Abstract
Objectives
Circulating anti-ENO1 and anti-H2A IgG2 have been identified as specific signatures of LN in a cross-over approach. We sought to show whether the same antibodies identify selected population of patients with LN with potentially different clinical outcomes.Methods
Here we report the prospective analysis over 36 months of circulating IgG2 levels in patients with newly diagnosed LN (n=91) and SLE (n=31) and in other patients with SLE recruited within 2 years from diagnosis (n=99). Anti-podocyte (ENO1), anti-nucleosome (DNA, histone 2 A, histone 3) and anti-circulating proteins (C1q, AnnexinA1-ANXA1) IgG2 antibodies were determined by home-made techniques.Results
LN patients were the main focus of the study. Anti-ENO1, anti-H2A and anti-ANXA1 IgG2 decreased in parallel to proteinuria and normalized within 12 months in the majority of patients while anti-dsDNA IgG2 remained high over the 36 months. Anti-ENO1 and anti-H2A had the highest association with proteinuria (Heat Map) and identified the highest number of patients with high proteinuria (68% and 71% respectively) and/or with reduced estimated glomerula filtration rate (eGFR) (58% for both antibodies) compared with 23% and 17% of anti-dsDNA (agreement analysis). Anti-ENO1 positive LN patients had higher proteinuria than negative patients at T0 and presented the maximal decrement within 12 months.Conclusions
Anti-ENO1, anti-H2A and anti-ANXA1 antibodies were associated with high proteinuria in LN patients and Anti-ENO1 also presented the maximal reduction within 12 months that paralleled the decrease of proteinuria. Anti-dsDNA were not associated with renal outcome parameters. New IgG2 antibody signatures should be utilized as tracers of personalized therapies in LN.Trial registration
The Zeus study was registered at https://clinicaltrials.gov (study number: NCT02403115).Free full text

Serum IgG2 antibody multi-composition in systemic lupus erythematosus and in lupus nephritis (Part 2): prospective study
Abstract
Objectives
Circulating anti-ENO1 and anti-H2A IgG2 have been identified as specific signatures of LN in a cross-over approach. We sought to show whether the same antibodies identify selected population of patients with LN with potentially different clinical outcomes.
Methods
Here we report the prospective analysis over 36 months of circulating IgG2 levels in patients with newly diagnosed LN (n=91) and SLE (n=31) and in other patients with SLE recruited within 2
months of circulating IgG2 levels in patients with newly diagnosed LN (n=91) and SLE (n=31) and in other patients with SLE recruited within 2 years from diagnosis (n=99). Anti-podocyte (ENO1), anti-nucleosome (DNA, histone 2
years from diagnosis (n=99). Anti-podocyte (ENO1), anti-nucleosome (DNA, histone 2 A, histone 3) and anti-circulating proteins (C1q, AnnexinA1-ANXA1) IgG2 antibodies were determined by home-made techniques.
A, histone 3) and anti-circulating proteins (C1q, AnnexinA1-ANXA1) IgG2 antibodies were determined by home-made techniques.
Results
LN patients were the main focus of the study. Anti-ENO1, anti-H2A and anti-ANXA1 IgG2 decreased in parallel to proteinuria and normalized within 12 months in the majority of patients while anti-dsDNA IgG2 remained high over the 36
months in the majority of patients while anti-dsDNA IgG2 remained high over the 36 months. Anti-ENO1 and anti-H2A had the highest association with proteinuria (Heat Map) and identified the highest number of patients with high proteinuria (68% and 71% respectively) and/or with reduced estimated glomerula filtration rate (eGFR) (58% for both antibodies) compared with 23% and 17% of anti-dsDNA (agreement analysis). Anti-ENO1 positive LN patients had higher proteinuria than negative patients at T0 and presented the maximal decrement within 12
months. Anti-ENO1 and anti-H2A had the highest association with proteinuria (Heat Map) and identified the highest number of patients with high proteinuria (68% and 71% respectively) and/or with reduced estimated glomerula filtration rate (eGFR) (58% for both antibodies) compared with 23% and 17% of anti-dsDNA (agreement analysis). Anti-ENO1 positive LN patients had higher proteinuria than negative patients at T0 and presented the maximal decrement within 12 months.
months.
Conclusions
Anti-ENO1, anti-H2A and anti-ANXA1 antibodies were associated with high proteinuria in LN patients and Anti-ENO1 also presented the maximal reduction within 12 months that paralleled the decrease of proteinuria. Anti-dsDNA were not associated with renal outcome parameters. New IgG2 antibody signatures should be utilized as tracers of personalized therapies in LN.
months that paralleled the decrease of proteinuria. Anti-dsDNA were not associated with renal outcome parameters. New IgG2 antibody signatures should be utilized as tracers of personalized therapies in LN.
Trial registration
The Zeus study was registered at https://clinicaltrials.gov (study number: NCT02403115).
Introduction
LN is one of the most serious organ complications of SLE. It affects almost 50% of patients with SLE, corresponding to 25–50 cases in a population of 100 000 [1]. LN is mainly characterized by the deposition of autoantibodies within glomeruli with different grades of proliferative activity [2]. LN may develop de novo or in patients with a previous diagnosis of SLE [3], in which case, substantial modification of therapies with addition of immunosuppressive agents beyond steroids is required.
