Abstract
Free full text

Approaches to characterize the transcriptional trajectory of human myogenesis
Abstract
Human pluripotent stem cells (hPSCs) have attracted considerable interest in understanding the cellular fate determination processes and modeling a number of intractable diseases. In vitro generation of skeletal muscle tissues using hPSCs provides an essential model to identify the molecular functions and gene regulatory networks controlling the differentiation of skeletal muscle progenitor cells. Such a genetic roadmap is not only beneficial to understanding human myogenesis but also to decipher the molecular pathology of many skeletal muscle diseases. The combination of established human in vitro myogenesis protocols and newly developed molecular profiling techniques offers extensive insight into the molecular signatures for the development of normal and disease human skeletal muscle tissues. In this review, we provide a comprehensive overview of the current progress of in vitro skeletal muscle generation from hPSCs and relevant examples of the transcriptional landscape and disease-related transcriptional aberrations involving signaling pathways during the development of skeletal muscle cells.
Introduction
Skeletal muscle is the most abundant tissue in the human body and constitutes approximately 40% of the total body mass [1]. The skeletal muscle tissues are controlled by the somatic nervous system, and the main function of skeletal muscle is to generate force, maintain posture, and control respiration and metabolism [2]. The function of skeletal muscle is mediated by a specialized contractile tissue that is composed of terminally differentiated multi-nucleated cells. Most skeletal muscles are composed of multiple bundles of muscle fibers that are connected to the bone through tendons (Fig. 1). Each muscle fiber is formed as a sequential chain of basic functional units called myofibrils. Myofibrils are composed of repeated units of sarcomeres, which are an arrangement of thin actin and thick myosin filaments, to generate the basic movement of muscle contraction. During embryonic development, skeletal muscle cells, mostly myogenic progenitor cells, form the myofibers through a cellular fusion process called myofusion, the process of forming multinucleated myotubes. The myogenic progenitor cells in the embryo originate from the somites, bilaterally paired blocks of paraxial mesoderm, that are located on both sides of the notochord and the neural tube. Specification of the myogenic progenitor cells initiates in response to signaling molecules from the neighboring tissues such as the neural tube, notochord, and dorsal ectoderm [3]. These signaling molecules include members of the WNT family, sonic hedgehog (SHH), and noggin as activators, and bone morphogenic protein (BMP) as an inhibitor. During somitogenesis, while secretions of SHH from the notochord and noggin from the floor of the neural tube produce the ventral part of the somite to generate the sclerotome for vertebral column formation, BMPs secreted from the lateral plate mesoderm direct the dorsomedial portion of the dermomyotome to form the muscle precursor cells of the primary myotome, which mostly become skeletal muscle tissues.
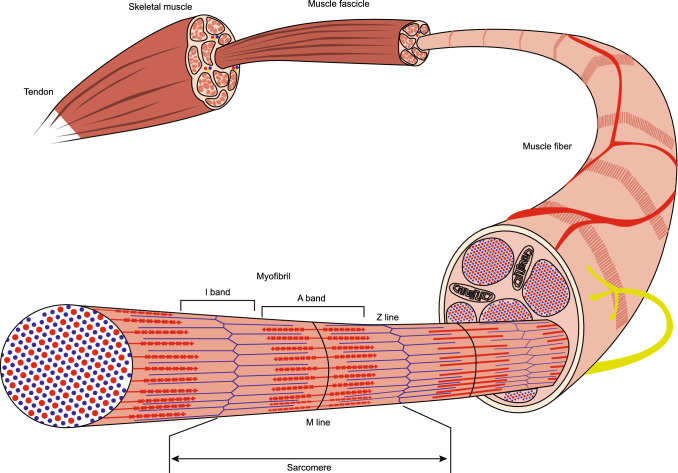
The structure of skeletal muscle. Striated muscle fiber consists of long fibres with each muscle fiber comprised of a bundle of myofibrils
Stem cell biology has initiated a new era for studying human developmental biology and advancing medical applications. Technologies using hPSCs and adult stem cells provide a robust source of cells for understanding the generation and mechanism of skeletal muscle development [4]. In contrast to the limited capabilities of adult stem cells, hPSCs, including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), have the ability to self-replicate indefinitely and differentiate into tissues of the three primary germ layers.
Human embryonic stem cells (hESCs) provide a potentially unlimited source of specialized cell types for regenerative medicine. However, the limited availability of embryos with the desired genotype and the moderate efficiency of hESC derivation make using these cells challenging. Another interesting strategy is the use of somatic cell nuclear transfer (SCNT), which has been successfully used for regenerative medicine approaches in mice [5–7]. However, despite the recent breakthrough in generating monkey SCNT lines [8], the application of SCNT for the derivation of hESCs is still not efficient. Furthermore, ethical and practical considerations surrounding egg donation make it questionable as to whether human SCNT technology will be suitable for routine disease modeling in humans.
