Abstract
Free full text

Allergenic components of the mRNA‐1273 vaccine for COVID‐19: Possible involvement of polyethylene glycol and IgG‐mediated complement activation
Abstract
Following the emergency use authorization of the mRNA‐1273 vaccine on the 18th of December 2020, two mRNA vaccines are in current use for the prevention of coronavirus disease 2019 (COVID‐19). For both mRNA vaccines, the phase III pivotal trials excluded individuals with a history of allergy to vaccine components. Immediately after the initiation of vaccination in the United Kingdom, Canada, and the United States, anaphylactic reactions were reported. While the culprit trigger requires investigation, initial reports suggested the excipient polyethylene glycol 2000 (PEG‐2000)—contained in both vaccines as the PEG‐micellar carrier system—as the potential culprit. Surface PEG chains form a hydrate shell to increase stability and prevent opsonization. Allergic reactions to such PEGylated lipids can be IgE‐mediated, but may also result from complement activation‐related pseudoallergy (CARPA) that has been described in similar liposomes. In addition, mRNA‐1273 also contains tromethamine (trometamol), which has been reported to cause anaphylaxis to substances such as gadolinium‐based contrast media. Skin prick, intradermal and epicutaneous tests, in vitro sIgE assessment, evaluation of sIgG/IgM, and basophil activation tests are being used to demonstrate allergic reactions to various components of the vaccines.
1. INTRODUCTION
On the 18th of December 2020, an emergency use authorization (EUA) provided by the US Food and Drug Administration (FDA) permitted the immediate use of the mRNA‐1273 vaccine developed by Moderna Therapeutics for the prevention of coronavirus disease 2019 (COVID‐19). The EUA allowed the immediate distribution and use of the mRNA‐1273 COVID‐19 vaccine in the United States in subjects 18 years of age and older. 1 It is the second vaccine to be granted an EUA by US regulators after the authorization received by Pfizer‐BioNTech on the 11th of December 2020 for the use of the BNT162b2 vaccine. 2 The authorization of mRNA‐1273 was based on early‐phase trials 3 , 4 and on the revision of the results of an ongoing phase III trial that involved 33,000 adult subjects, randomized 1:1 to receive them RNA‐1273 vaccine in a two‐dose regimen or placebo. The assessment performed by the FDA demonstrated that the vaccine was 94.1% effective for the prevention of COVID‐19 as determined 14 days after the administration of the second dose. 1 A total of 196 cases were evaluated for the efficacy analysis of which 185 cases of COVID‐19 were observed in the placebo group versus 11 in the mRNA‐1273 group. The secondary endpoint involved the assessment of severe cases of COVID‐19 and included 30 individuals. All of the severe cases occurred in the placebo group and none in the mRNA‐1273 vaccinated group. 5
The FDA stated that the potential benefits of mRNA‐1273 outweigh the potential risks. 1
Serious allergic reactions to the active components of the vaccine itself, or to other components, are one of the potential risks of every vaccination product. According to the New York Times 6 on the 25th of December, a physician in Boston developed an anaphylactic reaction to mRNA‐1273 soon after vaccination started in the United States. He used his own adrenaline autoinjector, carried for his shellfish allergy, and recovered well. On the 10th of January 2021, 4,041,396 people had received the first dose of the mRNA‐1273 vaccine and 1266 adverse events had been reported. A total of 10 cases were determined as being anaphylaxis, all of which recovered. 7
Anaphylactic reactions to BNT162b2 were also reported in the United Kingdom (UK), Canada, and the United States. 8 , 9
