroxybenzoic acid 2 and boric acid 3. It also presents a mass spectrum showing reaction products between collected microdroplets and 4-carboxyphenylboronic acid. We observed the cleavage of reactant 4-carboxyphenylboronic acid (deprotonated, m/z 165.04) by H2O2 contained in microdroplets to produce 4-hydroxybenzoic acid 2 (deprotonated, m/z 137.02) and boric acid 3 (deprotonated, m/z 61.01). These results confirm the production of H2O2 in condensed water microdroplets.
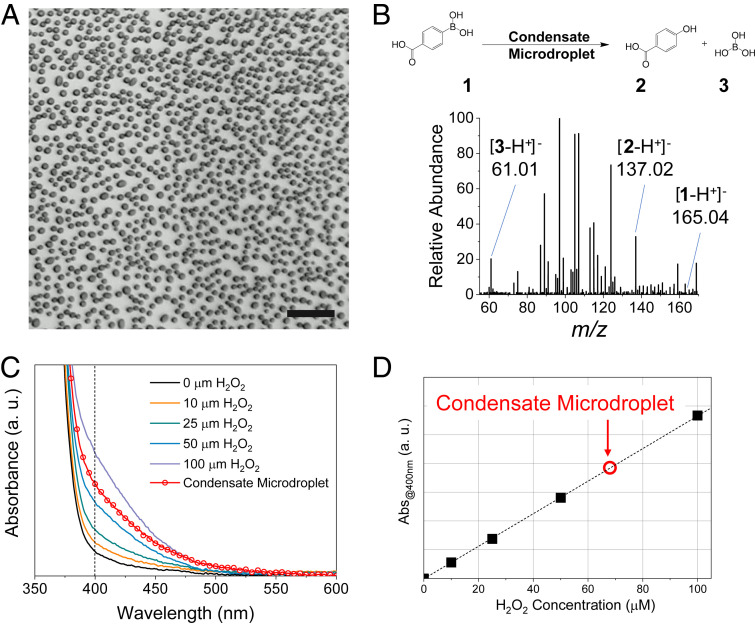
Generation of hydrogen peroxide in water microdroplets formed by water-vapor condensation. (A) Bright-field image of microdroplets formed on the cooled Si wafer surface. (B) Mass spectrum showing reaction products from reacting carboxyphenylboronic acid 1 with H2O2 generated in condensed water microdroplets to form 4-hydroxybenzoic acid 2 and boric acid 3. (C) The absorption spectrum of aqueous PTO solution with added H2O2 and a condensed microdroplet (red circle). (D) Calibration curve of the absorption at 400 nm acquired from spectra shown in C. The red circle represents the concentration of H2O2 generated from the condensed water microdroplet. (Scale bar, 50 µm.)
To quantify the concentration of H2O2 generated in water microdroplets, we carried out a titration with potassium titanium oxalate (PTO, K2TiO(C2O4)2· H2O). Fig. 2C shows the absorption spectra of 0.1 M PTO solution containing different concentrations of H2O2 as well as the collected water microdroplets. The calibration plot of the absorbance at 400 nm, acquired from Fig. 2C, shows the concentration of H2O2 generated in condensed water microdroplets to be ~68 µM, which is ~2.3 ppm (parts per million). The quantification was also performed using peroxide test strips (Movie S1). The agreement between the two different assays was confirmed in a previous study (6).
We recorded time-lapse imaging of microdroplet formation on a Si surface and compared the distribution of the microdroplet size with the H2O2 production yield to establish the relation between the two. Fig. 3 A–C shows the bright-field images and the size distribution of condensed water microdroplets at 0.5, 2, and 5 min after initiation of Si surface cooling at 3.5 °C. The relative humidity was maintained at 55%. Fig. 3D presents the H2O2 production yield as a function of time. At 0.5 min, the average microdroplet diameter was 4.0 ± 2.7 µm, and the H2O2 production yield was less than 1.0 ppm. Under continuous cooling, the microdroplet diameter increased to an average value of 7.7 ± 5.7 µm after 2 min. The H2O2 production yield reached a maximum value of ~3.9 ppm at 2 min. After 5 min, microdroplets continued to grow with an average diameter of 12.5 µm. At this time point, droplets with diameters of ~100 µm or larger were formed. Approximately 85% of the surface area was occupied by those large droplets or liquid films. Consequently, most of the liquid volume of collected microdroplets was dominated by these large droplets and liquid films. The concentration of the produced H2O2 at 5 min was reduced below the detection limit. A previous study using the H2O2-sensitive fluorescence dye, peroxyfluor-1, showed that H2O2 was mostly produced in water microdroplets less than 10 µm in diameter (6). This observation provides an explanation for why H2O2 production yield decreased when droplets continued to grow to form larger droplets or films, in which H2O2 is not effectively produced. The H2O2 produced at early time points when microdroplets are less than 10 µm becomes diluted, resulting in a decrease in the overall concentration. It may be desired to image microdroplets using H2O2-sensitive fluorescent dye, peroxyfluor-1, to establish a direct relationship between the microdroplet size and the H2O2 production yield. However, owing to the limitation of introducing dyes into microdroplets during condensation and the difficulty in normalizing the fluorescence intensity in growing droplets, this investigation could not be performed successfully.