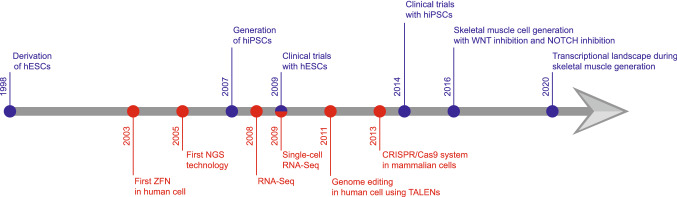
The induction of pluripotency in somatic cell types via the overexpression of reprogramming factors has been one of the great breakthroughs in stem cell biology. After the early pioneering studies by Shinya Yamanaka’s lab, questions remained as to whether mouse iPSCs were fully pluripotent [9] until subsequent work by Yamanaka and others conclusively demonstrated the germ line contribution and the generation of mouse iPSC-derived embryos using tetraploid complementation assays [10–12]. The success of applying human iPSC (hiPSCs) technology further supported the possibilities for this approach toward translational applications [13, 13, 14]. A subsequent key challenge was the derivation of hiPSCs without stable integration of reprogramming factors. This is particularly critical because the random integration of viral DNA can jeopardize further transplantation studies, and any remnant ‘Yamanaka factors’ could mask disease-related phenotypes. This issue can be addressed by the use of a RNA virus-based gene delivery system, a non-integrating viral vector system, direct protein transduction, or by identifying the factors capable of regulating extrinsically reprogramming factors [15]. hiPSCs have provided unique opportunities for modeling diseases as shown by others [14, 16, 17, 17–20, 20, 21] and our group [22–25]. New technologies have provided opportunities to improve knowledge in the field of developmental biology. With the development of various bioinformatics tools, next-generation sequencing (NGS) has become much more affordable [26]. High throughput sequencing has enabled us to understand the function and mechanism of developmental progress [27]. In this review, we examine the current progress of in vitro skeletal muscle generation using hESCs and hiPSCs in conjunction with newly developed technologies (Fig. 2). Furthermore, we discuss the potential of these approaches in future applications such as disease modeling and therapeutic treatment.
Skeletal muscle generation during embryonic development
During embryonic development, skeletal muscles originate from the mesodermal lineage, which starts in the primitive streak [28]. One of the major tasks of gastrulation is to generate a mesodermal layer of two bilateral bands that are located beside the neural tube and notochord [29]. These unsegmented bands are the paraxial mesoderm (presomitic mesoderm), which play a major role during the development of the skeletal muscle. During the process of somite formation (somitogenesis), the paraxial mesoderm progressively compacts and binds together into the symmetrical epithelium and is organized into the segmental plate of cells called somitomeres, which are then separated from the presomitic paraxial mesoderm, and form bilaterally paired block-like structures called somites [30]. Although somites are transient structures, these cells are important in not only being the precursors that give rise to different organs including the cartilage, skeletal muscle, and dermis, but also in determining the migratory paths of the neural crest cells. The multipotent mesodermal cells in the immature somite obtain tissue-specific cell fates in response to signals from the surrounding tissues during the development of the somite. As the somite tissues develop, the most dorsal compartment of cells remains as the epithelial layer and becomes a cell sheath called the dermomyotome, which develops into dermatome cells, forming the dermis of the back, and into myotome cells, which forms the muscles, while the most ventral portion of the somite transitions from the epithelial layer to the mesenchymal layer to form the sclerotome, which contributes to cartilage, bones, and tendons [3].
As the central part of the dermomyotome disintegrates, the muscle progenitors intercalate into the primary myotome, which gives rise to a fraction of the satellite cells during postnatal skeletal muscle generation. The cells from the two edges of the dermomyotome (the dorsomedial and ventrolateral lips) form the myotome, which is comprised of primitive skeletal muscle cells, and contribute to skeletal muscle formation. During skeletal muscle development, the myotome differentiates into a single nuclear myoblast that fuses to each other to make elongated, cylindrical, and multi-nucleated myotubes [31]. The myotube, then, builds up to organize actin and myosin filaments to form sarcomeres that are the functional units of skeletal muscle contractions, and the sequential chain of these sarcomeres, myofibrils, consist of myofiber that has the striated patterns of the skeletal muscle.
Gene regulatory network with signaling pathway
The process of skeletal muscle generation through embryo development initiates in the segmentation of the paraxial mesoderm with a clock-like rhythm (Fig. 3). As the embryo develops, newly formed mesodermal lineage cells are controlled by several signaling pathway molecules including WNT, SHH, and BMP from the surrounding tissues such as the dorsal ectoderm, neural tube, and notochord. WNT3A secreted from the neural tube directly regulates expression of T (brachyury) and TBX6, members of the T-box gene family that are DNA-binding transcription factors [32, 33]. MSGN1, a direct target of TBX6 and WNT signaling, is one of the essential proteins for the maturation of paraxial mesoderm and repression of neural fate [33].
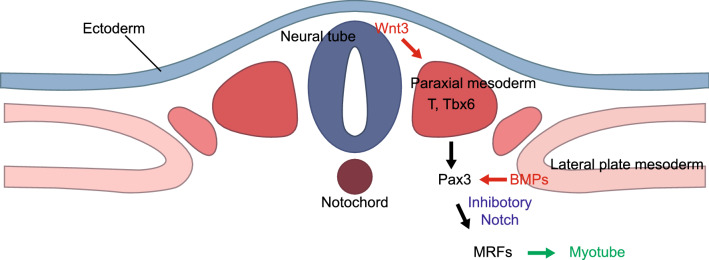
The formation of the skeletal muscle from paraxial mesoderm during human embryonic development. WNTs secreted from the dorsal neural tube enhance the expression of the members of the T-box gene family including T and TBX6. MSGN1 is a direct target of TBX6 and WNTs signaling and induces maturation of paraxial mesoderm. BMPs secreted from the lateral plate mesoderm regulate the proliferating skeletal muscle precursor cells expressing PAX3. PAX3 and NOTCH signaling inhibition directly and indirectly regulate the myogenic determination genes such as myogenic regulatory factors (MRF) involving MYF5 and MYOD1 in skeletal muscle progenitor cells. MYOG is controlled by MYF5, and MYOD1 initiates myotube formation by triggering myotube-specific gene expression.