Allergic reactions to vaccines including severe anaphylaxis are generally IgE‐mediated. 10 , 11 They usually occur within the first 30 min after vaccination and can be fatal. The symptoms include urticarial rashes, generalized pruritus, erythema, wheezing, coughing, dyspnea, throat, tongue, or eye swelling (angioedema), hypotension, dizziness, and vomiting. Furthermore, IgG‐ and complement‐mediated anaphylaxis‐like reactions have been described. 12 , 13 Clinical symptoms of this non‐IgE‐mediated hypersensitivity include, among others, hypo‐ and hypertension as well as airway obstruction with dyspnea. 14 Severe anaphylactic reactions to vaccines are rare, and the rate has been estimated at 1.31 (95% CI, 0.90–1.84) per million vaccine doses. 15
The frequency of allergic reactions to BNT162b2 was nearly the same in both the verum and placebo groups (0.6% vs. 0.5%). 16
Allergic reactions to the mRNA‐1273 vaccine have not been reported in detail. During the phase I trial of the mRNA‐1273 vaccine, one case of transient urticaria in the verum group treated with a vaccine dose of 25 µg was reported after the first injection. The total number of participants in this phase I trial was 45. 17
Both BioNTech/Pfizer and Moderna excluded individuals with a history of allergic reaction to vaccines or their components from the phase III trials. The exclusion criteria for mRNA‐1273 state: “History of anaphylaxis, urticaria, or other significant adverse reaction requiring medical intervention after receipt of a vaccine”. 18
The anaphylactic reactions to the BioNTech/Pfizer vaccine during the routine vaccination prompted the Medicines and Healthcare Products Regulatory Agency (MHRA) in the UK to issue an alert stating that individuals with a history of severe allergic reactions to vaccines, medicines, food, or to any component of these particular vaccines should be advised against their administration and that a second dose should not be given to anyone having experienced anaphylaxis after the first dose. 19 Currently, the MHRA only excludes those with a hypersensitivity to the active substance or to any of the excipients of the approved vaccines. Individuals having had a hypersensitive reaction to the first dose should not receive the second dose. For the PEG‐containing vaccines, a warning was also issued for individuals hypersensitive to polysorbates, due to a potential cross‐reactivity. 20 , 21 , 22 The EMA and the FDA both have the same recommendations for the BioNTech/Pfizer and Moderna vaccines. 23 , 24 , 25 , 26 , 27
Although the culprit trigger has yet to be determined, initial reports pointed at the excipient polyethylene glycol 2000 (PEG‐2000)—contained in the vaccine as a PEG‐micellar carrier system—as the potential cause of the anaphylactic reactions. 8 , 28 PEGylated microsomes used as the carrier of the vaccine can cause anaphylactic reactions in individuals with preexisting PEG allergies, as has been previously observed for PEGylated drugs used in cancer therapy and treatment of chronic diseases. 8 , 29 , 30 PEG is also used as an excipient in other medicinal products as well as in everyday products, such as deposteroids, tablets, toothpaste, cosmetics, shampoos, and some biologicals. 31
Lipid nanoparticles are similar to liposomes, which have been in use pharmaceutically for many years as carriers for drugs. Some of the approved liposome/LNP‐containing drugs also contain a PEGylated lipid (e.g., in Caelyx pegylated liposomal® or Onpattro®). The PEG chains on the surface form a hydrate shell around the liposome/LNP. This increases stability and prevents opsonization, that is, the mechanism by which the surface of foreign cells (e.g., bacteria, viruses) that have invaded the body is covered with antibodies and factors of the complement system. In addition, the stability and half‐life of the lipid particles are increased.
