Abstract
Free full text

Diagnosis and management of hemorrhagic complications of percutaneous transhepatic biliary drainage: a primer for residents
Abstract
Hemorrhagic complications are uncommon after percutaneous transhepatic biliary drainage. The presenting features include bleeding through or around the drainage catheter, hematemesis or melena. Diagnosis requires cholangiography, CT angiography or conventional angiography. Minor venous hemorrhage is managed by catheter repositioning, clamping or upgrading to a larger bore catheter. Major vascular injuries require percutaneous or endovascular procedures like embolization or stenting. A complete knowledge of these complications will direct the interventional radiologist to take adequate precautions to reduce their incidence and necessary steps in their management. This review presents and discusses various hemorrhagic complications occurring after percutaneous transhepatic biliary drainage along with their treatment options and suggests a detailed algorithm.
Introduction
Percutaneous transhepatic biliary drainage (PTBD) is a commonly performed procedure, aimed at relieving biliary obstruction and its complications.1 Performed under combined ultrasonographic and fluoroscopic guidance, it involves percutaneous puncture of the intrahepatic bile ducts followed by catheter placement for biliary drainage. A wide range of malignant or benign causes require biliary drainage, either to relieve biliary obstruction in cases of benign and malignant strictures or to divert bile in cases of post-operative bile leaks.1 The technical success and overall complication rates of PTBD are similar to endoscopic biliary drainage and are in the range of 48.4–93.9% and 12.9–67%, respectively.2 Furthermore, PTBD is the preferred technique in patients with obstruction in the proximal biliary tree, unsuccessful endoscopic drainage and surgically altered upper gastrointestinal tract anatomy.2
With an overall incidence of 10.8–23%, the various complications associated with PTBD include pain, bile leak, catheter dislodgement, occlusion, bleeding, cholangitis and sepsis.3–7 The incidence of hemorrhage after percutaneous biliary interventions varies from 1.1 to 9.9%.3–5,8 As recommended by the Society of Interventional Radiology (SIR), the acceptable threshold for all major complications of PTBD is 10%, while that for hemorrhagic complications is 5%.1 While hemorrhagic complications may vary in severity from minor self-limiting blood-stained bile to major and potentially lethal bleeding, their prompt recognition and management are necessary for a successful procedure. In addition, taking adequate precautions prior to PTBD will help reduce their incidence. This article comprehensively reviews the causes of PTBD related hemorrhagic complications, steps required to lower their occurrence and the ways to detect and treat them successfully.
Anatomy of the biliary tree and the portal tract
The knowledge of this anatomy helps understand the pathophysiology of hemorrhagic complications better. The biliary tree consists of extra- and intrahepatic components. The extrahepatic component consists of common bile duct, cystic duct and common hepatic duct, formed by the confluence of the right and the left hepatic ducts. The intrahepatic component, consisting of second-order sectoral bile ducts and their tributaries, form the target for percutaneous biliary interventions. The branches of hepatic artery and portal vein run alongside the bile ducts within a connective tissue framework referred to as the portal tract.9 Owing to the close proximity of the three structures, hepatic artery or portal vein branches are prone to injury during biliary interventions. Hemorrhagic complications may occur due to injury to the branches of the hepatic artery, the portal vein or the hepatic vein. Further, hemorrhage may also result from the lacerated liver parenchyma, tumor and from injured vessels lying along the percutaneous puncture tract, commonly the intercostal or internal mammary vessels.
Risk factors
Various patient- and procedure-related factors influence the incidence of hemorrhagic complications occurring after PTBD. The risk factors are mentioned in Table 1.
Table 1.