The proliferating skeletal muscle precursor cells generated from the somites express the paired box domain transcription factor, PAX3, and are regulated by BMPs secreted from the lateral plate mesoderm [34, 35]. BMPs also help the PAX3 positive precursor cells to maintain a proliferative status by enhancing the expression of PAX3. The PAX3 positive progenitor cells give rise to either muscle cells or vascular cells, which is determined by NOTCH signaling. PAX3 with inhibitory NOTCH signaling regulates the myogenic determination gene, MYF5, in skeletal muscle progenitor cells [36, 37]. The activation of NOTCH in the PAX3 positive cells promotes endothelial cell and smooth muscle cell fates [38]. It has been shown that activated NOTCH signaling inhibits the differentiation of myoblasts and maintains the immature myogenic status [39]. The formation of myotubes from myoblasts is controlled by a core network of skeletal muscle regulatory factors (MRFs) consisting of MYF5, MYOD1, MRF4, and MYOG, which are involved in myotube generation and trigger myotube-specific gene expression that results in the initiation of myotube formation.
Human stem cells as a source for studying skeletal muscle
Adult stem cells exist as a rare population in several tissues in niches connected by surrounding cells [40, 41]. The main function of adult stem cells is the maintenance of homeostasis and regeneration of their respective tissues. Stem cells can function in two complementary perspectives. The adult stem cells in organs with high replacement rates are constantly proliferating and differentiating to support the tissue. The other type of adult stem cells stays quiescent with a minute proliferation rate but undergoes asymmetric division to self-renew and gives rise to tissue-committed progenitors when the tissue is injured.
Skeletal muscle stem cells called satellite cells are excellent examples of quiescent adult stem cells [42]. Satellite cells [43] are localized between the skeletal muscle fiber and the basal lamina. Human satellite cells express several genetic marker genes including PAX7, M-CADHERIN, and NCAM [44]. In response to mechanical injury, quiescent satellite cells are released from the basal lamina to be activated, proliferate, and regenerate the skeletal muscle fibers [45]. The satellite cells provide the candidates for understanding skeletal muscle generation; however, it is challenging to isolate pure satellite cells from human tissues and to maintain/proliferate the satellite cells in an in vitro system without losing the myogenic capabilities.
hESCs derived from the inner cell mass at the blastocyst stage during human embryo development [46] and hiPSCs derived from human somatic cells with the ‘Yamanaka factors’ (OCT4, SOX2, KFL4, and MYC) [13] have similar gene expression profiles, the ability to self-replicate, and the potential to develop into the three primary germ layers including ectoderm, mesoderm, and endoderm and the possibility to generate a variety of differentiated tissues in vitro [47]. The derivation of hPSCs has presented the ability to generate skeletal muscle tissues in vitro; the skeletal muscle cells generated from hPSCs have been successfully used in in vivo skeletal muscle regeneration [48].
Application of biomedical engineering technologies to improve insight into skeletal muscle development
The application of new technologies such as a genetic reporter cell line and lineage-specific transcriptional profiling accelerate our understanding of the molecular mechanisms during skeletal muscle cell generation. Genetic reporting systems, including the use of tagging proteins, are valuable tools to allow non-invasive measurements of cell function [49], protein localization [50], gene regulation, and gene expression ratios in developmental biology [51, 52]. The use of fluorescent proteins with specific promoters can track targeted gene expression in a living substance to monitor its cellular biology and to purify a subset of cells of interest, leading us to address principal questions in cell biology [49–51]. Although gene regulatory elements for detecting gene expression can function at long distances, most studies with a plasmid-based reporter system used a relatively small DNA fragment for knock-in into genomic DNA [53]. While this limitation can be overcome by introducing a large-scale insertion of a construct including promoters and an enhancer into the genome, this approach is challenging due to the low efficiency of homologous recombination in human cells. The advance of genome editing technologies provides genetic changes in a site-specific manner [54]. The site-specific nucleases such as zinc-finger nucleases (ZFN) [55], transcription activator-like effector nucleases (TALENs) [56], and CRISPR/Cas9 system [57, 58], are efficient and precise tools for customizing genetic modification in human cells by inducing targeted DNA double-stranded breaks that stimulate the cellular DNA repair mechanisms. These approaches enable the generation of fluorescent protein knock-in hESC and hiPSC lines that are regulated by skeletal muscle lineage-specific promoters [59, 60].