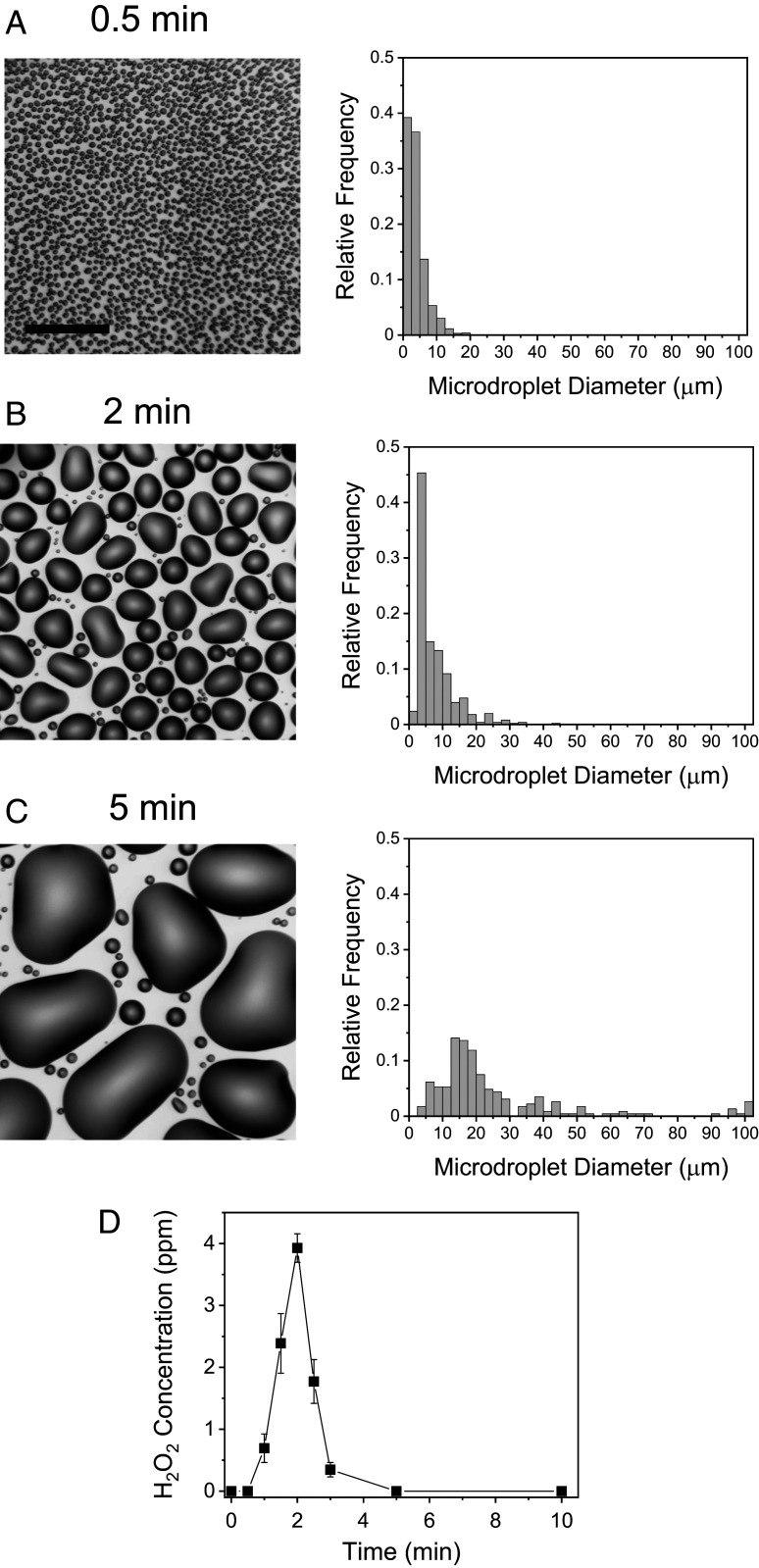
Time course of H2O2 formation in condensed water microdroplets. Time-lapse bright-field images (Left) and size distribution (Right) of microdroplets formed on a Si wafer after (A) 0.5 min, (B) 2 min, and (C) 5 min of cooling with a Peltier cooler. (D) Time course of H2O2 concentration. Error bars represent 1 SD from three independent measurements. (Scale bar, 50 µm.)