Risk factors for hemorrhagic complications after percutaneous transhepatic biliary drainage
| Patient related factors | Technique related factors |
|---|---|
· Advanced age (≥73 years) years) | · Large bore puncture needle (18G) |
| · Chronic kidney disease | · Central duct puncture |
| · Cirrhosis | · Minimally dilated or non-dilated biliary system |
| · Deranged coagulation parameters | · Multiple number of punctures and catheters |
| · Ongoing use of antithrombotic agents | · Presence of ascites |
Patient-related risk factors include chronic kidney disease and cirrhosis.10,11 Multiple factors including platelet dysfunction and anemia contribute to the increased bleeding risk in chronic kidney disease; relative deficiency of procoagulant factors and platelets increases the bleeding risk in cirrhotic patients. Also, older patients (aged ≥73 years) are at a higher risk, possibly because of the presence of multiple co-morbidities.12 Procedure-related factors include use of large bore puncture needle (18 vs 21/22G), central duct puncture, minimally dilated or non-dilated bile ducts, multiple number of passes, multiple catheter placement and presence of ascites.5,8,13–15 Complication rates can be reduced by the use of smaller bore (21/22G) puncture needles, especially for non-dilated systems and by the selection of peripheral biliary radicals (distal to second-order biliary radicals) for the puncture. Deranged coagulation parameters and ongoing use of antithrombotic agents also increase the incidence of bleeding.12,15 According to SIR consensus guidelines, PTBD is considered as a procedure with high bleeding risk and prothrombin time/International normalized ratio (PT/INR), platelet count and hemoglobin levels are recommended as routine pre-procedure investigations.16 The recommended thresholds to minimize the bleeding risk are INR less than 1.5 and platelet count greater than 50,000/mm3. The SIR also recommends withholding of antiplatelet and anticoagulant agents prior to the procedure.16 There is conflicting evidence regarding the difference in complication rates between right-sided and left-sided punctures.5,15,17,18
Clinical presentation
Direct injury to vascular structures occurs during the procedure either due to puncture by the needle or during manipulation of the hardware. Excessive bleeding through the tract during the procedure should raise the suspicion of a vascular injury. Transient drainage of blood-stained bile can be normally seen up to 48 h after the procedure. However, persistent passage of blood through the drainage catheter beyond 48
h after the procedure. However, persistent passage of blood through the drainage catheter beyond 48 h after the procedure, pericatheter bleeding, hematemesis or melena should necessitate further investigation.17 Right upper quadrant or epigastric pain may be the presenting complaint in case of a subcapsular hematoma. Pericatheter bleeding occurs as a result of injury to the vessels lying along the percutaneous puncture tract. This is usually self-limiting, unless larger vessels like the hepatic, intercostal or internal mammary vessels are injured.19 Bleeding into the pleural or peritoneal cavity may result in unexplained hypotension or respiratory distress.20,21
h after the procedure, pericatheter bleeding, hematemesis or melena should necessitate further investigation.17 Right upper quadrant or epigastric pain may be the presenting complaint in case of a subcapsular hematoma. Pericatheter bleeding occurs as a result of injury to the vessels lying along the percutaneous puncture tract. This is usually self-limiting, unless larger vessels like the hepatic, intercostal or internal mammary vessels are injured.19 Bleeding into the pleural or peritoneal cavity may result in unexplained hypotension or respiratory distress.20,21
Certain features may help in distinguishing between arterial and venous injuries. Hemorrhage due to venous injury manifests as drainage of blood tinged bile or dark red blood through or around the catheter.22 Significant hemobilia, manifesting as bright red blood in the catheter, melena, hematemesis and fall in hematocrit (by >13%), has high positive predictive value for arterial injury.8
Delayed presentation is also common, especially in cases of pseudoaneurysms and abnormal fistulous communication between biliary tract and vascular structures.17,23 Hemorrhage in such cases may be the result of an occult injury which occurred during the procedure, gradual erosion by the catheter or due to involvement of the portal triad by the primary tumor. Vascular injury may also present after accidental dislodgement of the catheter.
Management
The first step in the management of the patients presenting with hemorrhagic complications involves assessing the hemodynamic status by measuring the pulse rate, blood pressure and respiratory rate.24 Patients in shock are stabilized by administration of intravenous fluids, oxygenation and blood transfusion, if necessary. The blood investigations needed to be performed include hemoglobin level, hematocrit, INR and platelet count. Subsequently, work-up should be done to identify the cause of hemorrhage.
