Abstract
Free full text

Acute hepatic encephalopathy and multiorgan failure in sickle cell disease and COVID‐19
To the Editor,
Coronavirus disease 2019 (COVID‐19) due to severe acute respiratory syndrome‐coronavirus‐2 (SARS‐CoV‐2) in sickle cell disease (SCD) is still being described, with vaso‐occlusive crisis (VOC) and acute chest syndrome reported. 1 Reports suggest COVID‐19 causes hyperinflammation, vasculitis, coagulopathy, 2 acute respiratory distress syndrome, thromboembolism, and multiorgan failure syndrome (MOFS). 3 , 4 , 5 We present a case of post‐COVID‐19 encephalopathy, hyperammonemia, hyperinflammation, and MOFS in SCD.
A 19‐year‐old female with SCD (S/β0thalassemia), history of hepatic crisis, on chronic transfusions for 3 years (via erythrocytapheresis for 11 months) with iron overload (average ferritin 3465 ng/mL) requiring chelation with deferasirox (DFX, 1800 mg/day), was hospitalized for VOC when diagnosed with SARS‐CoV‐2 by PCR. She started hydroxychloroquine, was discharged with improved pain, but returned with emesis and transient aspartate aminotransferase (AST)/alanine aminotransferase (ALT) elevation, presumed due to hydroxychloroquine (thereafter discontinued). Four days after initial SARS‐COV‐2 diagnosis, she developed acute chest syndrome with fever, decreased hemoglobin, but no hypoxemia, which was managed with blood transfusion and antibiotics.
Four weeks later, she was re‐hospitalized with anorexia, fatigue, and worsening abdominal pain. She received analgesics (ketorolac, morphine) and continued home medications including DFX; repeat SARS‐CoV2 PCR negative. Fever developed on hospital day 3; blood culture was obtained, and ceftriaxone was administered. She became acutely encephalopathic 5 h later without focal neurologic deficit. Significant laboratory evaluations included ammonia 350 mmol/L, lactate 7.9 mmol/L, normal transaminases, total bilirubin (TB) 4.2 mg/dL, creatinine 1.3 mg/dL (baseline 0.7), international normalized ratio (INR) 1.47, and C‐reactive protein (CRP) 6.7 mg/dL (Figure 1). Computed tomography head and magnetic resonance imaging (MRI) brain were normal; abdominal ultrasound showed normal hepatoportal flow. She started lactulose and neomycin for hyperammonemia, and DFX discontinued due to possible toxicity. Electroencephalogram and clinical exam showed severe encephalopathy. She was intubated and underwent hemodialysis, with improved hyperammonemia (153 mmol/L) (Figure 1). She developed elevated transaminases (Figure 1), worsened synthetic liver function (INR 2.03, TB 10.7 mg/dL), increased creatinine (1.49 mg/dL), and thrombocytopenia (platelet 95 000) with coagulopathy (elevated D‐dimer 4.4 mcg/mL; Figure 1), and shock requiring vasopressors—consistent with MOFS—so red blood cell exchange (RBCX) was performed (pre‐/post‐RBCXHbS: 29%, 11% respectively). Despite RBCX, CRP increased (15.1 mg/dL), with persistent thrombocytopenia, fever, mildly decreased a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMSTS‐13) (66.2%), and von Willebrand factor (VWF) antigen level >196%, consistent with thrombocytopenia‐associated MOFS, so underwent therapeutic plasma exchange (TPE) 6 , 7 until platelet count was greater than 100 ,000. Following TPE, ammonia, AST/ALT, and INR normalized, CRP decreased (2.1 mg/dL) (Figure 1), fever resolved, and neurologic examination improved. Repeat brain MRI noted interval bilateral thalamic T2 hyperintensity, suggesting cytotoxic edema from hepatic encephalopathy for which hydrocortisone was administered. Nonocclusive catheter‐related femoral vein thrombus was detected, so enoxaparin was started (no earlier anticoagulation due to coagulopathy). Five days post‐TPE, she returned to neurologic baseline. She was discharged on hospital day 15 with improved pain, improved D‐dimer and CRP (Figure 1), and normal liver function tests.
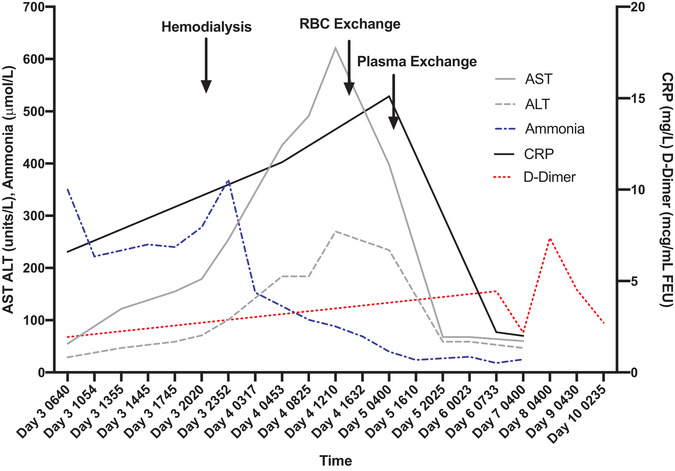
Liver function tests, ammonia, CRP, and D‐dimer during hospitalization with arrows outlining time of hemodialysis, red blood cell exchange transfusion, and therapeutic plasma exchange over 1 h (AST: aspartate aminotransferase; ALT: alanine aminotransferase)
MOFS is a known catastrophic SCD complication; however, this case with severe encephalopathy secondary to acute liver failure with hyperammonemia and neurologic insult is unusual. Often fatal without prompt RBCX, SCD‐MOFS typically evolves from VOC progressing to fever, altered sensorium, rapid significant anemia and thrombocytopenia, and acute liver, kidney, and/or respiratory failure. 8 , 9 While she had some classic features of SCD‐MOFS, hyperammonemia is not typical, and anemia and thrombocytopenia were mild. While her low baseline hemoglobin S (HbS) (<30%) is not fully protective, it suggests that MOFS was not only because of SCD.