To examine whether H2O2 can be formed on other surface types, we explored the production of H2O2 from water microdroplets condensed on plastic (Teflon), a glass slide, and polished copper. The surface temperature and the relative humidity were fixed at 3.5 °C and 55%. SI Appendix, Fig. S1A shows plots of H2O2 production yield on the different types of surfaces as a function of substrate cooling time. Although different surfaces exhibited different maximum H2O2 production yields, the qualitative temporal profiles remained almost the same, i.e., gradual rise and fall. The maximum H2O2 production yields are calculated from SI Appendix, Fig. S1A, showing that the Si wafer surface was the most efficient substrate for the production of H2O2 among the surfaces examined (SI Appendix, Fig. S1B). However, because these surfaces made of different materials have different physical properties such as surface roughness, hydrophobicity, and porosity, it could not be concluded whether the difference in the H2O2 production yield arises from different chemical effects or different physical surface properties of the materials. Therefore, we measured the H2O2 production yield on the same Si substrate that had different surface properties prepared by different surface treatments.
To make a fair comparison of the effect of different types of surfaces on the H2O2 production yield, we prepared Si wafers that were prepared differently: 1) hydrophobic treated, 2) hydrophilic treated, and 3) plasma etched. We chose Si as a base substrate because of the availability of various surface modification methods and the chemical inertness of the Si surface.
For preparing a hydrophobic surface, we silanized Si wafer surfaces using 1,7-dichloro-octamethyltetrasiloxane (Cl[Si(CH3)2O]3Si(CH3)2Cl) in heptane. The solvent and unbound residues were removed prior to condensation experiments. Fig. 4A shows a bright-field image of microdroplets condensed on a hydrophobic-treated Si surface taken at 2 min after the initiation of cooling. The number of microdroplets per unit area and the average microdroplet diameter were 18,709 per mm2 and 4.3 ± 1.2 µm. These values were much lower than those values of 24,255/mm2 and 7.7 ± 5.7 µm for microdroplets condensed on an untreated Si surface. This decrease in the formation of microdroplets on hydrophobic-treated surfaces might result in a decrease in the H2O2 production yield of 1.8 ppm at 2 min. The time-lapse images of microdroplets formed on the hydrophobic-treated Si wafer surface also showed that rates of both nucleation and droplet growth were slower compared to the untreated Si surface (SI Appendix, Fig. S2). This slow growth of microdroplets observed in images was consistent with the slow rise of H2O2 production yield shown in Fig. 4D.
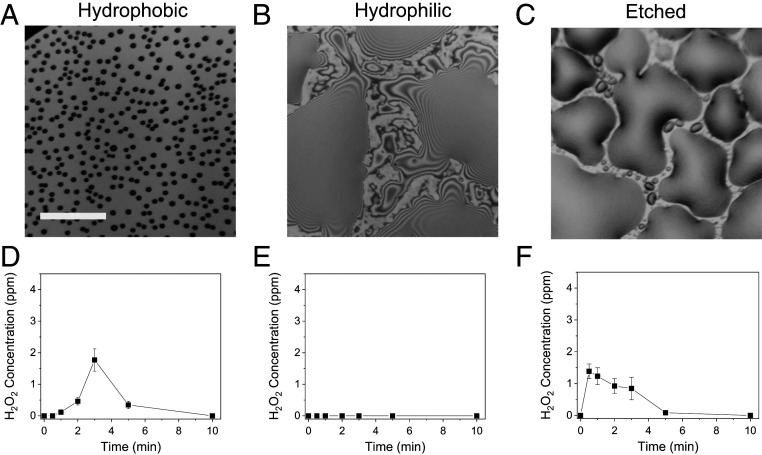
Dependence of H2O2 production on surface treatment conditions. Si wafers were treated to be hydrophobic (A and D), hydrophilic (B and E), and etched for increased surface roughness (C and F). (A–C) Bright-field images taken at 2 min after the initiation of cooling. (D–F) Time courses of H2O2 concentration on each treated surface. Error bars represent 1 SD from three independent measurements. (Scale bar, 50 µm.)