Cholangiography through the percutaneously placed catheter is usually the first investigation of choice in a stable patient to assess the position of the catheter and its side-holes and to visualize any vascular communication and filling defects.25 In the absence of any vascular opacification, a ‘pull-back cholangiogram’ is suggested, especially when venous bleed is suspected.22 In this technique, the PTBD catheter is removed over the wire and a vascular sheath (size 2F smaller than the catheter) is inserted. Then, the iodinated contrast agent is injected while the sheath is slowly pulled back to look for any vascular opacification. This procedure is often combined with ultrasonography (USG) to look for subcapsular or abdominal wall hematoma, hemoperitoneum, hemothorax or pseudoaneurysms. If no cause is evident on cholangiography and the patient continues to bleed, CT angiography or multiphase CT should be done to look for pseudoaneurysms, active contrast extravasation, fistulas between the biliary tract and any vascular channel, arterioportal fistula or proximity of the catheter to a vessel.26 Multiphase CT or CT angiography may also be done initially as a part of work-up. However, if an arterial bleed is suspected and the patient is hemodynamically unstable, a digital subtraction angiography (DSA) may be performed directly to identify the cause. One modification which may be needed during DSA is to obtain arteriography after removal of the biliary catheter over the wire, to remove the tamponade effect of the PTBD catheter and allow better visualization of the arterial pathology.27 Subsequent management depends on whether the source of bleeding is venous or arterial.
Venous injuries
Venous bleeding may occur due to injury to the portal vein branches, hepatic vein tributaries or hepatic capsular veins. Since the veins have low intravascular pressures, bleeding is usually not severe enough to cause a fall in hematocrit.22 However, severe hemorrhage with shock may occur if there is a major injury to the central large caliber portal vein branches.23 The common reason for bleeding is the traversing of the catheter through a peripheral portal venous branch. Sometimes, the PTBD catheter gets displaced so that the side-holes communicate with the venous channel.22 Diagnosis of venous injury is usually made by either a cholangiography through the catheter or a pull-back cholangiogram, as described above, when they show venous opacification. Subsequent management depends on the caliber of the injured vein and the severity of bleeding.
Small peripheral vein injury and minor bleeding
In cases of minor venous or parenchymal hemorrhage, depending on the findings on the cholangiogram, catheter repositioning, clamping of the catheter for 24–48 h or upgrading to a larger bore catheter may be performed to provide a tamponade effect (Figure 1).22 These measures control the bleeding in a majority of patients. Proper fixation of the catheter at the skin entry site using purse string sutures may be required to minimize the incidence of catheter displacement. If minor bleeding occurs during the procedure, an angioplasty balloon catheter can be inserted through the tract over a guidewire and temporarily inflated to provide a tamponade effect.
h or upgrading to a larger bore catheter may be performed to provide a tamponade effect (Figure 1).22 These measures control the bleeding in a majority of patients. Proper fixation of the catheter at the skin entry site using purse string sutures may be required to minimize the incidence of catheter displacement. If minor bleeding occurs during the procedure, an angioplasty balloon catheter can be inserted through the tract over a guidewire and temporarily inflated to provide a tamponade effect.

Injury to small portal vein branch. A 35-year-old lady with gallbladder cancer and PTBD performed for cholangitis, presenting with bleeding through the catheter after 24 h: (A) Cholangiogram through the catheter shows opacification of the portal vein branches (arrow). (B) - Cholangiogram performed after repositioning the catheter (arrow) shows no vascular opacification. Multiple cholangitic abscesses are also visualized (arrowheads). PTBD, percutaneous transhepatic biliary drainage.
h: (A) Cholangiogram through the catheter shows opacification of the portal vein branches (arrow). (B) - Cholangiogram performed after repositioning the catheter (arrow) shows no vascular opacification. Multiple cholangitic abscesses are also visualized (arrowheads). PTBD, percutaneous transhepatic biliary drainage.