The high-throughput sequencing technology has become a powerful tool for understanding genomic information and measuring the levels of gene expression [61, 62]. The RNA sequencing technology (RNA-Seq) based transcriptome analysis is a developing modern technology that is the application of next-generation sequencing or deep sequencing to analyze transcriptome profiling [63]. Additionally, the advantage of single-cell RNA-Seq provides opportunities for studying the gene expression profiles of distinct cell types in multicellular organs [64]. From the first attempt of profiling the transcriptome in individual cells using NGS technologies [65], single-cell RNA-Seq with an abundance of immature and mature mRNA for the transcriptional dynamics has developed the gene expression state called RNA velocity [66, 67]. RNA velocity can predict the sequential gene expression status by determining the amount of mRNA from synthesis to degradation at the single-cell level. As RNA-Seq has advantages, such as identifying and quantifying the expression of isoforms and unknown transcripts, isolated pure cell population using surface markers and/or genetic reporter system are becoming important tools to understand the step-wise generation of skeletal muscle cells from hESCs and hiPSCs.
Tissue engineering has provided bio-inspired biological scaffold and constructs that can be implanted as medical devices obtaining knowledge and technical advantages from related fields such as materials science [68], three-dimensional bioprinting technologies [69], nanotechnologies [70], cell biology, cell transplantation, and developmental biology [71]. Skeletal muscle tissue engineering combined with myogenic cells differentiated from hiPSCs is one of the most promising technologies to restore damaged skeletal muscle tissue [72]. The progress in the differentiation of skeletal muscle cells from hiPSCs is still on-going, however tissue engineering can enhance the ability of hiPSC-derived skeletal muscle cells to regenerate damaged muscle. Skeletal muscle tissue engineering will provide the structural support for proliferation and differentiation to skeletal muscle fibers for engraftment of transplanted muscle cells, suitable connection to the vascular system with the efficient conveyance for the metabolism, and formation of neuromuscular junctions with neural cells [73]. Tissue engineering with the convergence of multiple advantages, in particular from hiPSC-derived skeletal muscle cells, will provide an optimistic future for the therapeutic treatment of skeletal muscle disease.
In vitro differentiation of skeletal muscle cells from hPSCs
In human embryonic development, the skeletal muscle precursor cells originate from segmented paraxial mesoderm, proliferate, and fuse into multinucleated myotubes to form skeletal muscle tissues (Fig. 4). A number of differentiation protocols have been developed to induce hPSCs into skeletal muscle lineages. Among them, one of the first examples to induce human skeletal muscle progenitor cells was the generation of multipotent mesenchymal precursors derived from human ESCs [74]. These human mesenchymal precursors have the ability to be differentiated into chondrocytes, osteocytes, and myoblasts. Notably, the inhibition of glycogen synthesis kinase-3 (GSK-3), a WNT signaling activator, is an essential step to induce mesodermal lineages from human ESCs [75–77]. In these studies, the primitive streak and/or mesendoderm-like cells also appeared at the initial stages of GSK-3 inhibition, otherwise, the endodermal fate cells were induced [75]. The activation of WNT signaling induced T +
+ and MIXL1
and MIXL1 +
+ mesendoderm differentiation of hPSCs without additional exogenous factors [76]. Furthermore, the treatment of an GSK-3 inhibitor stimulated the generation of skeletal muscle precursor cells from human ESCs [77]. Subsequently, many groups successfully induced skeletal muscle progenitor cells from mouse and hPSCs [59, 60, 78, 79]. The inhibition of GSK-3 promoted mesoderm induction during differentiation and significantly enhanced the expression of mesodermal marker genes such as T, TBX6, and MSGN1 [59, 60, 78]. Remarkably, sequential treatment of the GSK-3 inhibitor followed by a gamma-secretase inhibitor (a NOTCH inhibitor), synergistically enhanced the efficiency of myogenic cell differentiation [59, 60]. This corroborated previous studies in mouse embryos showing that the NOTCH signaling pathway is closely involved in the early myogenic specification, as previously mentioned [36–39].
mesendoderm differentiation of hPSCs without additional exogenous factors [76]. Furthermore, the treatment of an GSK-3 inhibitor stimulated the generation of skeletal muscle precursor cells from human ESCs [77]. Subsequently, many groups successfully induced skeletal muscle progenitor cells from mouse and hPSCs [59, 60, 78, 79]. The inhibition of GSK-3 promoted mesoderm induction during differentiation and significantly enhanced the expression of mesodermal marker genes such as T, TBX6, and MSGN1 [59, 60, 78]. Remarkably, sequential treatment of the GSK-3 inhibitor followed by a gamma-secretase inhibitor (a NOTCH inhibitor), synergistically enhanced the efficiency of myogenic cell differentiation [59, 60]. This corroborated previous studies in mouse embryos showing that the NOTCH signaling pathway is closely involved in the early myogenic specification, as previously mentioned [36–39].