We prepared a hydrophilic Si surface by treating Si surfaces with an oxygen plasma cleaner, which induces onto the Si surface hydrophilic groups such as oxygen atoms in Si–O–Si bonds (22). Because of the instability of the hydrophilic Si surface from the high-strength strain in Si–O–Si bonds, condensation experiments using oxygen plasma-treated Si surfaces were carried out no later than 30 min after the completion of the oxygen plasma treatment. Fig. 4B and SI Appendix, Fig. S3 show bright-field images of condensates formed on hydrophobic-treated Si surfaces. Notably, the Si surface was dominantly covered by thin films with lateral sizes over hundreds of micrometers, which was caused by the high wettability of the hydrophilic Si surface. In these water condensate films, no H2O2 was produced (Fig. 4E). There were thin films with a few micrometers in thickness and tens of micrometers in lateral diameter. It is unclear whether H2O2 can be formed in these micrometer-sized thin condensed water films because most of the liquid collected for quantitative analyses was dominated by larger thin films. Again, direct imaging of the H2O2 production in these individual condensates by employing, for instance, H2O2-sensitive fluorescent dyes, is necessary to determine the production of H2O2 in these thin water films.
Nucleation and growth of dropwise water condensation are promoted on surfaces with a higher surface roughness. We increased the roughness of the Si surface by treating it with deep reactive-ion etching technique in an inductive coupled plasma etcher. The etched Si surface exhibited an increased surface root-mean-square (rms) surface roughness of 3.80 nm compared to 0.12 nm on the untreated Si surface (SI Appendix, Fig. S4). Time-lapse images of bright-field images of the water condensation on etched Si surfaces show the formation of small nuclei as early as 15 s after the initiation of cooling, followed by a rapid increase of water condensation (SI Appendix, Fig. S5). Due to the increased wettability of the etched Si surface, thin liquid films were observed rather than microdroplets at and after 2 min. The time course of H2O2 concentration on etched Si surfaces exhibits a similar trend (Fig. 4F). The H2O2 production yield reached a maximum value at 30 s and decreased gradually afterward. These studies of the effect of the surface properties on the H2O2 production yield indicate that the nucleation and the growth of condensed microdroplets determine the H2O2 production yield. Particularly, the surface density of microdroplets less than ~10 µm is important for achieving a high H2O2 production yield.
We also examined the dependence of H2O2 generation in condensed water microdroplets on environmental conditions, including relative humidity, ambient light level, and surface temperature. First, we measured the time course of H2O2 production yield under different relative humidity ranging from 40 to 70% in the environment control chamber (Fig. 5A). The surface temperature of untreated Si water was kept at 3.5 °C. At 40% relative humidity, the growth of microdroplet formation became slower than at 55% and 70%. Consequently, the time point for the maximum H2O2 production yield was ~3 min, which was slower compared to 2 min for the samples with 55 and 70% humidity. The time courses of H2O2 production yield under 55 and 70% humidity exhibit almost identical patterns, suggesting the H2O2 production was saturated beyond 55% relative humidity. These observations are also reflected in the plots of the maximum H2O2 production yield against relative humidity shown in Fig. 5B. The increase of relative humidity from 40 to 55% enhanced the H2O2 production yield from ~0.5 to ~4 ppm. The H2O2 production yield remains relatively constant above 55%.
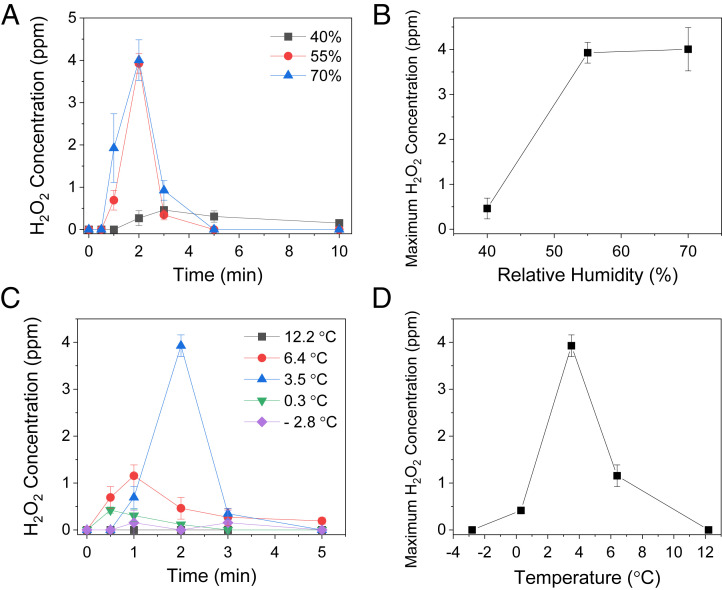
Dependence of H2O2 generation from condensed water microdroplets on the relative humidity and the substrate temperature. (A) Time course of H2O2 concentrations on a bare Si wafer under different relative humidities. (B) Maximum H2O2 concentrations at different relative humidities. (C) Time course of H2O2 concentrations at different temperatures of a bare Si wafer (D) Maximum H2O2 concentrations at different surface temperatures of a bare Si wafer.