Large central vein injury and major bleeding
In case of a major bleed due to venous injury, CT angiography is often needed to exclude an arterial source of bleeding, particularly if cholangiogram is inconclusive (Figure 2). Portobiliary fistula is a rare complication, which often develops few weeks following PTBD due to erosion of the vein by the catheter.23,28 It can be diagnosed by a catheter or a pull-back cholangiogram. Small fistulas usually heal spontaneously. Fistula with a centrally located portal vein branch necessitates removal of the catheter followed by embolization of the tract using gel foam, glue or coils.22 If the bleeding persists, embolization of the portal vein branch may be necessary through a percutaneous transhepatic route (Figure 2).29 Sometimes, larger fistulas may require percutaneous stent graft placement, either within the portal vein branch or within the bile duct (Figure 3).23,28,30 A safety margin of 1–2 cm on either side of the fistula is essential for technical success. Possible complications of stent grafts include hepatic infarction when placed within the portal vein and cholangitis when placed within the bile duct, both occurring due to isolation of their respective side-branches.28
cm on either side of the fistula is essential for technical success. Possible complications of stent grafts include hepatic infarction when placed within the portal vein and cholangitis when placed within the bile duct, both occurring due to isolation of their respective side-branches.28
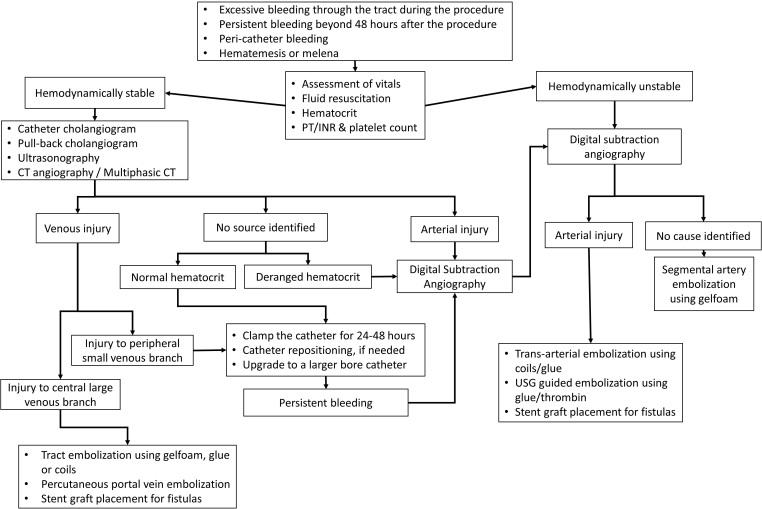
Active extravasation due to portal venous injury and arterio-portal fistula: A 56-year-old female with right sided PTBD performed for malignant hilar obstruction presenting with shock following accidental displacement of the catheter. (A) Coronal CT image in portal venous phase shows active contrast extravasation from a portal vein branch into the peritoneal cavity (arrow) resulting in hemoperitoneum (asterisks). (B) Selective DSA of the right hepatic artery shows opacification of a portal vein branch (arrowheads) suggesting arterio-portal fistula, with active extravasation (arrow). The hepatic artery branches are narrow in caliber as the patient was in shock. (C) DSA image following transhepatic embolization of the portal vein branch using n-butyl cyanoacrylate glue (arrow) shows no extravasation. DSA, digital subtraction angiography; PTBD, percutaneous transhepatic biliary drainage.
Portobiliary fistula. A 56-year-old male with gallbladder cancer presenting with melena and shock two weeks after PTBD. (A) Cholangiogram performed through the ring biliary catheter shows opacification of the portal vein branches (arrows), suggestive of portobiliary fistula. (B) Cholangiogram after insertion of a stent graft (arrowheads) into the left-sided bile duct and the common bile duct reveals no vascular opacification.23 PTBD, percutaneous transhepatic biliary drainage. [Reproduced with permission from the Indian National Association for Study of the Liver].