Many studies have previously attempted to define specific biomarkers of LN, suggesting the anti-dsDNA/anti-nucleosome antibodies as valid tools in the specific diagnosis of LN. In fact, several experimental observations supported their possible role in the pathogenesis of renal involvement: (i) anti-dsDNA were eluted from renal glomeruli of patients with LN [4, 5]; (ii) anti-dsDNA antibodies were shown to target chromatin fragments and non-nucleosome proteins in mesangial cells and in the GBM [4, 6–9]; and (iii) injection of anti-dsDNA IgGs in mice caused the deposition of chromatin-IgG complex within the GBM [10, 11].
However, despite these findings, the evidence of a clinical association between LN and circulating anti-dsDNA antibodies is still weak, and several findings from previous studies have proved contradictory [12–14]. A survey of the literature published before 2002 on the association between circulating anti-dsDNA antibodies in serum and LN indicated a likelihood ratio of only 1.7 indicating that the significance of anti-dsDNA as a means to discriminate patients with and without LN among SLE subjects is low [15]. In the last decade, anti-C1q antibodies have also been proposed as a second autoimmunity biomarker of LN, especially associated with proliferative glomerulonephritis [16, 17].
The primary reason why studies could not definitely demonstrate the specificity of anti-dsDNA antibodies for LN was the non-homogeneity of assays utilized in the different studies (i.e. Farr assays, Crytidia Lucillae, various ELISAs), and their non-specificity in respect to the IgG2 isotype: IgG2 represents, in fact, the prevalent isotype of antibodies micro-eluted from glomeruli of patients with LN and is therefore defined as a nephrogenic isotype [18, 19]. The issues reported here have been faced and addressed by the Part 1 of the study, consisting of a cross-over analysis of nephritogenic antibodies on a large population of 1052 SLE patients with (479) and without (573) LN and subdivided according to the time of disease onset. The laboratory approach consisted of the determination of antibodies of the IgG2 isotype vs the nucleosome components (i.e. anti-dsDNA, anti-Histone2A, anti-Histone3) as well as antibodies vs intracellular antigens (i.e. anti-alpha enolase) (anti-ENO1) and vs circulating proteins (i.e. anti-C1q, anti-Annexin A1) (antiANXA1). Of this panel, anti-ENO1 and anti-H2A IgG2 were associated with high SLEDAI and their presence discriminated between SLE and LN (ROC, Venn diagram); a main finding supporting this association was that high levels of anti-ENO1 and/or anti-H2A IgG2 could be found in 90% of LN patients vs fewer than 3% of SLE. In the second part of the study we defined the clinical meaning of the antibody signature (i.e. high anti-ENO1 and anti-H2A IgG2 vs anti-dsDNA): here, we report the prospective analysis of the serum levels of such antibodies in patients with LN, for a follow-up of 36 months.
months.
Materials and methods
Patients
This part of the study included a proportion of the large cohort of patients described in Part 1: specifically, the new study cohort included 91 patients with incident LN who had been recruited at the begin of the renal flare (T0–1m), 31 SLE patients who had been recruited at the same time and 99 incident SLE who had been recruited during the first and second year from the original diagnosis (T1–24m). The basic pre-requisite for enrolment in the prospective study was patient availability for a 36-month follow up period, during which we obtained serum samples every 6 months. All patients were recruited within the framework of the nationwide collaborative Zeus study https://clinicaltrials.gov (study number: NCT02403115). The data base and collected samples are located at the Giannina Gaslini Institute of Genoa (I) [20].
Inclusion criteria
Inclusion criteria have been reported in Part 1 of the study: age between 18 and 55 years, any sex and the availability of informed consent. Diagnosis of SLE was made according to the American College of Rheumatology systemic lupus classification criteria as revised by the Systemic Lupus International Collaborating Clinics (SLICC) [21]. The initial diagnosis of LN was suspected on the basis of urinary elements such as haematuria, proteinuria, and/or worsening of renal function and was confirmed by immune-histology including immunofluorescence and classical histology staining. For histological evaluation of kidney disease, Dubosq–Brasil solution-fixed tissues were embedded in paraffin, sectioned, and stained with haematoxylin/eosin, Masson’s trichrome, silver methenamine, and periodic–acid Schiff. Routine immunofluorescence studies on frozen sections were performed using anti-human IgG, IgA, IgM, C1q, C3, C4, C4d and fibrinogen antibodies.
years, any sex and the availability of informed consent. Diagnosis of SLE was made according to the American College of Rheumatology systemic lupus classification criteria as revised by the Systemic Lupus International Collaborating Clinics (SLICC) [21]. The initial diagnosis of LN was suspected on the basis of urinary elements such as haematuria, proteinuria, and/or worsening of renal function and was confirmed by immune-histology including immunofluorescence and classical histology staining. For histological evaluation of kidney disease, Dubosq–Brasil solution-fixed tissues were embedded in paraffin, sectioned, and stained with haematoxylin/eosin, Masson’s trichrome, silver methenamine, and periodic–acid Schiff. Routine immunofluorescence studies on frozen sections were performed using anti-human IgG, IgA, IgM, C1q, C3, C4, C4d and fibrinogen antibodies.
Exclusion criteria
Exclusion criteria were the presence of severe infections, malignancies, positivity for chronic HBV or HCV, breast-feeding or pregnancy.
