Abstract
Objective
Postprandial distress syndrome (PDS) and epigastric pain syndrome (EPS) are the two main forms of functional dyspepsia (FD). Probiotics are a promising therapy for FD, but current data remains heterogeneous. This work aims to evaluate a probiotic combination of Lacticaseibacillus rhamnosus LR04 (DSM 16605), Lactiplantibacillus pentosus LPS01 (DSM 21980), Lactiplantibacillus plantarum LP01 (LMG P-21021), and Lactobacillus delbrueckii subsp. delbruekii LDD01 (DMS 22106), alone or together with other pharmacological therapies, for clinical improvement of symptoms associated with FD.Methods
Patients with FD were enrolled and divided into two groups: PDS and EPS. Probiotic alone or combined with prokinetics, antacids, or proton-pump-inhibitors were administered for 30 days. A progressive-score scale was used to evaluate symptoms in all patients at the beginning of the trial and at 15 days after the end of treatment.Results
A cohort of 2676 patients were enrolled (1 357 with PDS; 1 319 with EPS). All patients showed significant improvements in dyspeptic symptoms following treatment. In patients with PDS, probiotic alone resulted in the lowest prevalence of symptoms following treatment, while patients with EPS showed no clear between-treatment differences.Conclusions
Dyspeptic symptoms were reduced following treatment in all patients.Free full text

Evaluation of main functional dyspepsia symptoms after probiotic administration in patients receiving conventional pharmacological therapies
Abstract
Objective
Postprandial distress syndrome (PDS) and epigastric pain syndrome (EPS) are the two main forms of functional dyspepsia (FD). Probiotics are a promising therapy for FD, but current data remains heterogeneous. This work aims to evaluate a probiotic combination of Lacticaseibacillus rhamnosus LR04 (DSM 16605), Lactiplantibacillus pentosus LPS01 (DSM 21980), Lactiplantibacillus plantarum LP01 (LMG P-21021), and Lactobacillus delbrueckii subsp. delbruekii LDD01 (DMS 22106), alone or together with other pharmacological therapies, for clinical improvement of symptoms associated with FD.
Methods
Patients with FD were enrolled and divided into two groups: PDS and EPS. Probiotic alone or combined with prokinetics, antacids, or proton-pump-inhibitors were administered for 30 days. A progressive-score scale was used to evaluate symptoms in all patients at the beginning of the trial and at 15 days after the end of treatment.
Results
A cohort of 2676 patients were enrolled (1 357 with PDS; 1
357 with PDS; 1 319 with EPS). All
patients showed significant improvements in dyspeptic symptoms following
treatment. In patients with PDS, probiotic alone resulted in the lowest
prevalence of symptoms following treatment, while patients with EPS showed
no clear between-treatment differences.
319 with EPS). All
patients showed significant improvements in dyspeptic symptoms following
treatment. In patients with PDS, probiotic alone resulted in the lowest
prevalence of symptoms following treatment, while patients with EPS showed
no clear between-treatment differences.
Conclusions
Dyspeptic symptoms were reduced following treatment in all patients.
Introduction
Functional gastrointestinal (GI) disorders are typical GI symptom complexes that can arise from different GI tract regions.1 Chronic symptoms that can be linked to functional GI disorders are commonly found in 10–30% of the population, and the majority of these patients have no evidence of organic causes and live in industrialized countries.2,3 Functional GI disorders are currently assigned to four categories: (I) functional dyspepsia (FD), comprising postprandial distress syndrome (PDS) and epigastric pain syndrome (EPS); (II) belching disorders; (III) chronic nausea and vomiting disorders, including chronic nausea vomiting syndrome, cyclic vomiting syndrome, and cannabinoid hyperemesis syndrome; and (IV) rumination syndrome.2 The prevalence of FD, calculated using large population-based studies in adults, ranges from 10% to 30% worldwide,4 while in children, prevalence varies between 3.5% and 27%.5 In the USA alone, social and economic costs are estimated to be approximately $18.4 billion/year for adults and $5.79 billion/year for children.4,6
Functional dyspepsia is a chronic GI disorder defined by upper abdominal symptoms originating from the gastroduodenal region and characterized by the absence of morphological disease on routine investigations, including upper GI endoscopy.1–3,7,8 The Rome IV criteria define dyspepsia as any combination of four specific symptoms: (I) postprandial filling; (II) early satiety; (III) epigastric pain; and (IV) epigastric burning. Such symptoms must be severe enough to interfere with the patient’s normal activities and have to occur with a frequency of at least 3 days per week over 3 months, with an onset of at least 6 months previously.2 In a recent publication, FD symptoms were reported with the following prevalence: epigastric pain and burning (60–70%), postprandial filling (80%), early satiety (60–70%), epigastric distension (80%), nausea (60%), and vomiting (40%).9 Diagnosis of FD is based on identification of the appropriate symptom and a negative upper GI endoscopy, which remains the gold standard to confirm the absence of lesions. Up to 75% of GI endoscopies in patients with known dyspeptic symptoms are confirmed to be healthy, and 20% show erosive oesophagitis.7