Injury to the hepatic vein tributary is a rare occurrence as it doesn’t form a part of the portal triad.31 Hemorrhage may result due to a biliovenous fistula, which can be visualized as hepatic vein opacification during cholangiogram (Figure 4). The right hepatic vein has higher chances of injury than the left one as the former frequently courses parallel to the segment VI bile duct. In the presence of a distal biliary obstruction, intraluminal pressures within the biliary system exceed that of the hepatic vein, resulting in bilhemia.31 This is manifested clinically as abnormally high blood levels of bilirubin, disproportionate to the severity of biliary dilation. Hemorrhage is rarely seen. Most biliovenous fistulas resolve spontaneously once the distal biliary obstruction is relieved (Figure 4). Sometimes, temporary clamping of the catheter or upgradation to a larger size may be necessary. Unlike portobiliary fistulas, these fistulas have long intrahepatic tracts, which are amenable for coil embolization following percutaneous access through the biliary radicals.31
Biliovenous fistula. A 39-year-old female with gallbladder cancer without any clinically evident bleeding after PTBD. (A) Cholangiogram performed through the external drainage catheter reveals an irregular filling defect in the bile duct (arrowhead) with opacification of middle hepatic vein (arrow), suggestive of biliovenous fistula. As there were no signs of bleeding, no active intervention was done. (B) Follow-up cholangiogram after 7 days shows reduction in the biliary dilation with spontaneous resolution of the fistula. PTBD, percutaneous transhepatic biliary drainage.
Arterial injuries
Injury to the hepatic arteries and their branches need more active intervention due to larger amount of blood loss.8 On CT angiography, which is the first investigation of choice, there may be active contrast extravasation, pseudoaneurysm, or arteriobiliary or arterioportal fistulas. Sometimes, the presence of the catheter causes beam-hardening artifacts and it becomes difficult to identify any arterial abnormality except for finding an arterial branch close to the PTBD catheter (Figure 5). Although arterial injuries usually present within a week after PTBD, delayed presentation after several weeks is also common, especially for pseudoaneurysms.17 This is due to the catheter constantly impinging on the nearby artery and resulting in its erosion. Arterial injuries are infrequently visualized on cholangiogram study because of the high pressure within the arterial system preventing its opacification. However, arterial blood entering the bile ducts may result in clearing of contrast within the central bile ducts (absent central bile duct sign) with retention in the peripheral ducts, providing an indirect indication of arterial injury.25 Pullback cholangiogram should be avoided whenever an arterial injury is suspected as this may result in torrential bleeding due to the removal of tamponade effect of the catheter.
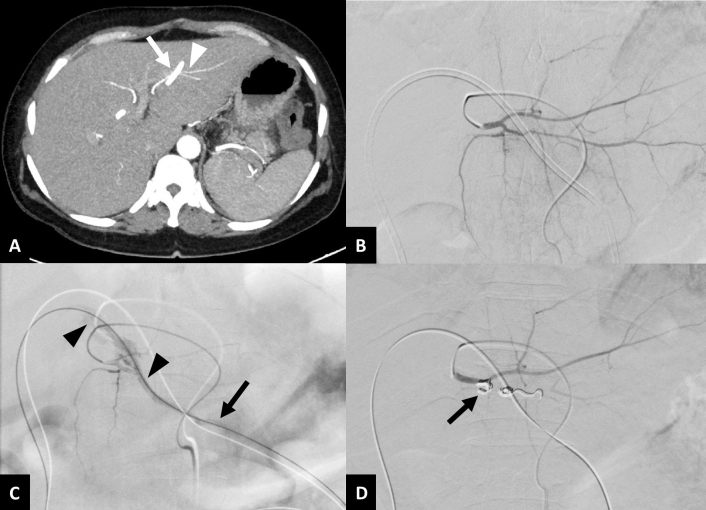
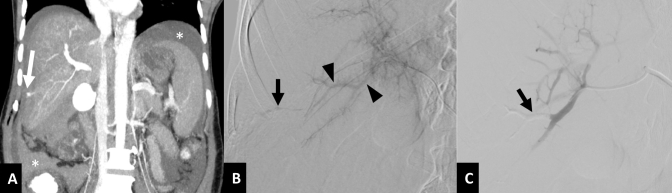
Arteriobiliary fistula. A 61-year-old female with gallbladder cancer presenting with pericatheter hemorrhage, one month after left-sided PTBD. (A) Axial contrast enhanced CT image in arterial phase shows the biliary catheter (arrow) in close proximity to the segment III hepatic artery branch (arrowhead). (B) Initial selective DSA of lateral segmental branch of left hepatic artery does not show the source of bleeding. (C) Repeat DSA following removal of the biliary catheter over a guidewire reveals extravasation of contrast into the biliary tree (arrowheads), suggestive of arteriobiliary fistula, and into the percutaneous tract (arrow). (D) DSA after coil embolization (arrow) of the segment III artery shows complete occlusion of the fistula. DSA, digital subtraction angiography; PTBD, percutaneous transhepatic biliary drainage.