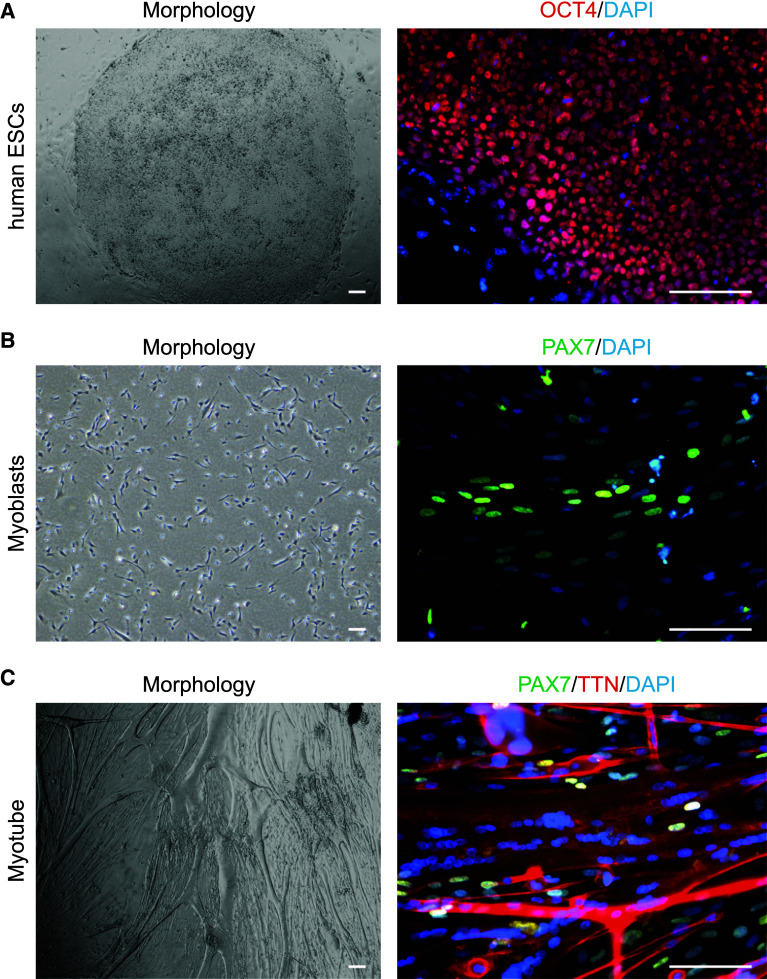
Representative images of skeletal muscle generation from hESCs to myotube [62, 63]. a Morphology of human ESCs and immunohistochemistry of pluripotency marker OCT4. b Morphology of skeletal muscle progenitor cells and immunohistochemistry of PAX7. c Morphology of multinucleated myotube and immunohistochemistry of PAX7 and TTN
Obtaining pure populations of skeletal muscle progenitor cells
A cell isolation strategy using surface markers and/or a genetic reporter system is needed to study the transcriptional dynamics during myogenesis. Cell surface markers are useful tools for the isolation of pure cell populations. A surface maker can be detected easily, and the number of surface markers have been determined to purify myogenic progenitor cells from hPSCs (Table (Table1).1). In one of the early studies, CD73+ mesenchymal precursors were isolated from human ESCs, and these cells were able to undergo multilineage differentiation into fat, cartilage, bone, and skeletal muscle cells [74]. A preliminary purification of skeletal muscle progenitor cells was achieved with CD73
mesenchymal precursors were isolated from human ESCs, and these cells were able to undergo multilineage differentiation into fat, cartilage, bone, and skeletal muscle cells [74]. A preliminary purification of skeletal muscle progenitor cells was achieved with CD73 +
+ following NCAM
following NCAM +
+ myoblasts derived from human ESCs, and these cells were engraftable into the tibialis anterior muscle of a mouse [80]. Subsequently, many groups succeeded in isolating pure human myogenic progenitors with the following surface markers: SMALD
myoblasts derived from human ESCs, and these cells were engraftable into the tibialis anterior muscle of a mouse [80]. Subsequently, many groups succeeded in isolating pure human myogenic progenitors with the following surface markers: SMALD +
+ /CD34- [81], CD56
/CD34- [81], CD56 +
+ /CD15- [82], PDFGR
/CD15- [82], PDFGR +
+ /KDR- [83], HNK1-/ACHR
/KDR- [83], HNK1-/ACHR +
+ /C-MET
/C-MET +
+ [77], CD34-CD56
[77], CD34-CD56 +
+ /ITGA7
/ITGA7 +
+ [84], CD133
[84], CD133 +
+ [85], PDGFR
[85], PDGFR +
+ [86], CD34
[86], CD34 +
+ [87], NCAM
[87], NCAM +
+ /HNK1- [59, 88], ERBB3
/HNK1- [59, 88], ERBB3 +
+ /NGFR
/NGFR +
+ [89], CD57-/CD108-/ERBB3
[89], CD57-/CD108-/ERBB3 +
+ /NGFR
/NGFR +
+ [90], CD10
[90], CD10 +
+ /CD24- [91], and CD271
/CD24- [91], and CD271 +
+ [60]. In particular, several groups discovered independently that NCAM [59, 80, 88] or NGFR (CD271) [60, 89, 90] were the most useful surface markers for isolating human myoblasts.
[60]. In particular, several groups discovered independently that NCAM [59, 80, 88] or NGFR (CD271) [60, 89, 90] were the most useful surface markers for isolating human myoblasts.