The Rome IV criteria also define the two subgroups of FD: (I) PDS; and (II) EPS. Postprandial filling and early satiety are the two pivotal symptoms associated with PDS. The presence of one of the two markers is sufficient for a final diagnosis of PDS, but according to the Rome IV criteria, it should occur at least three times a week.1,7,10 Secondary symptoms can be epigastric pain (immediately after meals), epigastric burning, upper abdominal swelling, and nausea. At the same time, epigastric pain and burning are the cardinal symptoms linked to EPS. As described for PDS diagnosis, the presence of one of the two specific markers, at least once a week, is also sufficient for diagnosis of EPS. PDS is considered a gastric motility disorder, together with impaired gastric accommodation, delayed gastric emptying, or gastric hypersensitivity to distension.7 To date, knowledge regarding EPS remains less well developed, but some authors have proposed that symptoms may be related to acid exposure, Helicobacter pylori infection, and duodenal hypersensitivity to lipids or acids.11–15 As stated by Oh et al.,16 the currently prescribed pharmacological therapies to treat FD-positive patients include: acid suppressant (e.g. proton-pump inhibitor, histamine type 2 receptor antagonists); prokinetics (e.g. dopamine D2 receptor antagonists, 5-hydroxytryptamine receptor 4 agonists, and motilin agonists); and antacids (e.g. sucralfate). While proton-pump inhibitors should be recommended as a first-line treatment for patients with EPS, prokinetics can be useful for patients with PDS.16 The availability of such different pharmacological therapies is one of the most important clinical problems to face; due to the natural chronicization of FD-related symptoms, physicians tend to prescribe various treatments simultaneously.17
The use of probiotics to control FD is a new treatment recently introduced in clinical studies.18 Probiotics are defined as ‘live microorganisms which, when administered in adequate amounts, confer a health benefit on the host’.19 The most known and fundamental mechanisms of action include antimicrobial molecule production, exclusion of pathogens through binding, competition for nutrients, and modulation of the immune system.20 Treatment with Lacticaseibacillus rhamnosus (formerly Lactobacillus rhamnosus) and Lactobacillus acidophilus has been shown to alleviate FD symptoms in children.5 In adults, the administration of probiotic yogurt containing Lactobacillus gasseri OLL2716 (LG21 yogurt) improved the symptoms of postprandial distress.21 One advantage exerted by the administration of probiotics as a treatment to control FD is the lack of adverse events reported in the available literature.18
The aim of the present study was to evaluate the effects of a probiotic combination of L. rhamnosus LR04 (DSM 16605), Lactiplantibacillus pentosus LPS01 (DSM 21980, formerly Lactobacillus pentosus), Lactiplantibacillus plantarum LP01 (LMG P-21021, formerly Lactobacillus plantarum), and Lactobacillus delbrueckii subsp. delbruekii LDD01 (DMS 22106) with N-acetylcysteine, alone or combined with conventional pharmacological therapies, in the clinical improvement of symptoms associated with FD.
Patients and methods
Study population and design
This pilot clinical study was conducted in Italian territory (with data analysed at the Department of Microbiology, University of Milan, Italy) between June 2018 and June 2019, and included sequentially enrolled outpatients with FD. Patient data were collected through a specific self-completed questionnaire that was dispensed to each patient during hospital visits at two study time-points, described below. Patients’ responses were entered into an online electronic repository by medical doctors (Gastrobiota Group). Patients were included based on typical FD symptoms reported during the initial visit or on the medical database, and were divided into the two most common forms of FD: patients with PDS (group A) and patients with EPS (group B). Patients whose existing medical records reported gastric or colon cancers, peptic ulcer diseases, pancreaticobiliary disease, thyroid disorder, and/or positive result for H. pylori by breath test, biopsy cultures, or faecal antigen test, were excluded from the study.
This study received ethics approval from the Italian Society of Digestive Endoscopy (SIED) and the Gastrobiota Group. Written informed consent was obtained from each study participant during the first visit.
Treatments, questionnaires and outcomes
All patients were randomly assigned to receive Abivisor probiotic formulation
(AURORA Biofarma, Milan, Italy) containing L. rhamnosus LR04
(DSM 16605; ≥109 Colony Forming Units [CFU]/Active Fluorescence Units
[AFU]), L. pentosus LPS01 (DSM 21980; ≥8 ×
× 108
CFU/AFU), L. plantarum LP01 (LMG P-21021; ≥3
108
CFU/AFU), L. plantarum LP01 (LMG P-21021; ≥3 ×
× 109
CFU/AFU), and L. delbrueckii subsp. delbruekii LDD01 (DMS
22106; ≥2
109
CFU/AFU), and L. delbrueckii subsp. delbruekii LDD01 (DMS
22106; ≥2 ×
× 108 CFU/AFU) with N-acetylcysteine, at a recommended
dosage of ≥5
108 CFU/AFU) with N-acetylcysteine, at a recommended
dosage of ≥5 ×109 CFU/AFU per day, alone or in combination with a
standard pharmacological therapy for FD (proton-pump inhibitors, prokinetics, or
antacid) for 30 days. All bacterial strains included in the probiotic
formulation are patented by Probiotical SpA (Novara, Italy), and were selected
for their particular resistance to the acidic pH of the stomach and for their
synergy. When co-cultured, all of the combined strains had an optimal growth
rate (in vitro data not shown).
×109 CFU/AFU per day, alone or in combination with a
standard pharmacological therapy for FD (proton-pump inhibitors, prokinetics, or
antacid) for 30 days. All bacterial strains included in the probiotic
formulation are patented by Probiotical SpA (Novara, Italy), and were selected
for their particular resistance to the acidic pH of the stomach and for their
synergy. When co-cultured, all of the combined strains had an optimal growth
rate (in vitro data not shown).