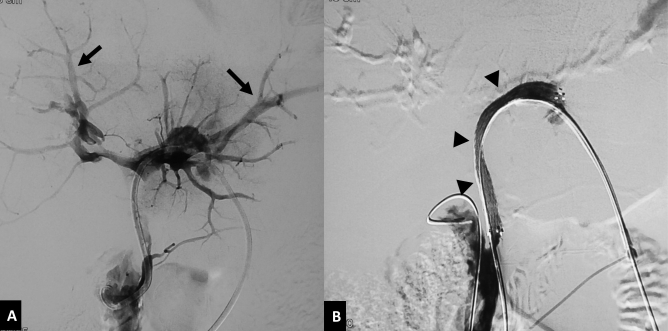
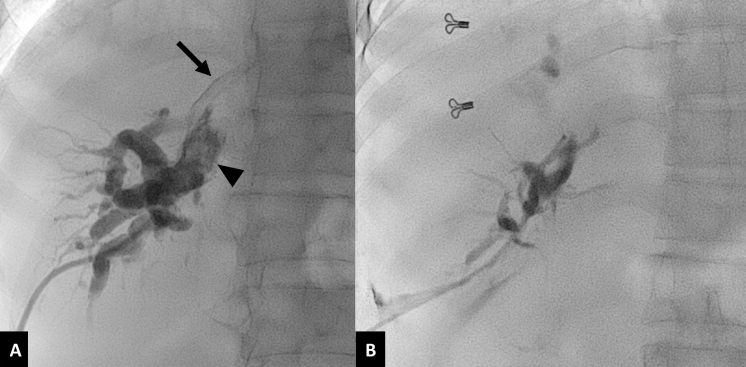
DSA is the modality of choice for treating arterial bleeding after PTBD. Selective angiography of the source artery is performed based on the CT angiography findings. If CT angiography is not performed, then celiac artery angiogram followed by selective hepatic artery angiogram should be performed to identify the site of bleed. Superior mesenteric artery angiogram is important in known or suspected cases of accessory or replaced right hepatic artery. In some cases, active extravasation or pseudoaneurysm is masked by the indwelling biliary drainage catheter due to its tamponade effect.13,17 In such cases a repeat angiogram after removal of the catheter over a guidewire is necessary to demonstrate the site of bleeding (Figure 5). Embolization, endovascular or direct percutaneous, is the treatment of choice for arterial bleeding.25 Transarterial embolization has a high technical success rate of over 95% with a low incidence of rebleeding.13,17,27 Super selective embolization using coils is the most commonly used method. For pseudoaneurysms and arterial fistulas, placement of coils in both the proximal and distal sites (sandwich technique) is essential to achieve complete exclusion of the lesion from circulation (Figures 6 and 7). In some cases, reaching distal to the site of bleed by the microcatheter may not be possible. Then, liquid embolic agents like n-butyl cyanoacrylate glue should be used for complete and successful embolization (Figure 8).32 If the pseudoaneurysm is visualized on USG, it may be directly punctured under USG guidance using a 22G Chiba needle and thrombin or n-butyl cyanoacrylate glue may be used for embolization (Figure 9).33 If no arterial bleeding source is evident on DSA, gel foam may be empirically injected to embolize the branch of the hepatic artery lying close to the PTBD catheter.25 Hepatic infarcts are rare after embolization of hepatic arteries due to the presence of extensive intrahepatic interlobar collaterals.17 Multiple cases of hepatic abscess formation have been reported, particularly when there is associated portal vein occlusion due to thrombosis or malignant invasion.27 In cases of transplanted liver or where there is portal vein thrombosis or injury to central hepatic arterial branches, stent graft is the preferred option to maintain vascular flow to the liver and avoid any ischemic and infective complications.34–36 Injuries to the intercostal or internal mammary arteries are visualized as active contrast extravasation or pseudoaneurysms on CT angiography and should be managed by transarterial embolization (Figure 10).20,21