Table 1
Surface markers for purification in human cells
| Authors | Year | References | Species | Surface markers | Isolated cell type | Cell types | Platform |
|---|---|---|---|---|---|---|---|
| Barberi et al. | 2005 | [74] | Human | CD73 + + | Mesencymal precursors | H1/H9 | Array |
| Barberi et al. | 2007 | [80] | Human | CD73 + + /NCAM /NCAM + + | Myoblast | H1/H9 | Array |
| Vauchez et al. | 2009 | [81] | Human | SMALD + + /CD34- /CD34- | Precursors of CD56 + + myoblast myoblast | Biopsies of muscle | N/A |
| Pisani et al. | 2010 | [82] | Human | CD56 + + /CD15- /CD15- | Myogenic progenitors | Biopsies of muscle | N/A |
| Sakurai et al. | 2012 | [83] | Human | PDGFR + + /KDR- /KDR- | Paraxial mesodermal progenitors | hiPSCs | N/A |
| Borchin et al. | 2013 | [77] | Human | HNK1-/ACHR + + /C-MET /C-MET + + | Skeletal muscle precursors | H9 | N/A |
| Castiglioni et al. | 2014 | [84] | Human | CD34-/CD56 + + /ITGA7 /ITGA7 + + | Muslce stem cells | Fetal muscle/adult muscle | Array |
| Meng et al. | 2014 | [85] | Human | CD133 + + | Muslce stem cells | Biopsies of muscle | N/A |
| Uezumi et al. | 2014 | [86] | Human | PDGFR + + | Mesencymal progenitors | Biopsies of muscle | N/A |
| Demestre et al. | 2015 | [87] | Human | CD34 + + | Myoblast-like cells | hiPSCs | N/A |
| Choi et al. | 2016 | [59] | Human | NCAM + + /HNK1- /HNK1- | Myoblast | H9, hiPSCs, DMD hiPSCs, corrected DMD hiPSCs | Array, Array, Array, N/A |
| Young et al. | 2016 | [88] | Human | NCAM + + /HNK1- /HNK1- | N/A | hiPSCs, DMD hiPSCs, corrected DMD hiPSCs | N/A, N/A, N/A |
| Hicks et al. | 2018 | [89] | Human | ERBB3 + + /NGFR /NGFR + + | Skeletal muscle progenitor cells | H9, hiPSCs, DMD hiPSCs, corrected DMD hiPSCs | RNA-Seq, N/A, N/A, N/A |
| Saki-Takemura et al. | 2018 | [90] | Human | CD57-/CD108-/ERBB3 + + /NGFR /NGFR + + | Skeletal muscle progenitor cells | hiPSCs | N/A |
| Wu et al. | 2018 | [91] | Human | CD10 + + CD24- CD24- | Skeletal myogenic progenitor cells | H1, H9, hiPSCs | N/A, N/A, N/A |
| Choi et al. | 2020 | [60] | Human | CD271 + + | Skeletal muscle progenitor cells | H9 | N/A |
N/A not available
Another important method for the isolation of skeletal muscle progenitor cells is the use of a genetic reporting system with tagging proteins. Genetic reporters with specific promoters have been successfully applied to isolate cell populations of differentiated cells from embryonic stem cells [52, 92]. Lineage-specific promoters have been used to drive the expression of a fluorescent protein such as GFP to detect gene expression (Table (Table2).2). In early studies, a bacterial artificial chromosome (BAC) library was used to induce homologous recombination into human ESC lines to generate cell-type-specific reporter lines that were stably expressing HES::GFP, DLL1::GFP, and HB9::GFP [52]. Combining genome editing technologies and the expression of fluorescent proteins has generated genetic reporter lines without destroying the endogenous target genes. For the detection of myogenic lineage markers, human ESC lines tagged with GFP/EGFP, tdTomato, mCherry, Achilles, or Venus and key myogenic markers such as MSGN1 [59, 60], HES7 [93], MESP2 [93], PAX7 [60, 91, 94, 95], MYF5 [91, 96], MYOG [60, 95], and RUNX1 [60] have been used to track the sequential differentiation of hPSCs.
Table 2
Genetic reporter line in human cells
| Authors | Year | References | Species | Targeted cell line | Cassette | Target region | Knock-in method | NGS platform |
|---|---|---|---|---|---|---|---|---|
| Choi et al | 2016 | [59] | Human | H9 | MSGN1::EGFP | Stop codon | CRISPR/Cas9 | N/A |
| Wu et al | 2016 | [94] | Human | 293T, hiPSCs | PAX7::GFP | Stop codon | CRISPR/Cas9 | N/A |
| Wu et al | 2016 | [96] | Human | 293T, hiPSCs | MYF5::GFP | Stop codon | CRISPR/Cas9 | N/A |
| Wu et al | 2018 | [91] | Human | H9/H1 | MYF5::EGFP;PAX7::tdTomato | Stop codon; Stop codon | CRISPR/Cas9; CRISPR/Cas9 | RNA-Seq |
| Choi et al | 2020 | [60] | Human | H9, H9, H9, H9, hiPSCs | OCT4::EGFP; MSGN1::EGFP; PAX7::EGFFP; MYOG::EGFP; RUNX1::EGFP | Stop codon; ;Stop codon; Stop codon; Stop codon; Stop codon | TALENs; CRISPR/Cas9; CRISPR/Cas9; CRISPR/Cas9; ZFN | RNA-Seq; RNA-Seq; RNA-Seq; RNA-Seq; N/A |
| Diaz-Cuadros et al | 2020 | [93] | Human | hiPSCs, hiPSCs, hiPSCs | HES7::Achilles; HES7::Achilles;pCAG::H2B::mCherry; HES7::Achilles;MESP2::mCherry | Stop codon; Stop codon; Stop codon | CRISPR/Cas9; CRISPR/Cas9; CRISPR/Cas9 | Single-cell RNA-Seq; Single-cell RNA-Seq; Single-cell RNA-Seq |
| Tanoury et al | 2020 | [95] | Human | hiPSCs, hiPSCs | PAX7::Venus; MYOG::Venus | Start codon; Stop codon | CRISPR/Cas9; CRISPR/Cas9 | Single-cell RNA-Seq; Single-cell RNA-Seq |
N/A not available
Transcriptional profiling of human myogenesis and its application for therapeutic treatment of skeletal muscle diseases
Significant progress has been achieved in the last decade to induce the formation of skeletal muscle cells from PSCs [97]. Several protocols for the generation of skeletal muscle cells from hPSCs have been well established based on the expression of skeletal muscle-specific gene isoforms [48]. However, no extensive transcriptional roadmap of how hPSCs become skeletal muscle cells in vitro was developed.