Renal activity stadiation
Renal activity stadiation was performed according to a modification of criteria already proposed by Moroni et al. [22]: (i) renal remission, estimated glomerula filtration rate (eGFR) >60 mL/min, proteinuria <0.3
mL/min, proteinuria <0.3 g/day; (ii) partial renal remission, proteinuria between 0.3 and 3.5
g/day; (ii) partial renal remission, proteinuria between 0.3 and 3.5 g/day; (iii) nephrotic syndrome, proteinuria >3.5
g/day; (iii) nephrotic syndrome, proteinuria >3.5 g/day and serum albumin <3.5
g/day and serum albumin <3.5 g%; (iv) chronic kidney disease (CKD), eGFR <60
g%; (iv) chronic kidney disease (CKD), eGFR <60 mL/min and inactive urinary sediment; (v) end-stage renal failure (ESRD), need of renal replacement therapy. eGFR was calculated by CKD-EPI.
mL/min and inactive urinary sediment; (v) end-stage renal failure (ESRD), need of renal replacement therapy. eGFR was calculated by CKD-EPI.
Groups of controls
The same 182 healthy subjects recruited among the hospital staff already reported in Part 1.
Ethics committee
Before initiation of the study, we obtained written approval of the protocol from the local Independent Ethics Committee (Comitato Etico Regione Liguria) on 24 October 2014 and from the Italian Drug Agency (Agenzia Italiana del Farmaco, AIFA). The study was registered at study number: NCT02403115.
Methods and antibody assays
They have been already described in Part 1 of the study.
Normal limits
Normal limits for all the tests above were calculated from ROC curves; the Cut Off represented the value that minimizes the geometric distance between 100% sensitivity and 100% specificity on the ROC curves [23].
High and low levels
Low levels corresponded to the values between the limit of normality and the median. High levels corresponded to levels higher than the median.
Statistics analysis
Auto-antibodies levels were expressed as median and interquartile range. Difference between antibodies titres at different observational times were determined using Friedman non-parametric test for paired measures. The difference between the variation in antibody levels in the first 12 months of follow up (ΔT0–T12) and variation of proteinuria in the same period were determined for each single antibody using Kruskal–Wallis non-parametric test for unpaired measures. In both cases, data were considered significative at two-tailed P-values ≤0.05 after Dunn’s correction for multiple comparison.
months of follow up (ΔT0–T12) and variation of proteinuria in the same period were determined for each single antibody using Kruskal–Wallis non-parametric test for unpaired measures. In both cases, data were considered significative at two-tailed P-values ≤0.05 after Dunn’s correction for multiple comparison.
Heat Maps were utilized to describe correlations between antibody levels and laboratory parameters (proteinuria and eGFR). In this case, a correlogram based on Spearman’s coefficient indicates correlations on a pseudocolor scale from red (maximal positive correlation), to blue (maximal negative correlation) and to white (o, null), respectively. The dendogram (Supplementary Fig. S3) displays the result of unsupervised hierarchical cluster analysis.
Data were analysed to evaluate the agreement between each antibody positivity/negativity and proteinuria (presence or absence of proteinuria >0.3 g/d or >3.5
g/d or >3.5 g/d) or eGFR (CKD-EPI > or <60 ml/min/1.73m2) for all patients at all times. The results were collected into a contingency table, and the agreement were calculated by dividing the sum of true positive and true negative for the sum of true positive, true negative, false-positive and false-negative. Results were considered significant at two-tailed P-values ≤0.05. All analyses were performed using software package R running the last version available at the time of experiments.
g/d) or eGFR (CKD-EPI > or <60 ml/min/1.73m2) for all patients at all times. The results were collected into a contingency table, and the agreement were calculated by dividing the sum of true positive and true negative for the sum of true positive, true negative, false-positive and false-negative. Results were considered significant at two-tailed P-values ≤0.05. All analyses were performed using software package R running the last version available at the time of experiments.
Results
Renal features of patients with LN
The main clinical characteristics of participants in the prospective study are reported in Table 1: the major cohort that was the focus of the study consisted of 91 incident LN patients who were recruited when demonstrating early signs of renal involvement (T0-1m); 31 incident SLE patients who were recruited at diagnosis and a further 99 recruited within 24 months from diagnosis (T0–12m and T1–24m) represented observational groups. All cohorts were followed for 36
months from diagnosis (T0–12m and T1–24m) represented observational groups. All cohorts were followed for 36 months with yearly blood sampling for the determination of the antibody levels and with continuous routine clinical tests.
months with yearly blood sampling for the determination of the antibody levels and with continuous routine clinical tests.
Table 1
Clinical data relative to patients with incident LN and SLE
| LN (n=91) | SLE (n=130) | |
|---|---|---|
| Sex M/F n(%) | 12(13) / 79(87) | 10(8) / 120(92) |
| Age | 35 (25–43) | 40 (25–54) |
| anti-dsDNA ratio | 3 (0.8–24) | 3 (1–15) |
| SLEDAI | 9 (7–11) | 2 (1–4) |
| C3 | 68 (45–89) | 85 (70–107) |
| C4 | 10 (5–17) | 13 (7–18) |
| Histological class | ||
| I n(%) | 1(1) | |
| II | 8(9) | |
| III | 13(14) | |
| IV | 36(40) | |
| V | 32(35) | |
| VI | 1(1) | |
| Proteinuria at T0 | 2.2 (0.7–4.2) | 0.1 (0.05–0.15) |
| Proteinuria at T12 | 0.5 (0.2–1.2) | 0.1 (0.05–0.14) |
| Proteinuria at T24 | 0.27 (0.2–0.8) | 0.1 (0.05–0.14) |
| eGFR ml/min/1.73m2 at T0 | 93 (71–119) | 110 (97–131) |
| eGFR ml/min/1.73m2 at T12 | 95 (77–122) | 111 (98–134) |
| eGFR ml/min/1.73m2 at T24 | 100 (77–125) | 102 (80–116) |
| Therapy n (%) | ||
| Steroids | 66 (72) | 42 (32) |
| CYC/CYA | 18 (20) | 5 (4) |
| MMF/AZA | 14 (15) | 6 (5) |
| Plaquenil | 26 (29) | 97 (75) |
| RTX | 1 (1) | 1 (0.8) |
LN patients were a part of those patients already presented in Part 1 of the study who were recruited at the onset of the renal disease (T0–1); SLE patients were a part of those patients who were recruited within a year from diagnosis. Data are given as median and interquartile ranges. CYA: ciclosporin A; eGFR: estimated glomerula filtration rate; RTX: rituximab.