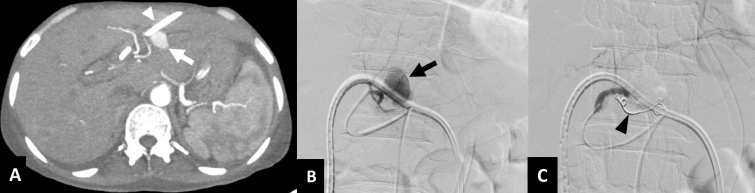
Hepatic artery pseudoaneurysm. A 42-year-old male with gallbladder cancer presenting with profuse bleeding through the catheter one week after PTBD. (A) Axial CT image in arterial phase shows left-sided PTBD catheter in position (arrowhead) with a contrast filled outpouching arising from the segment III branch of left hepatic artery, suggestive of a pseudoaneurysm (arrow). (B) Selective DSA of left hepatic artery reveals the pseudoaneurysm (arrow) close to the catheter. (C) DSA image after embolization with coils (arrowhead) shows complete exclusion of the pseudoaneurysm from the circulation. DSA, digital subtraction angiography; PTBD, percutaneous transhepatic biliary drainage.

Combined pseudoaneurysm and arterio-biliary fistula. A 56-year-old male with hilar cholangiocarcinoma presenting with bleeding through the catheter, three weeks following right PTBD. (A) Coronal maximum intensity projection CT image in arterial phase shows a contrast filled outpouching arising from the right hepatic artery, suggestive of a pseudoaneurysm (arrow), in close relation to the biliary catheter (arrowhead). (B) Initial DSA image of right hepatic artery shows a pseudoaneurysm (arrow) close to the catheter. (C) Subsequent DSA image shows rupture of the pseudoaneurysm with leak of contrast into the biliary tree (arrowheads), suggestive of arteriobiliary fistula. (D) Final DSA image after embolization using coils (arrow) shows complete occlusion of the artery. DSA, digital subtraction angiography; PTBD, percutaneous transhepatic biliary drainage.
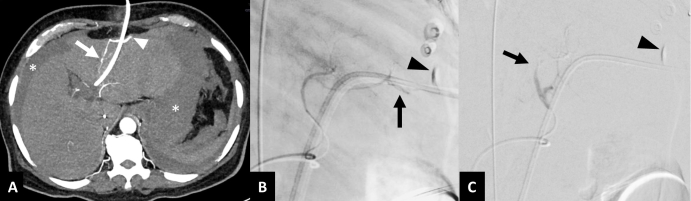
Active extravasation following arterial injury. A 68-year-old male with gallbladder cancer presenting with shock five days after left-sided PTBD. (A) Axial contrast-enhanced CT image in arterial phase shows contrast extravasation (arrowhead) from segment III branch of left hepatic artery (arrow) close to the PTBD catheter with hemoperitoneum (asterisks). (B) Selective lateral view DSA image of left hepatic artery shows linear track-like extravasation of contrast (arrow) into the perihepatic space. (C) DSA image after embolization using n-butyl cyanoacrylate glue shows complete occlusion of the segmental branch (arrow). An area of persistent contrast accumulation due to extravasation during the prior CT scan can also be seen (arrowheads in B, C). DSA, digital subtraction angiography; PTBD, percutaneous transhepatic biliary drainage.