A clinical symptom questionnaire, that had been previously approved by the Gastrobiota Group, was delivered to each patient during a hospital visit at the start of the study (T0) and at 15 days following the end of probiotic treatment (T1). The questionnaire was delivered by a clinician in a readable form for the patient, and comprised a comprehensive list of questions and a simple progressive score scale (from absent to severe) for severity of clinical symptoms.
The primary study outcome was evaluation of the presence of two specific clinical symptoms associated with PDS (postprandial filling and early satiety) and with EPS (epigastric pain and epigastric burning) at the beginning of the study (T0), and at the end of the study (T1). The secondary outcome was evaluation of the best therapy between probiotic alone or in combination with other pharmaceutical approaches, in terms of the capacity to ameliorate or eliminate the specified FD symptoms, and also secondary symptoms.
Statistical analyses
Data are presented as n or % prevalence. Statistical analyses,
together with graphic representation, were performed using GraphPad Prism
software, version 8.01 for Windows (GraphPad Software®, San Diego, CA, USA;
www.graphpad.com). Differences in symptom prevalence between the
two time points were analysed using χ2-test (or Yates' continuity
corrected χ2-test). Differences in symptom prevalence between
treatment groups was analysed using χ2-test. A
P-value <0.05 was considered statistically significant.
<0.05 was considered statistically significant.
Results
Overall data
A total of 2676 patients with clinical symptoms related to FD were enrolled in
the study. Group A (PDS) comprised 1 357/2676 patients (50.7%), while group B
(EPS) comprised 1
357/2676 patients (50.7%), while group B
(EPS) comprised 1 319/2676 patients (49.3%). Pharmacological therapies
administered before and during this study are summarised in Table 1 and Table 2. Patients who
dropped out of the study and did not attend the second visit at T1 comprised 729
patients (53.7%) in the PDS group and 799 patients (60.5%) in the EPS group.
319/2676 patients (49.3%). Pharmacological therapies
administered before and during this study are summarised in Table 1 and Table 2. Patients who
dropped out of the study and did not attend the second visit at T1 comprised 729
patients (53.7%) in the PDS group and 799 patients (60.5%) in the EPS group.
Table 1.
Ongoing pharmacological therapies in patients with postprandial distress syndrome (group A) or epigastric pain syndrome (group B), assessed at enrolment.
Study group | ||||
|---|---|---|---|---|
A | B | |||
n  = = 1357 1357 | n  = = 1319 1319 | |||
| Ongoing pharmacological therapy | n | % | n | % |
| Proton-pump inhibitors | 337 | 24.8 | 393 | 29.8 |
| Prokinetics | 173 | 12.7 | 71 | 5.4 |
| Antacids | 139 | 10.2 | 196 | 14.9 |
| Sucralfate | 22 | 1.6 | 34 | 2.6 |
| Other | 27 | 1.9 | 31 | 2.4 |
| Combination | 85 | 6.3 | 134 | 10.1 |
| No pharmacological therapy | 574 | 42.3 | 460 | 34.9 |
Table 2.
Experimental therapies prescribed to patients with postprandial distress syndrome (group A) and patients with epigastric pain syndrome (group B) during the study, assessed at T0 (start of the study period) and at T1 (15 days following the end of the 30-day treatment period).
Study group | ||||||||
|---|---|---|---|---|---|---|---|---|
A | B | |||||||
T0 n n = = 1357 1357 | T1 n n = = 628 628 | T0 n n = = 1319 1319 | T1 n n = = 520 520 | |||||
| Experimental therapy | n | % | n | % | n | % | n | % |
| Probiotic | 498 | 36.7 | 270 | 43 | 269 | 20.4 | 131 | 25.2 |
| Probiotic plus proton-pump inhibitor | 390 | 28.7 | 131 | 20.9 | 725 | 55 | 268 | 51.5 |
| Probiotic plus prokinetics | 327 | 24.1 | 146 | 23.2 | 152 | 11.5 | 58 | 11.2 |
| Probiotic plus antacids | 142 | 10.5 | 81 | 12.9 | 173 | 13.1 | 63 | 12.1 |
Probiotic was administered alone or in combination with proton-pump inhibitors, prokinetics, or antacids, as reported in Table 2.
Primary outcomes
Group A (PDS)
Statistics of the two main symptoms in patients with PDS treated with the
different pharmacological combinations (Table 2), are summarised in Figure 1. Probiotics
alone was administered to 498/1 357 patients (36.7%) at T0. The symptom of
postprandial filling was absent in 73/498 patients (14.6%) at T0 and 193/270
patients (71.5%) at T1 (P
357 patients (36.7%) at T0. The symptom of
postprandial filling was absent in 73/498 patients (14.6%) at T0 and 193/270
patients (71.5%) at T1 (P <
< 0.0001; Figure 1A). Early satiety showed a
similar trend in prevalence, and was absent in 13.8% of patients at T0 and
69.6% of patients at T1 (P
0.0001; Figure 1A). Early satiety showed a
similar trend in prevalence, and was absent in 13.8% of patients at T0 and
69.6% of patients at T1 (P <
< 0.0001; Figure 1B).
0.0001; Figure 1B).