Her acute and chronic liver disease—iron overload, history of hepatic crisis, 10 , 11 potential hydroxychloroquine, or DFX‐toxicity 12 , 13 —predispose to hepatic injury 14 ; however, these are unlikely the sole cause. Acute liver failure differential included acute SCD hepatopathy, which could be acute hepatic crisis, hepatic sequestration, or acute intra‐hepatic cholestasis. Though chronic RBCX maintained low HbS, this may not fully protect against hepatopathy. 7 , 10 Other organ involvement and hyperammonemia preceding increased AST/ALT argue against SCD hepatopathy alone. DFX‐induced liver failure and hyperammonemia are reported; however, her MOFS with hyperinflammation suggests cause beyond DFX toxicity alone. 8 , 9
Despite negative SARS‐CoV‐2 PCR immediately prior to MOFS, the short duration from COVID‐19 to MOFS is suggestive. Severe COVID‐19 disease is theoretically due to overproduction of pro‐inflammatory cytokines, leading to hyperinflammation, vascular permeability, vasculitis, and MOFS. 2 One such presentation is multisystem inflammatory syndrome in children (MIS‐C), similar to Kawasaki disease, occurring 1‐6 weeks post‐SARS‐CoV‐2, with gastrointestinal symptoms, risk for toxic‐shock syndrome, cardiac involvement (coronary aneurysms, myocarditis, heart failure), and macrophage activation syndrome (MAS). 15
COVID‐19‐related coagulopathy is also recognized, with severe cases showing increased D‐dimer, initial increase with late decrease in fibrinogen, thromboembolism, and/or disseminated intravascular coagulation. 16 Additional COVID‐19 manifestations include gastrointestinal symptoms and abnormal liver function tests. 12 , 13 , 17 Though this patient initially presented with VOC and respiratory symptoms of COVID‐19, her acute chest syndrome was mild; later manifestations were abdominal pain, hyperammonemia, liver failure, acute kidney injury (AKI), and hyperinflammation. While not consistent with MIS‐C given absence of aneurysm, myocarditis, or skin lesions, this case may represent another presentation of post‐COVID‐19 hyperinflammatory disease given her rapid improvement following TPE, as observed in other COVID‐19 MIS‐C patients. 18 , 19 MAS is also less likely given the normal level of triglycerides.
The pathophysiology of this case is likely multifactorial: COVID‐19‐related hyperinflammation triggering SCD‐MOFS and DFX‐induced hepatic injury with hyperammonemia in a patient with preceding liver disease. We recommend that RBCX and TPE be considered in SCD patients with MOFS and hyperinflammation following COVID‐19, and consideration of holding hepatotoxic drugs in those with underlying liver disease.
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.1002/pbc.28874
Read article for free, from open access legal sources, via Unpaywall:
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1002/pbc.28874
Citations & impact
Impact metrics
Article citations
Clinical outcomes of COVID-19 in patients with sickle cell disease and sickle cell trait: A critical appraisal of the literature.
Blood Rev, 53:100911, 20 Nov 2021
Cited by: 29 articles | PMID: 34838342 | PMCID: PMC8605823
Review Free full text in Europe PMC
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Vaso-occlusive crisis and acute chest syndrome in sickle cell disease due to 2019 novel coronavirus disease (COVID-19).
Am J Hematol, 95(6):725-726, 21 Apr 2020
Cited by: 57 articles | PMID: 32267016 | PMCID: PMC7262303
Care of patients with hemoglobin disorders during the COVID-19 pandemic: An overview of recommendations.
Am J Hematol, 95(8):E208-E210, 21 May 2020
Cited by: 14 articles | PMID: 32394480 | PMCID: PMC7272998
Impact of COVID-19 Infection on 24 Patients with Sickle Cell Disease. One Center Urban Experience, Detroit, MI, USA.
Hemoglobin, 44(4):284-289, 28 Jul 2020
Cited by: 27 articles | PMID: 32722950
Posterior Reversible Encephalopathy Syndrome.
Curr Pain Headache Rep, 25(3):19, 25 Feb 2021
Cited by: 41 articles | PMID: 33630183 | PMCID: PMC7905767
Review Free full text in Europe PMC

 2
2