Recently, our group published a transcriptional analysis of human myogenesis in vitro using multiple genetic reporter hESC lines. We performed an unbiased clustering analysis of the transcriptional trajectory during in vitro myogenesis with multiple genetic reporter hESCs; this presented a comprehensive insight into the stage-specific molecular signatures of skeletal muscle specification [60]. By means of stage-specific genetic reporter lines and myotube isolation strategy, OCT4::EGFP +
+ pluripotent stem cells, MSGN1::EGFP
pluripotent stem cells, MSGN1::EGFP +
+ presomite cells, PAX7::EGFP
presomite cells, PAX7::EGFP +
+ skeletal muscle progenitor cells, MYOG::EGFP
skeletal muscle progenitor cells, MYOG::EGFP +
+ myoblasts, and multinucleated myotubes were isolated during hESC-based skeletal muscle differentiation. With an unbiased analysis of RNA-Seq, K-mean clustering was then performed and presented a transcriptional roadmap during skeletal muscle generation. CD271 was identified in putative skeletal muscle stem/progenitor cells that can replace the PAX7::EGFP reporter system, which can be a new marker for isolating satellite-like cells, and CD271
myoblasts, and multinucleated myotubes were isolated during hESC-based skeletal muscle differentiation. With an unbiased analysis of RNA-Seq, K-mean clustering was then performed and presented a transcriptional roadmap during skeletal muscle generation. CD271 was identified in putative skeletal muscle stem/progenitor cells that can replace the PAX7::EGFP reporter system, which can be a new marker for isolating satellite-like cells, and CD271 +
+ cells presented high levels of multinucleated myotube formation. Similar approaches revealed undefined roles of RUNX1 in skeletal muscle lineages. The expression level of RUNX1 gradually increased throughout myogenesis and peaked during the formation of the myotubes. Another important finding was identifying TWIST1 grouped into the PAX7::EGFP
cells presented high levels of multinucleated myotube formation. Similar approaches revealed undefined roles of RUNX1 in skeletal muscle lineages. The expression level of RUNX1 gradually increased throughout myogenesis and peaked during the formation of the myotubes. Another important finding was identifying TWIST1 grouped into the PAX7::EGFP +
+ putative skeletal muscle progenitor cell category. Importantly, TWIST1 mutations cause Saethre-Chotzen syndrome, an autosomal dominant craniosynostosis syndrome with skeletal muscle deformation [98, 99]. Although further investigation is needed, TWIST1 may play a role in skeletal muscle progenitor cells and/or PAX7
putative skeletal muscle progenitor cell category. Importantly, TWIST1 mutations cause Saethre-Chotzen syndrome, an autosomal dominant craniosynostosis syndrome with skeletal muscle deformation [98, 99]. Although further investigation is needed, TWIST1 may play a role in skeletal muscle progenitor cells and/or PAX7 +
+ satellite cells, related to the pathogenic events that result in the musculoskeletal symptoms of Saethre-Chotzen syndrome. Our study emulated human in vitro myogenesis and provided insight into the potential pathogenic mechanism in congenital musculoskeletal diseases.
satellite cells, related to the pathogenic events that result in the musculoskeletal symptoms of Saethre-Chotzen syndrome. Our study emulated human in vitro myogenesis and provided insight into the potential pathogenic mechanism in congenital musculoskeletal diseases.
Another approach to modeling musculoskeletal diseases is to generate disease-specific stem cells. For example, deriving mouse ESCs of an animal disease model could be a useful approach to study in vitro phenotypes that could be complementary to in vivo models. Chal et al., published a disease modeling study using mouse ESCs from mdx mice, the animal model of Duchenne muscular dystrophy (DMD) in which the mice have a mutation in the dystrophin gene [79]. In this study, the mouse ESC lines from the blastocysts of mdx mice were derived to generate skeletal muscle cells for disease modeling. These skeletal muscle cells produced multinucleated myotubes based on the protein expression of fast MyHC. These fast MyHC +
+ myotubes exhibited more abnormal branches without any defects in membrane integrity compared with the control. The increased branching of the skeletal muscle cells may induce abnormal morphogenesis during skeletal muscle regeneration in mdx mice.
myotubes exhibited more abnormal branches without any defects in membrane integrity compared with the control. The increased branching of the skeletal muscle cells may induce abnormal morphogenesis during skeletal muscle regeneration in mdx mice.
While the mdx mouse has been a widely accepted model for DMD, there are over 1,000 genetic variations in the dystrophin gene found in the patients of DMD and Becker muscular dystrophies. The generation of new mouse models for each mutation found in the dystrophin gene is an option; alternatively, it is possible to develop patient-specific hiPSC lines.