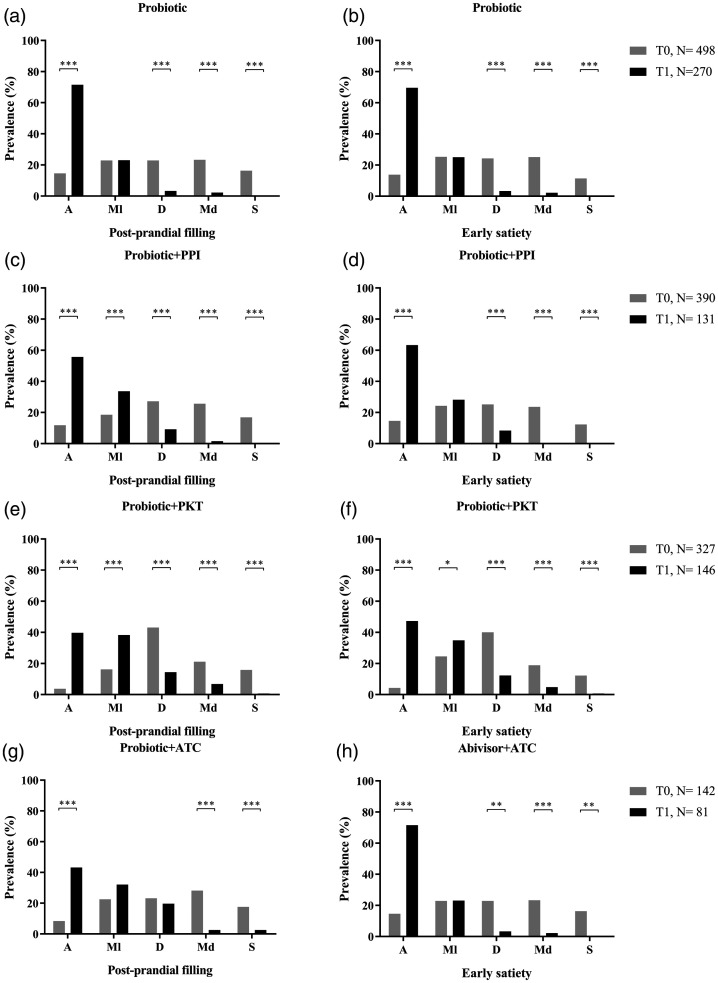
Analysis of postprandial filling and early satiety in patients with
postprandial distress syndrome treated for 30 days with one of four
pharmacological combinations: (a and b) probiotic alone; (c and d)
probiotic plus proton-pump inhibitor; (e and f) probiotic plus
prokinetics; and (g and h) probiotic plus antacid. T0, baseline
timepoint; T1, 15 days following completion of 30-day treatment
period; A, absent; Ml, mild; D, discreet; Md, moderate; S, severe;
PPI, proton-pump inhibitor; PKT, prokinetics; ATC, antacids:
*P ≤
≤ 0.05; **P
0.05; **P <
< 0.01;
***P
0.01;
***P <
< 0.001.
0.001.
Probiotics combined with proton-pump inhibitor was given to 390/1 357
patients (28.7%). Both postprandial filling and early satiety showed a
statistically significant improvement between the beginning and end of
treatment. Postprandial filling was absent in 46/390 patients (11.8%) at T0
and 73/131 patients (55.7%) at T1 (P
357
patients (28.7%). Both postprandial filling and early satiety showed a
statistically significant improvement between the beginning and end of
treatment. Postprandial filling was absent in 46/390 patients (11.8%) at T0
and 73/131 patients (55.7%) at T1 (P <
< 0.0001; Figure 1C), while
early satiety was absent in 14.6% of patients at T0 and 63.6% at T1
(P
0.0001; Figure 1C), while
early satiety was absent in 14.6% of patients at T0 and 63.6% at T1
(P <
< 0.0001; Figure 1D).
0.0001; Figure 1D).
Probiotics plus prokinetics were administered to 327/1 357 patients (24.1%).
This combination was shown to significantly improve the absence of
postprandial filling and early satiety from T0 to T1 (Figure 1E and 1F). Postprandial
filling was absent in 12/327 patients (3.7%) at T0, and this increased to
58/146 patients (39.7%) at T1 (P
357 patients (24.1%).
This combination was shown to significantly improve the absence of
postprandial filling and early satiety from T0 to T1 (Figure 1E and 1F). Postprandial
filling was absent in 12/327 patients (3.7%) at T0, and this increased to
58/146 patients (39.7%) at T1 (P <
< 0.0001). A
statistically significant increase in the absence of early satiety was
reported by patients between T0 (14.6%) and T1 (63.6%;
P
0.0001). A
statistically significant increase in the absence of early satiety was
reported by patients between T0 (14.6%) and T1 (63.6%;
P <
< 0.0001).
0.0001).
The last pharmacological combination (probiotic plus antacid) was prescribed
to 142/1 357 patients (10.5%). Postprandial filling was absent in 12/142
patients (8.4%) at T0 and 35/81 patients (43.2%) at T1
(P
357 patients (10.5%). Postprandial filling was absent in 12/142
patients (8.4%) at T0 and 35/81 patients (43.2%) at T1
(P <
< 0.0001; Figure 1G), and the absence of early
satiety also increased from 12.7% at T0 to 51.8% at T1
(P
0.0001; Figure 1G), and the absence of early
satiety also increased from 12.7% at T0 to 51.8% at T1
(P <
< 0.0001; Figure 1H).
0.0001; Figure 1H).
For all pharmacological treatments, all patients reported the presence of a severe postprandial filling or early satiety at T1.