We then explored the possible effect of visible-light irradiation on H2O2 production. SI Appendix, Fig. S6 shows there was almost no difference in the H2O2 production yield under different visible-light intensities from 0 to 4 mW/cm2. This result indicates that the H2O2 generation from condensed water microdroplets was not induced or influenced by irradiation with visible light.
We also investigated the effect of the surface temperature at Si wafer surfaces on the H2O2 production yield (Fig. 5 C and D). The temperature of Si surfaces varied from −2.8 to 12.2 °C, while the relative humidity was fixed at 55%. Fig. 4C shows the time courses of H2O2 production yield under different temperatures. At −2.8 °C, condensed water was frozen to form thin ice layers at the surface, and no H2O2 production was detected. At 12.2 °C, almost no condensation occurred because of the lack of enough thermal gradient between water vapor, which was at room temperature, and the Si surface. The H2O2 production yield reached a maximum value of ~3.9 ppm at 3.5 °C (Fig. 5D). These observations show that H2O2 production from condensed water microdroplets is sensitive to substrate surface temperature and humidity but not to ambient light levels.
Discussion
We report the spontaneous production of H2O2 at several ppm levels in water microdroplets formed by the condensation of water vapor on low-temperature surfaces in air. The results in this work suggest that the spontaneous oxidation of water to form H2O2 in water microdroplets is a general phenomenon regardless of how microdroplets are created, either through condensation (bottom up) or atomization (top down). We found that the nucleation and growth processes are important for the H2O2 production yield because these processes determine the density and the size distribution of condensed water microdroplets. Surface temperature and relative humidity are also important factors influencing the production yield.
Although the maximum production yield and the time in which a maximum value appears to vary with the type of surfaces and with environmental factors such as temperature and relative humidity, the overall temporal profile of H2O2 production exhibits a bell-shaped behavior consisting of a simple rise and fall. This observation is consistent with our previous finding that H2O2 is dominantly produced in water microdroplets with diameters less than ~10 µm (6). Fig. 6 A–D presents suggested processes explaining the typical rise and fall of H2O2 production in condensed water microdroplets. Before the initiation of surface cooling, water molecules in the vapor phase do not form any surface nuclei (Fig. 6A). No H2O2 is produced at this stage. As the surface temperature is lowered, small nuclei are formed on the cooled surface (Fig. 6B), and H2O2 starts being generated. Small nuclei grow to form microdroplets through different possible processes, including a simple growth, coalescence, and shedding of larger droplets onto smaller droplets (Fig. 6C). More H2O2 molecules are produced at this stage. It is unclear how these different routes for the growth of microdroplets influence the generation of H2O2 owing to the lack of a method for probing the production of H2O2 from individual microdroplets. As condensed water microdroplets continue to grow to form large droplets, the production of H2O2 decreases and then stops because the size of droplets becomes larger than the typical H2O2 production size limit, ~10 µm (Fig. 6D). At this stage, H2O2 produced in previous stages is diluted; consequently, the overall concentration of H2O2 decreases. Eventually, condensed water forms thin liquid films on the surface, and the H2O2 concentration essentially drops below our detection limit.
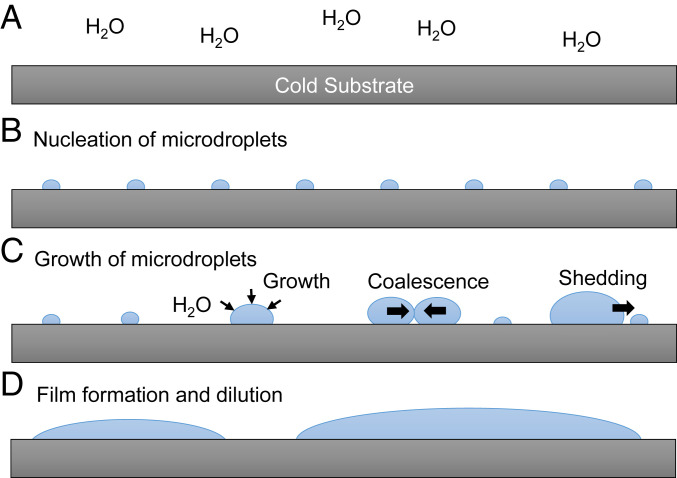
Schematic of the time course of H2O2 formation on cold surfaces by condensation of water vapor: (A) capture of water vapor on cold substrate, (B) nucleation formation, (C) growth of microdroplets, and (D) formation of thin water film and the dilution of H2O2.