We developed a hiPSC-based disease model and drug validation study has been reported by our group [59]. We developed a protocol to direct the hiPSCs of DMD with multiple mutations (exon deletions, duplications, point mutations, etc.) and isolated myoblasts with a NCAM +
+ HNK1- sorting strategy. Following a comparative transcriptional analysis of healthy controls and DMD hiPSC-derived NCAM
HNK1- sorting strategy. Following a comparative transcriptional analysis of healthy controls and DMD hiPSC-derived NCAM +
+ HNK1- myoblasts, we identified aberrant activation of the BMP and TGFβ signaling pathways in the patient myoblasts. In addition, we found that the DMD hiPSC-derived NCAM
HNK1- myoblasts, we identified aberrant activation of the BMP and TGFβ signaling pathways in the patient myoblasts. In addition, we found that the DMD hiPSC-derived NCAM +
+ HNK1- myoblasts showed significantly decreased levels of myotube formation, which was rescued by the genetic addition of dystrophin (either by artificial chromosome or genetic correction by CRISPR/Cas9 system) and the pharmacological inhibition of SMAD signaling pathway [59].
HNK1- myoblasts showed significantly decreased levels of myotube formation, which was rescued by the genetic addition of dystrophin (either by artificial chromosome or genetic correction by CRISPR/Cas9 system) and the pharmacological inhibition of SMAD signaling pathway [59].
The transplantation of hPSC derived skeletal muscle progenitor cells into the skeletal muscle of animal muscle tissue improved physiological muscle function in dystrophic mice [59, 100, 101]. Although the transplantation of hPSC-derived skeletal muscle cells could be replicated, there are still challenges such as migration into the skeletal muscle niche areas, long-term engraftment, transition to dormant or quiescent status, and cellular maturation of the hPSCs-derived skeletal muscle progenitor cells into the adult stage. The establishment and identification of skeletal muscle stem cells with functional engraftment capabilities will be crucial for addressing such critical challenges; this will elucidate the molecular mechanisms and transcriptional trajectory of regeneration. Recent advances in skeletal muscle tissue generation from hPSCs will increase our knowledge of human skeletal muscle generation and insight into a number of muscular dystrophies.
Conclusion
Much progress has been made in the establishment of a transcriptional draft of human myogenesis from several studies to dissect the complicated molecular regulations of the skeletal muscle fate determination process, as well as the molecular pathology of muscular dystrophies. Because elucidating disease mechanisms has a crucial role in drug discovery and therapeutic treatment, further studies with hiPSCs of muscular dystrophies should focus on the comprehensive transcriptional profiling of specific subsets of the skeletal muscle lineage, which can lead to uncovering abnormal aspects of signaling molecules or gene regulation network as potential drug targets. The hope is that integrative biological experimentation, in conjunction with newly developed technologies, will decipher the transcriptional regulatory mechanisms of skeletal muscle generation and facilitate finding new treatments for musculoskeletal diseases in near future.
Authors’ contributions
HTL analyzed the published data and wrote the manuscript. IYC wrote the manuscript. HK wrote the manuscript. SHH over-saw data interpretation and contributed to writing the manuscript. GL designed the review paper and wrote the manuscript.
Funding
This work was supported by NIH grants R01NS093213 (GL), R01AR070751 (GL), the Maryland Stem Cell Research Fund (MSCRF) (GL), the Muscular Dystrophy Association (MDA) (GL), and the Global Research Development Center Program from the National Research Foundation of Korea (NRF) (2017K1A4A3014959, G.L. & S.-H.H.).
Compliance with ethical standards
GL is a founder of the Vita Therapeutics. IYC, HTL and GL are shareholders of the Vita Therapeutics. SHH and HK declare no potential conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Articles from Cellular and Molecular Life Sciences: CMLS are provided here courtesy of Springer
Citations & impact
This article has not been cited yet.
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/156794459
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Recapitulating human myogenesis ex vivo using human pluripotent stem cells.
Exp Cell Res, 411(2):112990, 30 Dec 2021
Cited by: 1 article | PMID: 34973262 | PMCID: PMC8996775
Review Free full text in Europe PMC
Transcriptional landscape of myogenesis from human pluripotent stem cells reveals a key role of TWIST1 in maintenance of skeletal muscle progenitors.
Elife, 9:e46981, 03 Feb 2020
Cited by: 29 articles | PMID: 32011235 | PMCID: PMC6996923
Myogenic progenitor specification from pluripotent stem cells.
Semin Cell Dev Biol, 72:87-98, 01 Dec 2017
Cited by: 22 articles | PMID: 29107681 | PMCID: PMC5723218
Review Free full text in Europe PMC
Making muscle: skeletal myogenesis in vivo and in vitro.
Development, 144(12):2104-2122, 01 Jun 2017
Cited by: 376 articles | PMID: 28634270
Review
Funding
Funders who supported this work.
Maryland Stem Cell Research Fund (1)
Grant ID: 2017-MSCRFD-3941
Muscular Dystrophy Association (1)
Grant ID: 381465
NIAMS NIH HHS (2)
Grant ID: R01 AR070751
Grant ID: R01AR070751
NINDS NIH HHS (1)
Grant ID: R01 NS093213
National Institute of Arthritis and Musculoskeletal and Skin Diseases (1)
Grant ID: R01AR070751
National Institute of Neurological Disorders and Stroke (1)
Grant ID: R01NS093213
National Institute of Neurological Disorders and Stroke (US) (1)
Grant ID: R01NS093213
 2,4,5
2,4,5