Group B (EPS)
Statistical analyses of the two main symptoms in patients with EPS treated
with different pharmacological combinations (Table 2) are shown in Figure 2. Probiotics
alone were administered to 269/1 319 patients (20.4%) at T0 (Figure 2A and B).
Epigastric pain was absent in 38/269 patients (14.1%) at T0 and absent in
70/131 patients (53.4%) at T1 (P
319 patients (20.4%) at T0 (Figure 2A and B).
Epigastric pain was absent in 38/269 patients (14.1%) at T0 and absent in
70/131 patients (53.4%) at T1 (P <
< 0.0001). Epigastric
burning showed a similar increase in absence of symptoms, with absence
prevalence of 26.4% at T0 and 65.1% at T1
(P
0.0001). Epigastric
burning showed a similar increase in absence of symptoms, with absence
prevalence of 26.4% at T0 and 65.1% at T1
(P <
< 0.0001).
0.0001).
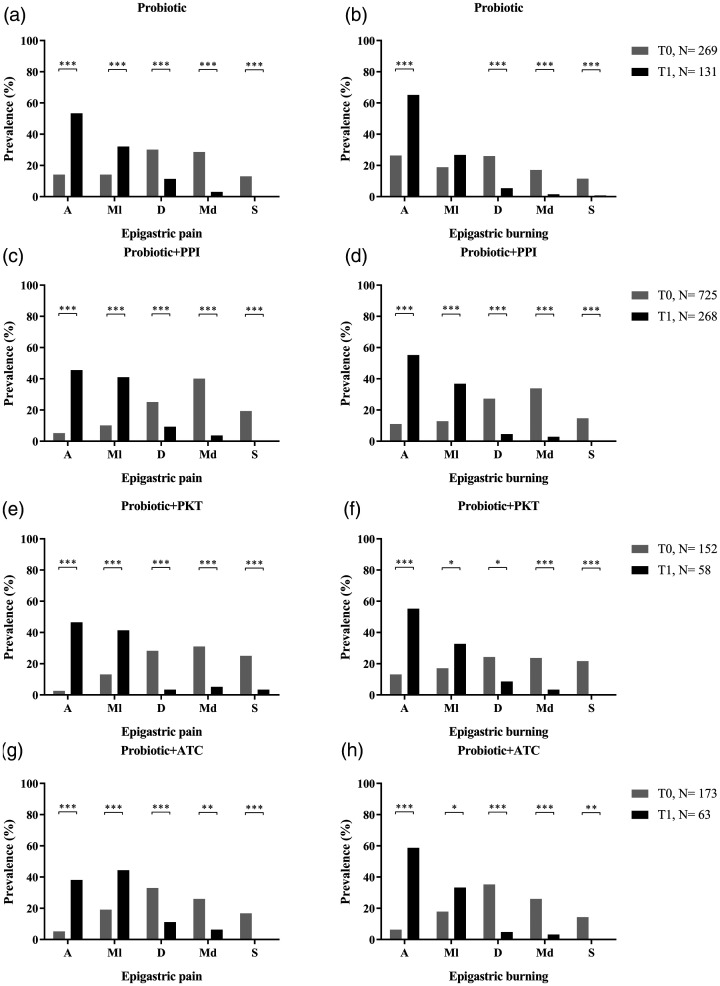
Analysis of epigastric pain and epigastric burning in patients with
epigastric pain syndrome treated for 30 days with one of four
pharmacological combinations: (a and b) probiotic alone; (c and d)
probiotic plus proton-pump inhibitor; (e and f) probiotic plus
prokinetics; and (g and h) probiotic plus antacid. T0, baseline
timepoint; T1, 15 days following completion of 30-day treatment
period; A, absent; Ml, mild; D, discreet; Md, moderate; S, severe;
PPI, proton-pump inhibitor; PKT, prokinetics; ATC, antacids:
*P ≤
≤ 0.05; **P
0.05; **P <
< 0.01;
***P
0.01;
***P <
< 0.001.
0.001.
The combination of probiotics plus proton-pump inhibitor was given to
725/1 319 patients (54.9%). A statistical improvement between T0 and T1 was
observed in both epigastric pain and epigastric burning (Figure 2C and D).
Epigastric pain was absent in 38/725 patients (5.2%) at T0 and 122/268
patients (45.5%) at T1 (P
319 patients (54.9%). A statistical improvement between T0 and T1 was
observed in both epigastric pain and epigastric burning (Figure 2C and D).
Epigastric pain was absent in 38/725 patients (5.2%) at T0 and 122/268
patients (45.5%) at T1 (P <
< 0.0001), and a similar
statistically significant improvement in symptoms was seen in terms of
epigastric burning, which was shown to be absent in 11% of patients at T0
and 55.2% of patients at T1 (P
0.0001), and a similar
statistically significant improvement in symptoms was seen in terms of
epigastric burning, which was shown to be absent in 11% of patients at T0
and 55.2% of patients at T1 (P <
< 0.0001).
0.0001).