These observations provide a clue for enhancing the production yield of H2O2 beyond the ppm level. The increase of the density of micrometer-sized droplets with the delayed growth of these droplets through appropriate surface modification method (23) can lead to the improvement of the H2O2 production yield. This proposition was demonstrated by studies we made with modified Si surfaces, presented in Fig. 4. The etched Si surface exhibited a more rapid rise of H2O2 production yield to reach a maximum value as early as 30 s compared to 2 min in the untreated Si surface. A higher roughness of the etched Si surface (SI Appendix, Fig. S4), which promotes the nucleation (24), may be responsible for the rapid rise of the H2O2 concentration. However, the etched surface also increases surface wettability, which resulted in the formation of thin liquid films. Therefore, the overall H2O2 production yield decreased because of dilution of the previously produced H2O2 (SI Appendix, Fig. S5). In contrast, both nucleation and growth on the hydrophobic Si surface were inefficient compared to the bare Si surface, exhibiting the slower H2O2 formation with lower overall production yield. These observations suggested that to increase H2O2 yield, a better-designed structured surface is needed to promote the nucleation to form smaller droplets without having a liquid film being formed.
The mechanism for the formation of hydrogen peroxide in water microdroplets is presently not known, but some aspects seem well established. It has been shown that OH as well as other reactive oxygen species are present in freshly formed microdroplets (4, 6). It then seems reasonable to assume that H2O2 is formed from the recombination of OH radicals in water. The question is where does the OH come from? Many experiments have shown that water microdroplets surrounded by some hydrophobic medium, such as air or oil, are negatively charged (25, 26), although scrupulously purified water microdroplets are reported to be uncharged (27). The negatively charged water microdroplets have been attributed to the preferential adsorption of OH− at the interface (25, 28, 29), but this has been strongly disputed (30, 31). Another possibility for explaining negative charging is the preferential adsorption at the microdroplet periphery of some unknown anionic surfactant (32), such as the adsorption of bicarbonate anion (HCO3−) from the absorption by water of carbon dioxide (CO2) by water microdroplets in contact with the air (27). Whatever the case, the presence of negatively charged ions at the interface causes the formation of an electric double layer, which in turn gives rise to a strong electric field. Recently, Xiong et al. (14) determined the electric field to be on the order of 107 V/cm at the periphery of a water microdroplet in oil. This was accomplished by measuring the Stark shift of a probe molecule at the microdroplet interface compared to that at the center of the microdroplet. It might then be speculated that this strong electric field at the periphery of the water microdroplet assists the conversion of OH− to OH or promotes a reaction that forms OH. The answer to what is the origin of the OH in water is presently under investigation.
The findings in this work carry the following notable implications. Because the spontaneous water oxidation to generate hydrogen peroxide occurs in water microdroplets formed by the condensation of water vapor, this work provides evidence that the water oxidation in water microdroplets is not caused by any process involved in the atomization of bulk water, for instance, a high-pressure gas nebulization, the friction between water and air, and the electrification of moving water flow over different materials. Because no complex equipment or series of chemical processes are necessary to produce H2O2 and only vapor water is needed as a raw material for the production of H2O2, this work may provide a simple and economical method for on-demand production of H2O2 and other reactive oxygen species, which can be used for green disinfection and sterilization of surfaces.
Moreover, this work may have important implications for atmospheric chemistry because it represents another possible source for making hydrogen peroxide that does not need radiation as a driving energy source. It is well known that rainwater contains H2O2 (33–35). We suggest that some of this might be produced simply by water vapor condensation. At first, it might be thought that the concentration of H2O2 is too small to be important, but if the H2O2 is removed by some chemical reaction, it will be regenerated on the surface of the microdroplet in air. We also note that upon visible and ultraviolet photolysis, H2O2 yields OH radicals. It is thought that hydrogen peroxide plays an important role in the troposphere, such as the photochemical oxidation of sulfur dioxide to sulfuric acid in the formation of acid rain (36).