One of the main features of the LN study group was that proteinuria had a marked decrease within the first 12 months and it then decreased steadily in the following 24
months and it then decreased steadily in the following 24 months (Supplementary Fig. S1, available at Rheumatology online). eGFR was more stable and maintained the same median and interquartile limits. Therapy mainly consisted of steroids and hydroxylchloroquine in SLE patients and steroids plus cytotoxic drugs or ciclosporin in LN patients (Table 1).
months (Supplementary Fig. S1, available at Rheumatology online). eGFR was more stable and maintained the same median and interquartile limits. Therapy mainly consisted of steroids and hydroxylchloroquine in SLE patients and steroids plus cytotoxic drugs or ciclosporin in LN patients (Table 1).
Serum IgG2 antibody levels during the follow up
Antibody levels at different time points of the study are reported in Figs 1 and and22 for LN patients and in Supplementary Figs S2––S4 (available at Rheumatology online) for SLE patients. Anti-dsDNA IgG2 serum levels were high in LN and SLE patients at T0 compared with healthy people and did not modify during the follow-up to 36 months. Anti-Histone2A and Anti-Histone 3IgG2 serum levels were higher in LN compared with both SLE patients and healthy people at T0; in LN, their serum levels reached normal levels at T12 and maintained stable during the follow-up of 36 months. Only anti-H2 IgG2 presented modifications in the two groups of SLE patients during the follow-up.
months. Only anti-H2 IgG2 presented modifications in the two groups of SLE patients during the follow-up.
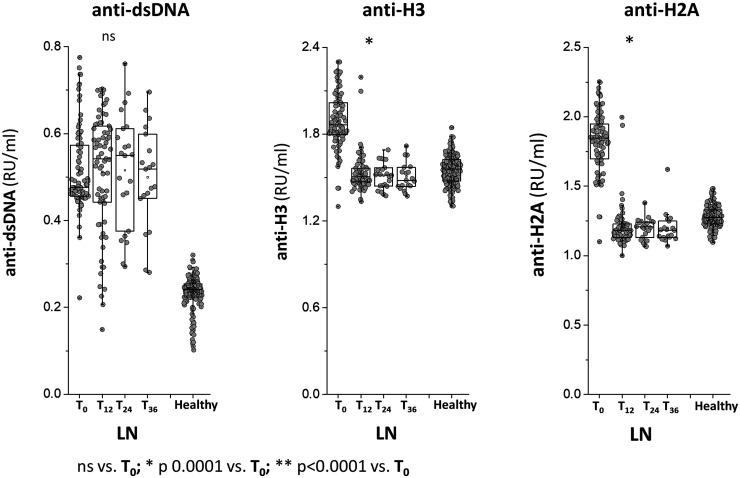
Circulating levels of anti-dsDNA, anti-histone 2 A and anti-histone 3 IgG2
A and anti-histone 3 IgG2
Circulating levels of anti-dsDNA, anti-histone 2A and anti-histone 3 IgG2 were determined in a cohort of 91 LN patients recruited at the time of the diagnosis and were then followed for 36 months. In all cases, antibodies were of the IgG2 isotype and levels were calculated as Relative Intensity value (RU/ml) given the absence of WHO international standards. * indicates a statistical difference with T0. WHO: World Health Organisation.
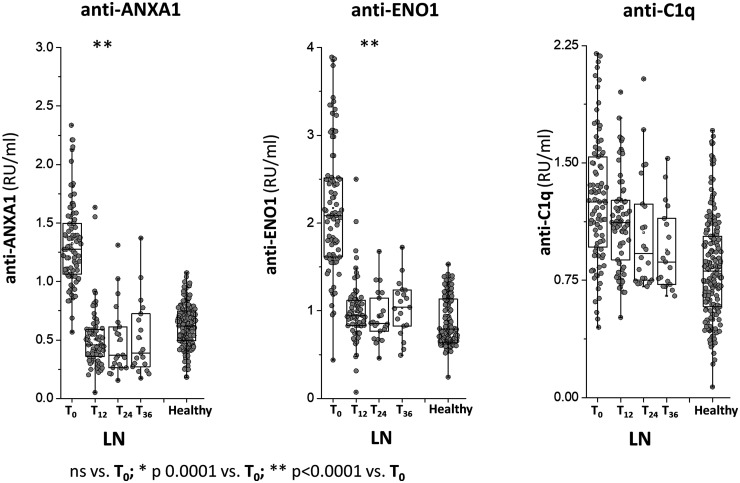
Circulating levels of anti-ENO1, anti-annexin A1 and anti-C1q IgG2
Circulating levels of anti-ENO1, anti-ANXA1 IgG2 and anti-C1q IgG2 were determined in the same group of LN patients of Fig. 1. In all cases, antibodies were of the IgG2 isotype and levels were calculated as Relative Intensity value (RU/ml) given the absence of WHO international standards .* indicates a statistical difference with T0. WHO: World Health Organisation.