Probiotics plus prokinetics were given to 152/1 319 patients (11.5%), and
symptoms of epigastric pain and epigastric burning were shown to be
clinically improved from the start to the end of the study. At T0, 14/152
patients (2.6%) reported the absence of epigastric pain, compared with 27/58
patients (46.5%) at T1 (P
319 patients (11.5%), and
symptoms of epigastric pain and epigastric burning were shown to be
clinically improved from the start to the end of the study. At T0, 14/152
patients (2.6%) reported the absence of epigastric pain, compared with 27/58
patients (46.5%) at T1 (P <
< 0.0001; Figure 2E). A reported absence of
epigastric burning showed a similar trend between T0 (13.1% absence) and T1
(55.2% absence; P
0.0001; Figure 2E). A reported absence of
epigastric burning showed a similar trend between T0 (13.1% absence) and T1
(55.2% absence; P <
< 0.0001; Figure 2F).
0.0001; Figure 2F).
The last pharmacological combination (probiotic plus antacid) was prescribed
to 173/1 319 patients (13.1%). The absence of epigastric pain increased from
9/173 patients (5.2%) at T0 to 24/63 patients (38.1%) at T1
(P
319 patients (13.1%). The absence of epigastric pain increased from
9/173 patients (5.2%) at T0 to 24/63 patients (38.1%) at T1
(P <
< 0.0001; Figure 2G), and this improving trend
was similar regarding absence of epigastric burning (11/173 patients [12.7%]
at T0 and 37/63 patients [51.8%] patients at T1;
P
0.0001; Figure 2G), and this improving trend
was similar regarding absence of epigastric burning (11/173 patients [12.7%]
at T0 and 37/63 patients [51.8%] patients at T1;
P <
< 0.0001; Figure 2H).
0.0001; Figure 2H).
Secondary outcomes
Minor symptoms related to FD
Minor symptoms related to PDS (epigastric pain, epigastric burning, and abdominal swelling), and those of EPS (postprandial filling, early satiety, and abdominal swelling) were evaluated as described for the pivotal symptoms.
For all PDS-positive patients, epigastric pain and epigastric burning showed similar positive trends in the absence of severe symptoms between the two time-points for all pharmacological combinations. Abdominal swelling showed variable results at T1; two of the treatment combinations (probiotic plus prokinetics, and probiotic plus antacid) did not show the level of improvement in abdominal swelling between T0 and T1 that was observed for other clinical signs and treatment combinations (see Supplemental Figure 1 and Supplemental Table 1A).
All four pharmacological combinations prescribed to patients with EPS improved the perception of all minor symptoms at T1 (see Supplemental Figure 2 and Supplemental Table 1B).
Overall pharmacological combination
The prevalence of absence of clinical symptoms at T1 was compared between all
pharmacological combinations used to treat clinical symptoms associated with
PDS and EPS (Figure
3). In patients with PDS (group A), probiotic alone significantly
reduced the prevalence of all symptoms (Supplemental Table 1A). At T1, the
use of the probiotic alone showed the highest prevalence of no postprandial
filling symptoms, followed by probiotic combined with proton-pump inhibitor,
then probiotic combined with either prokinetics or antacids (all
P <
< 0.01; Figure 3A). Probiotic alone also
showed the highest prevalence of no epigastric burning compared with all
other treatment groups (P
0.01; Figure 3A). Probiotic alone also
showed the highest prevalence of no epigastric burning compared with all
other treatment groups (P <
< 0.01), and the highest
prevalence of no early satiety symptoms and no abdominal swelling versus
probiotic plus prokinetics or antacids (P
0.01), and the highest
prevalence of no early satiety symptoms and no abdominal swelling versus
probiotic plus prokinetics or antacids (P <
< 0.01; Figure 3A). Probiotic
alone showed a higher prevalence of no epigastric pain, a minor sign in
patients with PDS, versus probiotic combined with antacids
(P
0.01; Figure 3A). Probiotic
alone showed a higher prevalence of no epigastric pain, a minor sign in
patients with PDS, versus probiotic combined with antacids
(P =
= 0.0042).
0.0042).
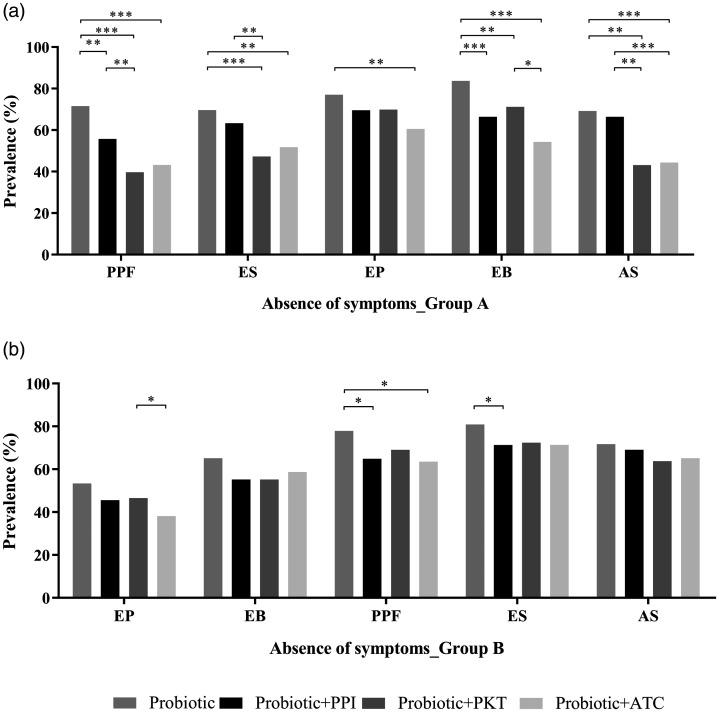
Comparison of the prevalence of absence of symptoms following
treatment (T1) between different therapy groups in patients with (a)
postprandial distress syndrome (group a); and (b) epigastric pain
syndrome (group b). PPF, postprandial filling; ES, early satiety;
EP, epigastric pain; EB, epigastric burning; AS, abdominal swelling;
PPI, proton-pump inhibitor; PKT, prokinetics; ATC, antacids:
*P ≤ 0.05; **P <
< 0.01;
***P
0.01;
***P <
< 0.001.