We also wish to point out that the spontaneous formation of hydrogen peroxide in fogs, mists, and raindrops may be a missing part of the story of how the building blocks of life were formed on Earth. Clearly, this process can play an important role in redox chemistry without the need to have a biological catalyst present. It may also provide a new pathway for the origin of atmospheric oxygen on Earth. Conventionally, atmospheric oxygen is thought to originate in the photocatalytic splitting of vapor water (37). Because H2O2 self-decomposes into H2O and O2, the results in this work suggest that water microdroplets, which are abundant in nature, might be an alternative source of atmospheric oxygen during the prebiotic era. This speculation is supported by our previous study reporting the generation of oxygen atoms in water microdroplets (5). In addition, the results of this study suggest that the biological ecosystem, particularly the microbiome, might be influenced by spontaneously generated reactive oxygen species in water microdroplets.
Materials and Methods
Chemicals.
High-performance liquid chromatography (HPLC)-grade water was purchased from Fisher Scientific Co. Potassium titanium oxalate [K2TiO(C2O4)2·H2O] and 4-carboxyphenylboronic acid were purchased from Sigma-Aldrich.
Microdroplet Generation and Characterization.
A custom-designed chamber was fabricated for maintaining environmental conditions, including temperature and relative humidity, for generating microdroplets formed by water vapor condensation. The surface temperature of wafers was maintained by polyimide resistive heater films and a thermoelectric Peltier cooling module (TEC1-12706), which were connected to a temperature controller (ITC-308, Inkbird Tech). The ~10-g thermal compound paste was applied between the Peltier cooling module and a Si wafer to promote heat transfer. The relative humidity of the environment control chamber was manipulated using a household humidifier and a dehumidifier, which were connected to a programmable plug digital humidity controller (IHC-200, Inkbird Tech). The mist generated from the ultrasonic humidifier was placed away from the condensation module in order to avoid any direct deposit of droplets generated from the humidifier onto substrates. The microdroplet condensation chamber was equipped with an optical microscope (Nikon) for monitoring the condensation on substrates. Image analyses for the size distribution and the number of microdroplets were carried out using ImageJ (NIH). The visible-light irradiance of fluorescent room light and white-light light emitting diode light source was measured at 550 nm using a laser power meter (FieldMate 1098297, Coherent).
Mass Spectrometric Analysis.
Thermo Scientific LTQ Orbitrap XL Hybrid Ion Trap-Orbitrap mass spectrometer was used for mass spectrometric analysis of the reaction products collected from the microdroplet condensate solution and 4-carboxyphenylboronic acid. The volume of 200 μl from the microdroplet condensate solution was mixed with 200 µl of 100 µM 4-carboxyphenylboronic acid to form a solution that was introduced into the mass spectrometer inlet without forming aerosol microdroplets in order to avoid possible reactions occurring in aerosol microdroplets. No voltage was applied to the solution. The heated capillary inlet to the mass spectrometer was maintained at ~275 °C. The capillary and tube lens voltages were set as −44 and −60 V, respectively.
Quantification of H2O2 Production.
The H2O2 concentration in microdroplets was determined by PTO and spectrophotometric analysis with a maximum response at 400 nm. A 0.1 M PTO (K2TiO(C2O4)2·H2O; ≥99.0%, Sigma-Aldrich) solution was prepared. To develop the calibration curve, 200 μL of a H2O2 standard solution with a concentration between 0 and 100 µM was added into the 200-μL PTO solution. From this mixture, a 300-μL aliquot was removed, and the absorbance at 400 nm was measured using a Tecan Infinite M1000 Plate Reader (Tecan Benelux BVBA). An identical procedure was conducted on microdroplet samples where 200 μL of collected microdroplets was combined with PTO. The H2O2 concentration of microdroplet samples was determined from the calibration curve acquired from the standard H2O2 solution. The H2O2 concentration of microdroplets was also confirmed using peroxide test strips (Quantofix, Macherey-Nagel), which covered the range of 0.5–25 ppm H2O2.
Si Surface Treatment.
