Abstract
Free full text

DAMPening mortality in COVID19 – Therapeutic insights from basic cardiometabolic studies on S100A8/A9
With subsequent waves of the COVID19 pandemic driving ever-climbing mortality, we are currently using dexamethasone as one of the few beneficial treatments for patients with COVID19 requiring oxygen supplementation, providing proof of principle that a dysregulated inflammatory response underlies poor outcomes in COVID19. Therefore, optimization of the anti-inflammatory approach is likely to save lives. Although great excitement has surrounded the announcement of the potential effectiveness of several vaccines, not much is known yet about the durability of their efficacy, and several logistical issues surrounding mass vaccination efforts have been raised. Therefore, studies into the pathophysiology of the dysregulated immune-response that drives COVID19, and formal testing of already available anti-inflammatory agents, is still warranted to drive down COVID19-associated mortality.
Recent studies have identified a dysregulated myeloid cell compartment as a key characteristic of severe COVID19, and Silvin et al in their recent publication in Cell reveal that excess production of DAMPs (damage-associated molecular patterns) at least in part drives this association. In a meticulous study using state of the art flow cytometry and single cell sequencing, they showed that elevated neutrophil-derived calprotectin (S100A8/A9) levels are linked to immature neutrophils and non-classical monocytes, which discriminate mild from severe COVID19, directly implicating the S100A8/A9-driven expansion of myeloid cells in poor outcomes in COVID19. S100A8/A9 may therefore be a key agent, leading to a potent activation of NF-κB through interaction with the receptor for advanced glycation endproducts (RAGE) and Toll-like receptor 4 (TLR4), and expansion of inflammatory monocytes, neutrophils and platelets that, in turn, wreak havoc on the diseased lung tissue and contribute to the hypercoagulable state that contributes to COVID19 mortality (Figure).1
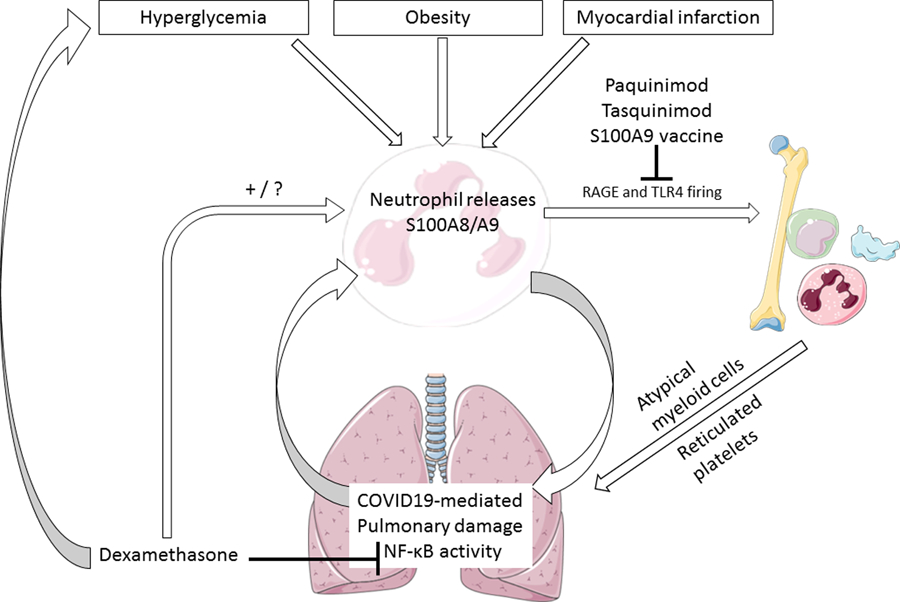
Interactions between obesity, hyperglycemia, dexamethasone and S100A8/A9 in COVID19. RAGE: receptor for advanced glycation endproducts, TLR4: toll-like receptor 4. We hypothesize that dexamethasone may increase S100A8/A9 levels by increasing circulating neutrophil levels. This figure was created using Servier Medical Art templates, which are licensed under a Creative Commons Attribution 3.0 Unported License.
We hypothesize that these findings also explain why individuals with diabetes, obesity and cardiovascular disease are at such high risk of poor outcome by COVID19, and that these findings have several potential implications for clinical practice. We recently found that both chronic and intermittent hyperglycemia lead to increased production of inflammatory monocytes and neutrophils through enhanced S100A8/A9 signaling on RAGE, in a NF-κB dependent pathway.2 We hypothesize that dexamethasone use may further elevate S100A8/A9 levels (Figure), as dexamethasone potently increases circulating neutrophil levels by “softening” them and releasing them into the circulation through rearrangement of the cytoskeleton.3 This is an urgent issue as there currently exists no clear consensus on the effect of dexamethasone on S100A8/A9 levels. We suggest that dexamethasone may be able to reduce at least some of the excess risk of poor outcome in COVID19 infection in diabetes and that its beneficial effects are hampered by increased hyperglycemia leading to further myeloid dysregulation through the S100A8/A9 – RAGE pathway. To our knowledge, few studies have evaluated whether the benefit of dexamethasone is greater, lesser or in fact equal in individuals with diabetes.