0.001.
In patients with EPS (group B), probiotic alone significantly reduced the
prevalence of all symptoms (Supplemental Table 1B). At T1,
probiotic alone showed the highest prevalence of no postprandial filling
compared with probiotic combined with proton-pump inhibitor
(P =
= 0.0109) or probiotic plus antacid
(P
0.0109) or probiotic plus antacid
(P =
= 0.0391), and the highest prevalence of no early
satiety versus probiotic combined with proton-pump inhibitor
(P
0.0391), and the highest prevalence of no early
satiety versus probiotic combined with proton-pump inhibitor
(P <
< 0.05; Figure 3B). Although probiotic alone
showed numerically higher prevalence of no other symptoms versus other
treatment groups, the differences were not statistically significant.
0.05; Figure 3B). Although probiotic alone
showed numerically higher prevalence of no other symptoms versus other
treatment groups, the differences were not statistically significant.
Discussion
The pathophysiology of FD is not fully understood, but the main cofactors are thought to be: (I) altered GI motility; (II) visceral sensitivity; and (III) psychosocial factors.22 The final diagnosis of FD remains based on a series of conventional diagnostic exams repeated over time in case of negative results. The lack of appropriate explanations of the nature of FD may leave patients worried about their clinical condition and symptoms; thus, they might have decided to abandon clinical trials and examinations.2
To date, the role of H. pylori in the development of FD remains unclear. In particular, H. pylori has not been associated with specific FD symptoms and its eradication is not clearly associated with any improvement of clinical FD symptoms.1,23,24 Diet and dietary components, such as coffee, alcohol, high-fat meals, carbohydrates, carbonated drinks and some vegetables, have been considered to play a role in triggering or exacerbating specific FD symptoms.22,25–27
The beneficial effects of probiotic supplementation in reducing FD symptoms (including nausea, postprandial fullness, and upper GI pain) have been studied previously.22,24 According to Agah et al.,22 four studies have reported investigating a single bacterial strain: one that used L. reuteri,28 and three that used L. gasseri OLL2716 (LG21),21,29,30 while two studies reported using combinations of probiotic strains.31,32
Overall, the present results suggest that the combination of probiotics (L.
rhamnosus LR04 [DSM 16605], L. pentosus LPS01 [DSM
21980], L. plantarum LP01 [LMG P-21021], and L.
delbrueckii subsp. delbruekii LDD01 [DMS 22106]) has beneficial effects
in reducing PDS symptoms after treatment for 30 days. The microorganisms were
administered as a microencapsulated preparation, which extends the viability of
bacteria, allowing survival in a low pH environment.33,34 The present results were
consistent with the literature,31,32 even with slight differences,
e.g., one study focused on the eradication of H. pylori using a
probiotic combination,32 while another used a combination of probiotics and an olive-oil treatment.31 In the present study, administration of a probiotic combination of four
Lactobacilli species showed the synergistic effects exerted by
a cocktail of well characterized probiotic strains, corroborating previously
published results.35 To the best of the authors’ knowledge, this is the first study to
specifically investigate the use of probiotics alone or combined with standard
pharmacological therapy (prokinetic, antacid, or proton-pump inhibitor). The main
symptoms of PDS (postprandial filling and early satiety) were significantly reduced
at the end of the trial, and probiotic alone showed the highest prevalence of no
symptoms. Taking into account that PDS is specifically related to motility disturbance,3 the combination of probiotic plus prokinetics was not an ideal treatment. As
a possible explanation, PDS is characterized by fasting and postprandial gastric hyper-mechano-sensitivity.3 The results in patients with EPS were more difficult to interpret, since all
of the treatments improved or eradicated specific symptoms (epigastric pain and
epigastric burning), but no treatment statistically outperformed the others. Another
important aspect is the potential competition for nutrients exerted by
Lactobacilli on the resident, and potentially dangerous
intestinal flora.20 It is well known that eubiotic microflora in the small intestine consist of
mixed aerobe and facultative anaerobic bacterial populations (≤104 CFU/ml
in healthy patients), that can easily overgrow causing small intestinal bacterial
overgrowth syndrome.36
Lactobacilli and other probiotics can rapidly colonize intestinal
mucosa acting as protective agents or they can acidify the surrounding intestinal
ecosystem making this environment uncomfortable for pathogen replication.37
CFU/ml
in healthy patients), that can easily overgrow causing small intestinal bacterial
overgrowth syndrome.36
Lactobacilli and other probiotics can rapidly colonize intestinal
mucosa acting as protective agents or they can acidify the surrounding intestinal
ecosystem making this environment uncomfortable for pathogen replication.37
The results of the present study may be limited by several factors. First, patients were enrolled on a volunteer basis and some patients did not complete the trial after the initial visit, perhaps due to temporary improvement of their health condition, or a lack of health benefit, which may have demotivated them to warrant further commitment. Thus, a high proportion of patients were lost to follow-up (did not attend T1 visit), however, despite the high drop-out rate, the results remained highly significant. Secondly, the lack of a unique diet for all patients enrolled during the clinical trial may have introduced some bias in terms of pharmacological alterations. As previously reported, diet is one of the most critical parameters that may exacerbate clinical symptoms associated with FD.22,25–27 Thirdly, changes or alterations in the intestinal microbiota that could explain both PDS and EPS features were not investigated. The fourth limitation is connected to the third; the evolution of intestinal microbiota may affect the faeces consistency, which was another aspect not investigated in the present study. Finally, since the study was not planned as a clinical trial, a placebo group was not included. However, no adverse events during probiotic treatment for dropped-out patients was reported.