2. PATHOPHYSIOLOGY
Allergic reactions to such PEGylated lipids are IgE‐mediated. 10 , 11 However, non‐IgE‐mediated reactions should also be considered. 32 , 33 , 34
IgE activates mast cells and basophilic granulocytes via cross‐links of high‐affinity IgE receptors, which is indirectly measurable in an increased expression of surface markers (CD63, CD203c) on basophils. 9 , 35
The symptoms of anaphylactic reactions are particularly caused by mediators released mainly from mast cells and basophilic granulocytes such as histamine, prostaglandins, leukotrienes (LTB4, LTC4, and LTD4), tryptase, platelet‐activating factor (PAF), heparin, proteases, serotonin, and cytokines. 9 , 35 Besides IgE, other antibody classes may trigger similar symptomatology or amplify an IgE‐mediated reaction. 9 , 35 Possible non‐IgE‐mediated reactions include complement activation‐related pseudoallergy (CARPA) and have been described in the context of liposomes. 36 , 37 , 38
Updating the Gell and Coombs scheme of type I–IV hypersensitivity reactions (HSRs), 39 CARPA may be regarded as an independent category within type I reactions, representing “receptor‐mediated” mast cell activations. 38
CARPA is partly attributed to the binding of preexisting anti‐PEG IgM to the liposomes with subsequent complement activation. 40 Clinical symptoms of this non‐IgE‐mediated hypersensitivity have been described as hypo‐ and hypertension, airway obstruction with dyspnea, and other anaphylaxis symptoms shortly after the intravenous administration of liposome‐containing drugs. 12 , 33 , 34 , 41 , 42 Independent of PEGylation, liposomes have the potential to activate complement, non‐specifically depending on their different surface structures and charge. 12 , 36 Complement products C3a, C4a, and C5a (anaphylatoxins) are considered to be particularly important mediators. In addition to basophils, neutrophils and macrophages are also considered to be relevant effector cells that can be activated via immune complex receptors (CD16, CD32, and CD64, respectively). 9 , 12 , 13 , 35 Anaphylatoxins are liberated during complement activation. 38 , 43
Possible sensitization to PEG by previous use of cosmetics or drugs containing PEG is conceivable. Little is known about the prevalence of anti‐PEG antibodies in the population. Certain reports state that as much as 72% of the population have at least some IgG or IgM antibodies against PEG, 44 while others report high levels in healthy people and even in those without documented treatment with PEGylated products. 37 , 45 , 46 However, there is no evidence for allergy or anaphylaxis depending on IgG in man. Evidence for a possible role of IgE in triggering PEG‐induced hypersensitivity is also being discussed. 47 , 48 , 49 Allergic reactions following the use of PEG as an excipient in a variety of products have been described. This is also referred to as a “hidden” allergen. 47 , 48 , 49 , 50
Similar to BNT162b2, the mRNA‐1273 COVID‐19 vaccine is a messenger ribonucleic acid (mRNA) vaccine encoding the viral spike (S) glycoprotein of SARS‐CoV‐2. The list of excipients of both vaccines shares certain components but also differs in others. Interestingly, PEG‐2000 can also be found as an excipient in the mRNA‐1273 COVID‐19 vaccine (Figure 1). It should be noted that PEGs have never been used in any vaccine and that both Pfizer‐BioNTech and Moderna are the first to apply this substance. PEG is a hydrophilic polyether compound used as an additive in medical products and cosmetics. 51 It is branded under different names, for example, macrogol. The molecular weight of the different PEG excipients varies from 300 to 10,000 g/mol or higher. Furthermore, hypersensitivity reactions may occur to the PEG of all molecular weights, with a higher rate of reaction to molecular weights of between 3350 and 6000 g/mol. 47 However, it has been suggested that the molecular weight threshold for PEG immediate reactions is still undetermined. 48 , 52