Anti-ENO1 and Anti-Annexin A1IgG2 serum levels were higher in LN compared with both SLE patients and healthy people at T0; in LN patients, serum levels of both antibodies decreased to low levels at T12 and maintained stable during the follow-up of 36 months. In the two groups of SLE patients, both antibodies presented minimal variations during the follow-up.
months. In the two groups of SLE patients, both antibodies presented minimal variations during the follow-up.
Anti-C1q IgG2 serum levels were slightly higher in LN compared with healthy subjects and SLE patients at T0 and then decreased in the LN group to reach low levels at T24; in SLE patients, anti-C1q levels were variable, i.e. high at T12 and low at T24.
Decrements of each antibody at various times of the follow-up in patients with LN are summarized in Fig. 3, showing major median decrements at T12m for anti-ENO1 and anti-Annexin A1 (−55% and −70%, respectively) and less evident but constant decrements for anti-H2A and anti-H3 (−35% and −15%, respectively); the median decrement of anti-dsDNA was 0.
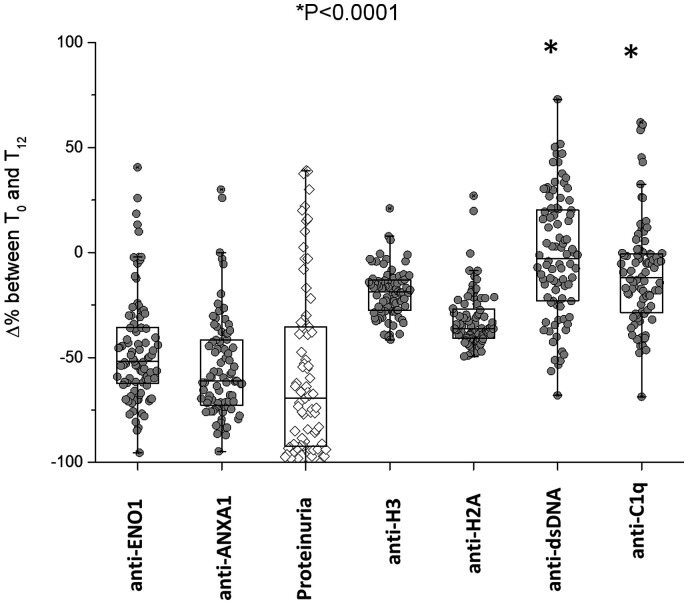
Variations of circulating antibodies within 12 months of follow-up in LN patients
months of follow-up in LN patients
Variations of circulating levels of each antibody from T0 were determined after 12 months of follow-up in LN patients. The decrement of proteinuria is reported for comparison. It is shown that anti-ENO1 and anti-ANXA1 had the maximal decrement and that anti-dsDNA had no variation at all. Results are shown as median and interquartile range. *indicates a statistical difference between anti-dsDNA and anti-ANXA1 with anti-ENO1.
Correlations of antibody levels with proteinuria and renal function
Fig. 4 shows the levels of each antibody of the panel at T0–36m and, in parallel, also shows proteinuria at the same times. Anti-ENO1 and anti-ANXA1 IgG2 decreased at each point similarly to proteinuria, anti-H2A and anti-H3 IgG2 decreased slightly in the first 12 months, while anti-dsDNA IgG2 remained high during the whole period. Because eGFR was stable during the follow-up, the outcome was separate from antibody levels (Supplementary Fig. S5, available at Rheumatology online). The Heat Map shown in Supplementary Fig. S6, available at Rheumatology online, indicates the association between each antibody level and continuous variable of clinical interest (proteinuria and eGFR) or with discrete variables (clinical groups, LN or SLE). The highest association was between proteinuria and anti-ENO1 and anti-H2A; in this context it is clear that the two antibodies are associated with LN (red) and not with SLE (blue).
months, while anti-dsDNA IgG2 remained high during the whole period. Because eGFR was stable during the follow-up, the outcome was separate from antibody levels (Supplementary Fig. S5, available at Rheumatology online). The Heat Map shown in Supplementary Fig. S6, available at Rheumatology online, indicates the association between each antibody level and continuous variable of clinical interest (proteinuria and eGFR) or with discrete variables (clinical groups, LN or SLE). The highest association was between proteinuria and anti-ENO1 and anti-H2A; in this context it is clear that the two antibodies are associated with LN (red) and not with SLE (blue).