Undoped Si wafers with 4-in. diameter and 500-µm thickness were used as a base substrate for studies about the dependence of surface conditions on the H2O2 production. Si waters were cleaned prior to each treatment. For preparing a hydrophobic Si surface, wafers were treated with Sigmacote (Sigma-Aldrich) following the manufacturer’s protocol. Briefly, cleaned and dried wafers were covered with undiluted Sigmacote solution for 30 min at room temperature and air dried. Treated wafers were rinsed three times with HPLC-grade water and dried before condensation experiments. Hydrophilic Si wafers were prepared by treating precleaned wafers in an oxygen plasma cleaner at 30 W for 30 s. Hydrophilic wafers were used for condensation experiments within 30 min after the plasma treatment. Etched Si wafers were prepared by deep reactive-ion etching in an inductive coupled plasma (ICP) etcher from Plasma-Therm at the Stanford Nanofabrication Facilities. The samples are etched for 100 cycles using a standard recipe with a Si etch rate of 0.83 µm/cycle without any mask. Each cycle of the recipe includes three main steps: 1) deposition of polymer step (150 sccm of C4F8, 30 sccm of Ar, 25 mTorr, 2,000-W ICP power, 10-V bias voltage, 2.5 s); 2) polymer etch A step (150 sccm SF6, 30 sccm Ar, 40 mTorr, 2,000-W ICP power, 250-V bias voltage, 1.5 s); and 3) Si etch B step (300 sccm SF6, 30 sccm Ar, 75 mTorr, 3,000-W ICP power, 10-V bias voltage, 3.0 s). The pressure of the backside He was maintained at 4 Torr, while temperatures of the electrode, liner, lid, and spool were kept at 15, 70, 150, and 180 °C, respectively.
Roughness Measurement.
The sample roughness measurement was carried out with an atomic force microscope tool from Park XE-70 system at the Stanford Nano Shared Facilities. Standard NSC15 cantilever probe with a typical resonance frequency of 325 kHz and a spring constant of 40 N/m were used in noncontact mode.
Supplementary Material
Supplementary File
Supplementary File
Acknowledgments
H.S.H. acknowledges support from the Volkswagen Group of America. This work was funded by the Air Force Office of Scientific Research through Basic Research Initiative Grant AFOSR FA9550-12-1-0400.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/10.1073/pnas.2020158117/-/DCSupplemental.
Data Availability.
All study data are included in the article and SI Appendix.
References
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.2020158117
Read article for free, from open access legal sources, via Unpaywall:
https://www.pnas.org/doi/pdf/10.1073/pnas.2020158117
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1073/pnas.2020158117
Article citations
pH Affects the Spontaneous Formation of H<sub>2</sub>O<sub>2</sub> at the Air-Water Interfaces.
J Am Chem Soc, 146(38):25889-25893, 16 Sep 2024
Cited by: 0 articles | PMID: 39283298 | PMCID: PMC11440490
Soft X-ray Induced Radiation Damage in Dip-and-Pull Photon Absorption and Photoelectron Emission Experiments.
J Phys Chem C Nanomater Interfaces, 128(34):14381-14387, 20 Aug 2024
Cited by: 0 articles | PMID: 39257861 | PMCID: PMC11382277
Spontaneous Production of I2 at the Surface of Aqueous Iodide Solutions.
J Phys Chem A, 128(32):6739-6744, 02 Aug 2024
Cited by: 0 articles | PMID: 39092462
Microdroplets initiate organic-inorganic interactions and mass transfer in thermal hydrous geosystems.
Nat Commun, 15(1):4960, 11 Jun 2024
Cited by: 0 articles | PMID: 38862499
The role of charge in microdroplet redox chemistry.
Nat Commun, 15(1):3670, 30 Apr 2024
Cited by: 1 article | PMID: 38693110 | PMCID: PMC11519639
Go to all (36) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Spontaneous generation of hydrogen peroxide from aqueous microdroplets.
Proc Natl Acad Sci U S A, 116(39):19294-19298, 26 Aug 2019
Cited by: 108 articles | PMID: 31451646 | PMCID: PMC6765303
The Air-Water Interface of Water Microdroplets Formed by Ultrasonication or Condensation Does Not Produce H2O2.
J Phys Chem Lett, 12(46):11422-11429, 18 Nov 2021
Cited by: 12 articles | PMID: 34792369
Revisiting the Effect of the Air-Water Interface of Ultrasonically Atomized Water Microdroplets on H2O2 Formation.
J Phys Chem B, 126(16):3180-3185, 19 Apr 2022
Cited by: 5 articles | PMID: 35439005
Spontaneous Oxidation in Aqueous Microdroplets: Water Radical Cation as Primary Oxidizing Agent.
Angew Chem Int Ed Engl, 63(17):e202400118, 19 Feb 2024
Cited by: 6 articles | PMID: 38302696
Review
Funding
Funders who supported this work.
DOD | USAF | AFMC | Air Force Office of Scientific Research (1)
Grant ID: AFOSR FA9550-12-1-0400