In conclusion, dyspeptic symptoms were reduced in all patients following treatment with probiotics alone, or combined with conventional pharmacological therapy. This pilot study aimed to represent a snapshot of events during the clinical management of FD that included a combination of probiotics, and is intended to be preparatory to a subsequent clinical trial. Nevertheless, the results are consistent and statistically well-supported. The present preliminary findings require validation with additional data from further investigations, including a double-blinded and randomized clinical trial.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_0300060520982657 for Evaluation of main functional dyspepsia symptoms after probiotic administration in patients receiving conventional pharmacological therapies by Lorenzo Drago, Gabriele Meroni, Dario Pistone, Luigi Pasquale, Giuseppe Milazzo, Fabio Monica, Salvatore Aragona, Leonardo Ficano, Roberto Vassallo and Gastrobiota Group in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_0300060520982657 for Evaluation of main functional dyspepsia symptoms after probiotic administration in patients receiving conventional pharmacological therapies by Lorenzo Drago, Gabriele Meroni, Dario Pistone, Luigi Pasquale, Giuseppe Milazzo, Fabio Monica, Salvatore Aragona, Leonardo Ficano, Roberto Vassallo and Gastrobiota Group in Journal of International Medical Research
Footnotes
Declaration of conflicting interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All authors received grants for analysis of data in the present study, and as members of the Advisory Board for Aurora Biofarma and for Probiotical.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was partly supported by grants from Aurora Biofarma and from Probiotical.
ORCID iDs: Gabriele Meroni https://orcid.org/0000-0003-2772-6410
Dario Pistone https://orcid.org/0000-0002-1703-7470
Supplemental material: Supplemental material for this article is available online.
References
Articles from The Journal of International Medical Research are provided here courtesy of SAGE Publications
Full text links
Read article at publisher's site: https://doi.org/10.1177/0300060520982657
Read article for free, from open access legal sources, via Unpaywall:
https://journals.sagepub.com/doi/pdf/10.1177/0300060520982657
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/119546205
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1177/0300060520982657
Article citations
Relative Frequency of Gastrointestinal Functional Disorders in Patients with Inflammatory Bowel Disease Based on Rome IV: A Case-Control Study.
Adv Biomed Res, 13:43, 29 Jul 2024
Cited by: 0 articles | PMID: 39224402 | PMCID: PMC11368227
Research Progress for Probiotics Regulating Intestinal Flora to Improve Functional Dyspepsia: A Review.
Foods, 13(1):151, 02 Jan 2024
Cited by: 5 articles | PMID: 38201179 | PMCID: PMC10778471
Review Free full text in Europe PMC
Food, Dietary Patterns, or Is Eating Behavior to Blame? Analyzing the Nutritional Aspects of Functional Dyspepsia.
Nutrients, 15(6):1544, 22 Mar 2023
Cited by: 3 articles | PMID: 36986274 | PMCID: PMC10059716
Review Free full text in Europe PMC
Gut Microbiome-Brain Alliance: A Landscape View into Mental and Gastrointestinal Health and Disorders.
ACS Chem Neurosci, 14(10):1717-1763, 08 May 2023
Cited by: 30 articles | PMID: 37156006 | PMCID: PMC10197139
Review Free full text in Europe PMC
Functional Gastrointestinal Disorders with Psychiatric Symptoms: Involvement of the Microbiome-Gut-Brain Axis in the Pathophysiology and Case Management.
Microorganisms, 10(11):2199, 07 Nov 2022
Cited by: 5 articles | PMID: 36363791 | PMCID: PMC9694215
Review Free full text in Europe PMC
Go to all (10) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The innovative potential of Lactobacillus rhamnosus LR06, Lactobacillus pentosus LPS01, Lactobacillus plantarum LP01, and Lactobacillus delbrueckii Subsp. delbrueckii LDD01 to restore the "gastric barrier effect" in patients chronically treated with PPI: a pilot study.
J Clin Gastroenterol, 46 Suppl:S18-26, 01 Oct 2012
Cited by: 10 articles | PMID: 22955351
Rome III functional dyspepsia subdivision in PDS and EPS: recognizing postprandial symptoms reduces overlap.
Neurogastroenterol Motil, 27(8):1069-1074, 01 Aug 2015
Cited by: 29 articles | PMID: 26220647
Discriminant value of Rome III questionnaire in dyspeptic patients.
Saudi J Gastroenterol, 17(2):129-133, 01 Mar 2011
Cited by: 16 articles | PMID: 21372351 | PMCID: PMC3099059
Current and emerging therapeutic options for the management of functional dyspepsia.
Expert Opin Pharmacother, 21(3):365-376, 03 Jan 2020
Cited by: 6 articles | PMID: 31899982
Review
Funding
Funders who supported this work.