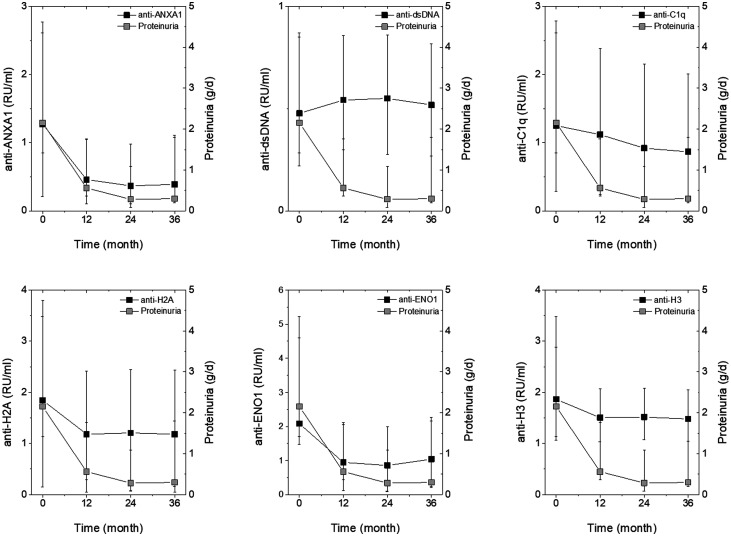
Time course of proteinuria and of circulating levels of antibodies in LN patients
Circulating levels of each antibody were determined every 12 months for 3 years in LN patients. Results are reported together with proteinuria determined at the same time points. Statistical significance of the decrement in circulating antibodies is reported in Figs 1–3. Statistical significance of proteinuria decrements is reported in Supplementary Fig. S1, available at Rheumatology online.
The table of agreement (Supplementary Table S1, available at Rheumatology online) confirms the capability of the same two antibodies to identify the association with proteinuria and eGFR. With this analysis, it is demonstrated that anti-ENO1 identifies 68% and 73% patients with proteinuria >0.3 g/day and 3.5
g/day and 3.5 g/day, respectively and 58% of patients with a reduced eGFR, and that anti-H2A identifies 70%, 71% and 58%, respectively. The performance of anti-dsDNA was by far worse, as it identified 50%, 23% and 17% of patients with proteinuria >0.3
g/day, respectively and 58% of patients with a reduced eGFR, and that anti-H2A identifies 70%, 71% and 58%, respectively. The performance of anti-dsDNA was by far worse, as it identified 50%, 23% and 17% of patients with proteinuria >0.3 g/day, 3.5
g/day, 3.5 g/day and reduced eGFR, respectively.
g/day and reduced eGFR, respectively.
Anti-ENO1 positivity identifies different classes of LN
At T0–1m, anti-ENO1 IgG2 levels were higher than normal people in 90% of the LN cohort; as the unique example among all the antibodies of the panel, anti-ENO1positivity was associated with a higher proteinuria than in the negative group (Fig. 5). On the other hand, the decrement of proteinuria in the anti-ENO1 positive group during the first 12 months of follow-up was more evident than in patients who were anti-ENO1 negative, then leading to normalization of proteinuria. Patients who were positive and negative to other antibodies did not present different decrement of proteinuria (Supplementary Figs S7 and S8, available at Rheumatology online).
months of follow-up was more evident than in patients who were anti-ENO1 negative, then leading to normalization of proteinuria. Patients who were positive and negative to other antibodies did not present different decrement of proteinuria (Supplementary Figs S7 and S8, available at Rheumatology online).
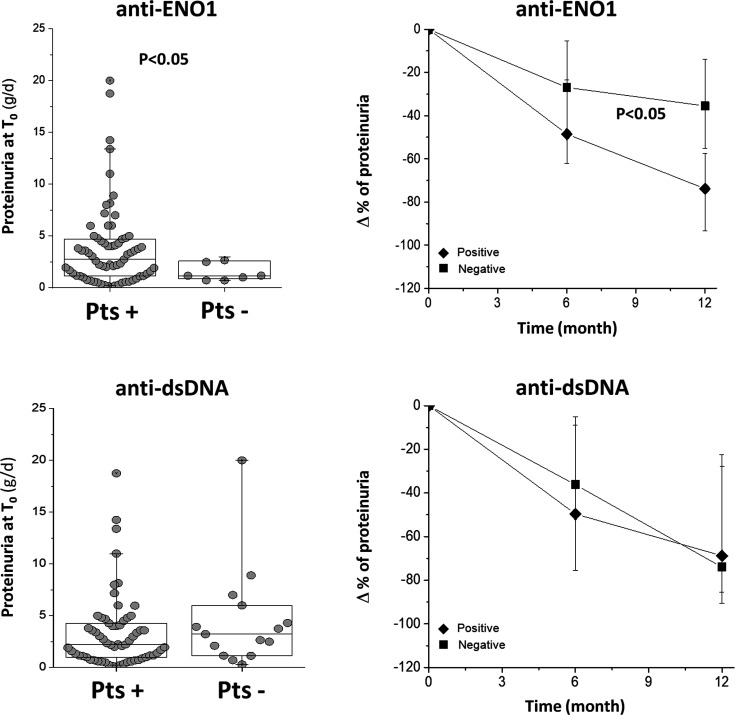
Fig. 5 Proteinuria at T0 and modifications during the follow-up in anti-ENO1 positive and negative LN patients
Proteinuria at T0 and % changes during the first 12 months of follow-up are reported for patients positive and negative for anti-ENO1. In the former case (proteinuria levels) a statistical difference was observed between the two groups. Also, the decrement of proteinuria was more evident in anti-ENO1 positive patients. The same parameters are reported sub-dividing the whole LN group in respect to positivity for anti-dsDNA IgG2.