USG-guided direct embolization of pseudoaneurysm. A 48-year-old male with hilar cholangiocarcinoma presenting with fever, melena and catheter dislodgement, one month after left PTBD. (A) Bedside color Doppler USG image of the left lobe of liver shows a pseudoaneurysm with turbulent flow within (arrow). (B) USG image shows 22 G needle (arrows) used to puncture the pseudoaneurysm. (C) Color Doppler USG image after embolization with n-butyl cyanoacrylate glue shows complete thrombosis of the pseudoaneurysm (arrow). PTBD, percutaneous transhepatic biliary drainage; USG, ultrasound.

Pseudoaneurysm of internal mammary artery. A 30-year-old male with cirrhosis and choledocholithiasis, presenting with profuse pericatheter bleeding two weeks after PTBD. (A) Coronal maximum intensity projection CT image in arterial phase shows a contrast filled outpouching (white arrow) arising from the left internal mammary artery (arrowhead), suggestive of a pseudoaneurysm, adjoining the biliary catheter (black arrow). (B) DSA image of left internal mammary artery (arrowhead) shows the pseudoaneurysm (arrow). (C) Spot radiograph image after embolization with n-butyl cyanoacrylate glue shows glue cast in the target artery (arrow). DSA, digital subtraction angiography; PTBD, percutaneous transhepatic biliary drainage.
An algorithm for the management of patients presenting with hemorrhage after PTBD has been summarized in Figure 11.
Conclusion
Hemorrhagic complications are infrequent after PTBD. However, major hemorrhage can become life threatening. Therefore, identifying the risk factors, taking necessary pre-procedure precautions and following a meticulous technique are the pre-requisites to reduce the occurrence of such complications. Thorough knowledge of the possible causes of these complications is essential for their prompt identification and appropriate management. Venous injuries are more common than arterial injuries, but usually self-limiting. Arterial injuries result in greater blood loss and often require active management. Both arterial and venous injuries can be managed by radiological interventions with high success rates. Also, formulating and following an institutional standardized protocol is critical to avoid associated morbidity and mortality.
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
Funding: This study was not supported by any funding.
REFERENCES
Articles from The British Journal of Radiology are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1259/bjr.20200879
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8010549
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/113925508
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1259/bjr.20200879
Article citations
A case of an intraabdominal, but extrahepatic ruptured percutaneous transhepatic biliary drainage and its following rescue. A case report and literature review.
Radiol Case Rep, 19(11):5452-5458, 03 Sep 2024
Cited by: 0 articles | PMID: 39285960 | PMCID: PMC11403903
Efficacy and safety analysis of continued nursing of complications in discharged patients after percutaneous transhepatic biliary drainage.
World J Clin Cases, 12(19):3898-3907, 01 Jul 2024
Cited by: 0 articles | PMID: 38994318
Percutaneous transhepatic choledochoscopy in the management of hepatolithiasis: a narrative review.
Quant Imaging Med Surg, 14(7):5164-5175, 20 Jun 2024
Cited by: 0 articles | PMID: 39022230 | PMCID: PMC11250287
Review Free full text in Europe PMC
Bloody Bile and Rescue Intervention-A Case Series of Post-PTBD Hemorrhagic Complications With a Review of the Literature.
J Clin Exp Hepatol, 14(4):101392, 05 Mar 2024
Cited by: 0 articles | PMID: 38558862
The impact of pre-operative cholecystostomy on laparoscopic excision of choledochal cyst in paediatric patients.
Pediatr Surg Int, 39(1):282, 17 Oct 2023
Cited by: 0 articles | PMID: 37847409
Go to all (8) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Living donor liver transplantation: complications in donors and interventional management.
Radiology, 230(2):443-449, 29 Dec 2003
Cited by: 29 articles | PMID: 14699180
Interventional biliary radiology.
AJR Am J Roentgenol, 142(1):31-34, 01 Jan 1984
Cited by: 14 articles | PMID: 6606961
Percutaneous transhepatic biliary drainage: technique, results, and applications.
Radiology, 135(1):1-13, 01 Apr 1980
Cited by: 132 articles | PMID: 7360943
Percutaneous management of benign biliary strictures.
Tech Vasc Interv Radiol, 4(3):141-146, 01 Sep 2001
Cited by: 3 articles | PMID: 11748552
Review
 1
1