The S100A8/A9 – RAGE interaction induced by hyperglycemia and COVID19 may also be relevant in the setting of the very high risk in situ immunothrombosis, causing pulmonary embolism and ischemic strokes in severe COVID19. Of note, a therapeutic vaccine against S100A9 has been developed that inhibits thrombosis in a rodent stroke model without affecting bleeding risk, an intriguing finding of potential interest.4
Based on preclinical studies demonstrating S100A8/A9-driven myeloid cell activation in the setting of hyperglycemia and myocardial infarction, we propose a rationale that argues for further evaluation of the use of dexamethasone in these patient groups to optimize its potential benefits. Therefore, we propose that large observational and interventional studies that monitor the effect of intensified glycemic control on outcomes in individuals with COVID19 and diabetes that are treated with dexamethasone be urgently performed. Furthermore, compounds with S100A8/A9 inhibiting properties already have been developed and tested in humans, such as tasquinimod and paquinimod. We found that paquinimod reduces monocytosis and atherosclerosis induced by S100A8/A9 release driven by hyperglycemia in mice,2 and these findings may also apply to monocyte dysregulation in COVID19. Together with the well-established observation that dexamethasone significantly elevates effective white blood cell counts in patients with COVID (and thus might increase S100A8/A9 levels), we hypothesize that S100A8/A9 inhibition may help to reduce excess mortality in individuals with COVID19 and diabetes, and may further amplify the beneficial effects of dexamethasone in this vulnerable patient group. Because it is debated how much of the on-target effect of tasquinimod is attributable to S100A9 inhibition in the setting of prostate carcinoma (for which the drug has been developed),5 we propose performance of preclinical studies comparing head-to-head paquinimod and tasquinimod in mice exposed to either hyperglycemia or SARS-CoV2, with and without S100A9 deficiency to address this issue. This mouse model is already in use, and after this preclinical efficacy study clinical trials with the best performing compound may be performed in a relatively short timeframe.
These clinical hypotheses should be urgently tested to further curtail the impact of the severe COVID19 pandemic. With only modest efficacy of current direct viral inhibitors, and no clear insight into the impact of a global vaccination program, further targeting of inflammatory dysregulation is likely to remain a cornerstone of treatment of severe COVID19. Although several other hyperinflammatory pathways may act as therapeutic targets in COVID19, such as NETosis, contact activation, and complement activation, inhibition of DAMPs such as S100A8/A9 may contribute to the achievement of this goal as potential drugs in the form of paquinimod and tasquinimod are already available. Performing carefully designed studies with these compounds in vulnerable cardiovascular populations may further reduce mortality by COVID19 in a relatively timely matter.
Acknowledgements
NMJH, BS, PN and AJM wrote and edited the manuscript.
Funding
PRN is supported by NIH (HL137799). AJM is supported by an National Health and Medical Research Council Investigator grant (APP1194329). NMJH is supported by a Diabetes Fonds / Diabetes Onderzoek Nederland grant (2020.10.002).
Footnotes
Conflict of Interest Disclosures
Dr Hanssen has received honorarium from Boehringer Ingelheim. Drs Spaetgens, Nagareddy and Murphy report none.
References.
Full text links
Read article at publisher's site: https://doi.org/10.1161/circulationaha.120.053025
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7940571
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1161/circulationaha.120.053025
Article citations
Neutrophils in cardiovascular disease: warmongers, peacemakers, or both?
Cardiovasc Res, 118(12):2596-2609, 01 Sep 2022
Cited by: 18 articles | PMID: 34534269 | PMCID: PMC9890471
Review Free full text in Europe PMC
Uncovering the genetic links of SARS-CoV-2 infections on heart failure co-morbidity by a systems biology approach.
ESC Heart Fail, 9(5):2937-2954, 21 Jun 2022
Cited by: 11 articles | PMID: 35727093 | PMCID: PMC9349450
Circulating biomarkers of inflammaging as potential predictors of COVID-19 severe outcomes.
Mech Ageing Dev, 204:111667, 25 Mar 2022
Cited by: 10 articles | PMID: 35341896 | PMCID: PMC8949647
Review Free full text in Europe PMC
Hematopoietic progenitor cell liabilities and alarmins S100A8/A9-related inflammaging associate with frailty and predict poor cardiovascular outcomes in older adults.
Aging Cell, 21(3):e13545, 15 Feb 2022
Cited by: 5 articles | PMID: 35166014 | PMCID: PMC8920446
Circulating levels of calprotectin, a signature of neutrophil activation in prediction of severe respiratory failure in COVID-19 patients: a multicenter, prospective study (CalCov study).
Inflamm Res, 71(1):57-67, 30 Oct 2021
Cited by: 17 articles | PMID: 34718856 | PMCID: PMC8556860
Go to all (6) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
FPR1 signaling aberrantly regulates S100A8/A9 production by CD14<sup>+</sup>FCN1<sup>hi</sup> macrophages and aggravates pulmonary pathology in severe COVID-19.
Commun Biol, 7(1):1321, 14 Oct 2024
Cited by: 0 articles | PMID: 39402337 | PMCID: PMC11473795
Emerging roles of neutrophil-borne S100A8/A9 in cardiovascular inflammation.
Pharmacol Res, 161:105212, 28 Sep 2020
Cited by: 32 articles | PMID: 32991974 | PMCID: PMC7755830
Review Free full text in Europe PMC
Lipocalin-2, S100A8/A9, and cystatin C: Potential predictive biomarkers of cardiovascular complications in COVID-19.
Exp Biol Med (Maywood), 247(14):1205-1213, 23 Apr 2022
Cited by: 15 articles | PMID: 35466734 | PMCID: PMC9379606
Pathogenic roles of neutrophil-derived alarmins (S100A8/A9) in heart failure: From molecular mechanisms to therapeutic insights.
Br J Pharmacol, 180(5):573-588, 26 Dec 2022
Cited by: 5 articles | PMID: 36464854
Review