Discussion
In Part 1 of this study, we reported that several antibodies of IgG2 isotype (anti-dsDNA, anti-H2A, anti-H3, anti-ANXA1 and anti-ENO1) represent a new and specific signature of SLE/LN when compared with healthy subjects. Compared with classical SLE/LN biomarkers, such as anti-dsDNA and anti-C1q IgGs, the new antibodies were much more sensitive in identifying those patients with mild or initial forms of SLE/LN. High levels of anti-ENO1 and anti-H2A IgG2 also discriminated LN from SLE.
The main focus of Part 2 was the modifications of antibody titres in patients with incident LN who were followed longitudinally from the beginning of the disease up to 36 months and the evaluation of how these changes could be associated with the outcome of proteinuria and of renal function. The objective was to define whether any specific antibody could identify cohorts of patients with different renal characteristics.
months and the evaluation of how these changes could be associated with the outcome of proteinuria and of renal function. The objective was to define whether any specific antibody could identify cohorts of patients with different renal characteristics.
The main finding of this study was that each IgG2 antibody had different trends during the follow-up: only a few presented a significant decrease within 12 months from the onset of LN, while others had limited modifications and/or maintained high levels during the whole period of the study. In particular, anti-dsDNA and anti-C1q antibodies belonged to the second group. Anti-histones had a stable but limited decrease (−15% and −35% for anti-H3 and anti-H2A, respectively) while anti-ENO1 and anti-ANXA1 IgG2 levels normalized during the first 12
months from the onset of LN, while others had limited modifications and/or maintained high levels during the whole period of the study. In particular, anti-dsDNA and anti-C1q antibodies belonged to the second group. Anti-histones had a stable but limited decrease (−15% and −35% for anti-H3 and anti-H2A, respectively) while anti-ENO1 and anti-ANXA1 IgG2 levels normalized during the first 12 months and remained within the normal range for the 36
months and remained within the normal range for the 36 months of the follow-up. Normalization in the short term and maintenance of normal levels of both antibodies were paralleled by specular and synchronous normalization of proteinuria. Agreement analysis further confirmed the capability of anti-ENO1 antibodies to identify patients with or without proteinuria and high or low eGFR. Of note, anti-dsDNA had no correlation with any parameter of renal outcome. Overall, these results confirmed what was already observed in Part 1 of the study and further strengthened the poor association of anti-dsDNA with LN in patients followed prospectively for years.
months of the follow-up. Normalization in the short term and maintenance of normal levels of both antibodies were paralleled by specular and synchronous normalization of proteinuria. Agreement analysis further confirmed the capability of anti-ENO1 antibodies to identify patients with or without proteinuria and high or low eGFR. Of note, anti-dsDNA had no correlation with any parameter of renal outcome. Overall, these results confirmed what was already observed in Part 1 of the study and further strengthened the poor association of anti-dsDNA with LN in patients followed prospectively for years.
It is clear that an association between two phenomena such as those here described, i.e. concomitant decrease of proteinuria and of anti-ENO1 circulating levels, does not imply any direct pathogenetic role. In fact, both anti-ENO1 levels and proteinuria may reflect a change of disease activity as in Part 1 of the study has been reported.
However, in spite of the limited number of patients and potentially variable effects of medications, our observations indicate that by monitoring serum levels of anti-ENO1, it is possible to identify a category of LN patients whose clinical outcome is characterized by normalization of proteinuiria within 1 year: an indirect sign of resolution of LN. Outcome prediction for LN patients based on levels of a circulating marker is clinically relevant and offers a practical way of handling and monitoring the effects of therapies using simple laboratory data. Studies on innovative drugs should therefore consider utilizing new antibody fingerprints for increasing the statistical power, thereby opening, de facto, a new season in the search for treatments of LN based on personalized medicine. This evolution would justify the use of a new antibody fingerprint as an adjunctive tool to predict outcome in every single patient.
year: an indirect sign of resolution of LN. Outcome prediction for LN patients based on levels of a circulating marker is clinically relevant and offers a practical way of handling and monitoring the effects of therapies using simple laboratory data. Studies on innovative drugs should therefore consider utilizing new antibody fingerprints for increasing the statistical power, thereby opening, de facto, a new season in the search for treatments of LN based on personalized medicine. This evolution would justify the use of a new antibody fingerprint as an adjunctive tool to predict outcome in every single patient.
How could the different decreases in antibody levels be explained? We speculate that anti-ENO1 and anti-ANXA1 IgG2 respond more rapidly to therapies compared with anti-dsDNA due to different autoimmune processes involved in their generation. Both DNA and Enolase and partially also Annexin A1 [24] are intracellular components and need to be externalized to achieve an immune response. One of the mechanisms for externalization is the formation of Neutrophil Extracellular Traps (NETs), a particular form of apoptosis that contains both DNA and soluble proteins including Enolase and annexins (see below) [25]. Fragments of DNA of different sizes are also transported in microparticles that derive from apopotic cells [26, 27]. Removal of DNA from NETs and from microparticles requires DNases [28, 29] and takes place with different modalities; protein components of NETs are, instead, removed by proteases. It is conceivable that the removal of DNA and of soluble proteins takes place in different times and that the exposure of DNA and proteins to the environment is different, implying different antibody kinetics.
At least for anti-ENO1, recent advances have clarified a possible mechanism for auto-antibody formation that involves an oxidized isoform of ENO1 (with a methionine sulphoxide 93) and NETs [25] that accumulate in serum for a defective removal linked to DNases [30, 31]. Experimental studies support the implication of NETs in lupus, and oxidized components of NETs contributed to determining lupus-like disease in mice; in addition, oxidation inhibitors reduced the severity of renal lesions in the same mouse model of SLE and nephritis [32, 33]. Last but not least, ENO1 and NETs can justify the relevance of the IgG2 isotype of antibodies: studies in vitro with B cells demonstrated that the NETs-DNA-ENO1 complex stimulates TLR8 and TLR9 to proliferate and produce IgG2 (personal unpublished observation). Blocking or reducing NETs formation or enhancing their removal are two potential new ways of intervention to modulate the formation of anti-ENO1 antibodies. TLR8-TLR9 may represent potential targets to reduce the production of nephritogenic anti-ENO1 IgG2 antibodies.
In conclusion, the results of this study offer the opportunity to approach the broad field of LN in terms of personalized medicine, in which newly developed specific biomarkers of renal flare could be utilized in early diagnosis and, above all, for governing treatments. In this view, patients with high anti-ENO1 IgG2 levels have more chances to undergo remission of proteinuria. Determining all these parameters offers chances for a more effective personalized approach to LN. Studies on mechanisms underlying the formation of autoantibodies against enolase and on isotype switching leading to IgG2 offer chances for developing unexplored concepts about the pathogenesis of LN.
Acknowledgements
The Giannina Gaslini Institute (trial sponsor) had provided logistic and financial support to the study through grants from the ministry of health (‘Cinque per mille of IRPEF-Finanziamento della ricerca sanitaria’) and from the ministry of Research (‘Ricerca Corrente’). People working at the project on LN belong to the “Fondazione Malattie Renali del Bambino” of which we acknowledge the financial support. Grant ROL 9849 was received from Compagnia di San Paolo. Thanks to all the Zeus study participants (doctors, nurses, laboratory personnel) and to all patients who accepted to be enrolled. Thanks to Miss Anna Capurro for reviewing grammar and English style. G.M.G. and A.R. were the principal investigators (PI) of the study. They were involved in the study design and coordination, patients’ recruitment, data managing and supervision, manuscript writing and discussion. M.B. had a key role in lab analysis, proteomics, supervision, statistics and data managing; G.C., A.P., M.P. were involved in lab analysis; G.M., R.A.S., F.F., M.F., A.V., L.C., F.P., P.M., F.L., G.Pa., G.Pe., M.B., A.M., G.A.R., P.E., G.M., S.N., L.C., B.T., G.E., I.C., V.B., M.dA., P.F., I.P., G.G., C.M., D.S., F.S., S.V., M.M., A.T., E.V., A.A. were involved in patient recruitment, data managing, manuscript discussion.
Disclosure statement: The authors have declared no conflicts of interest.
Funding: No specific funding was received from any funding agency in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Data availability statement
Specific data are available upon request. Please write to gro.inilsag@ireggihgocramg.
Supplementary data
Supplementary data are available at Rheumatology online.
References
Articles from Rheumatology (Oxford, England) are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/rheumatology/keaa793
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/rheumatology/article-pdf/60/7/3388/38849946/keaa793.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1093/rheumatology/keaa793
Article citations
Anti-C1q antibodies: a biomarker for diagnosis and management of lupus nephritis. A narrative review.
Front Immunol, 15:1410032, 13 Jun 2024
Cited by: 0 articles | PMID: 38938561
Review
Immune podocytes in the immune microenvironment of lupus nephritis (Review).
Mol Med Rep, 28(5):204, 15 Sep 2023
Cited by: 7 articles | PMID: 37711069 | PMCID: PMC10540031
Review Free full text in Europe PMC
[Relationship between anti-ENO1 antibody and systemic lupus erythematosus patients with retinopathy].
Beijing Da Xue Xue Bao Yi Xue Ban, 54(6):1099-1105, 01 Dec 2022
Cited by: 0 articles | PMID: 36533339 | PMCID: PMC9761819
Global Phosphoproteomics Unveils Kinase-Regulated Networks in Systemic Lupus Erythematosus.
Mol Cell Proteomics, 21(12):100434, 27 Oct 2022
Cited by: 1 article | PMID: 36309313 | PMCID: PMC9712766
Current Insights on Biomarkers in Lupus Nephritis: A Systematic Review of the Literature.
J Clin Med, 11(19):5759, 28 Sep 2022
Cited by: 18 articles | PMID: 36233628 | PMCID: PMC9570701
Review Free full text in Europe PMC
Go to all (7) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (2 citations) ClinicalTrials.gov - NCT02403115
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Serum IgG2 antibody multicomposition in systemic lupus erythematosus and lupus nephritis (Part 1): cross-sectional analysis.
Rheumatology (Oxford), 60(7):3176-3188, 01 Jul 2021
Cited by: 8 articles | PMID: 33374003 | PMCID: PMC8487649
Glomerular autoimmune multicomponents of human lupus nephritis in vivo: α-enolase and annexin AI.
J Am Soc Nephrol, 25(11):2483-2498, 01 May 2014
Cited by: 66 articles | PMID: 24790181 | PMCID: PMC4214525
C-reactive protein, immunoglobulin G and complement co-localize in renal immune deposits of proliferative lupus nephritis.
Autoimmunity, 46(3):205-214, 25 Feb 2013
Cited by: 23 articles | PMID: 23331132
A critical view on autoantibodies in lupus nephritis: Concrete knowledge based on evidence.
Autoimmun Rev, 23(5):103535, 27 Mar 2024
Cited by: 3 articles | PMID: 38552995
Review