Representation of the Moderna COVID‐19 vaccine: The principle of the PEGylated‐lipid nanoparticles as a delivery system for the mRNA is illustrated together with the full list of ingredients contained in the vaccine. PEG‐2000 and tromethamine/trometamol as potential triggers of allergic reactions are indicated in red and shown on the left side. Different ways of exposition to PEG and PEG analogues are illustrated on the right side. Biorender software was used to create the figure under an academic license
Cross‐reactivity between PEG and its derivatives—that is, structurally related polymers such as polysorbates—exists due to shared moieties (=CH2CH2 and =CH2CH2OH). 47 Severe allergic reactions to PEG, although rare, have been described after the administration of medications that contain this excipient. PEG has even been described as the high‐risk hidden allergen, since it is difficult to detect as a possible cause of allergic reaction. 30 , 47 , 48 , 52 , 53 PEGylation is used successfully for drug delivery to protect the drug from any damage by the immune system and to deliver it to the targeted location. In addition, PEG is an additive in cosmetics and shampoos. Both a primary cutaneous sensitization pathway and sensitization after systemic administration are possible. 48
Additionally, and unlike the Pfizer‐BioNTech vaccine, mRNA‐1273 contains tromethamine, also called trometamol (molecular formula: C4H11NO3), an organic amine that is widely used in several medications for topical, enteral, or parenteral administration. Tromethamine/trometamol is also used in cosmetic products as an emulsifier, and contact sensitization and allergy to this compound have been described. 54 Recently, the first case of anaphylaxis to trometamol as an excipient in a gadolinium‐based contrast agent (GBCA) was reported. 55 The reaction occurred immediately after the GBCA injection in a 23‐year‐old woman, and IgE‐mediated trometamol allergy was detected in this patient. 55
3. DIAGNOSTIC OPTIONS
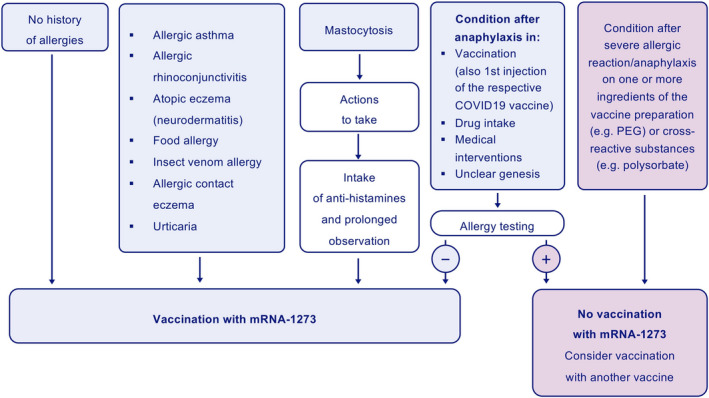
To avoid anaphylactic or anaphylactoid reactions to vaccines, individuals at possible risk should be identified by asking for their medical history and by diagnostic procedures. Reactions to PEG in substances such as depot steroids, laxatives, gels, wound dressings, lotions, toothpaste, mouthwash, cosmetics, and shampoos may be indicative. 27 , 31 , 48 , 52 Depending on the risk profile, a decision must be made as to the conditions under which vaccination is possible (Figure 2). 35 , 56 , 57
Risk factors exacerbating allergic reactions need to be taken into account when noting the medical history, such as previous anaphylaxis, mastocytosis, or other mast cell diseases (Figure 2).
Allergy testing should be performed in specialized allergy centers. Skin prick tests should be performed very carefully:initial dilutions from 0.001% up to 10% and a 30‐minute observation period after every dose step to minimize the risk of a systemic reaction to PEG. Since it has been speculated that the individual threshold for positive reactions to PEG of different molecular weights varies, 52 testing should be performed with PEG excipients of 2000 g/mol molecular weight that are used in both vaccines. Published algorithms should also be followed. 52 Skin tests should be performed not earlier than 2 weeks after the hypersensitivity reaction. In addition, basophil activation tests (BATs) 8 , 40 and screening for specific IgE to PEG in blood serum 8 , 40 may be performed in high‐risk individuals and in patients with suspected allergy to excipients of the vaccine. 8 If PEG allergy can be confirmed, an emergency kit should be prescribed, and a PEG‐allergy information sheet provided. If not, intradermal testing with PEG of different molecular weights at a dilution of 0.01% can be carefully considered. High‐risk patients should be excluded from those testing because systemic reactions can occur. 8 , 52 In some settings, oral provocation tests can be performed if necessary. 47
Trometamol as a contact sensitizer is usually tested epicutaneously for allergic reactions of the delayed type. Testing for suspected type 1 reactions can be done by the skin prick procedure (concentration 1:1) followed by intradermal testing with dilutions of trometamol from 1:1000 to 1:10. 55 , 59
Although allergic reactions tomRNA‐1273 components such as PEG and trometamol have not been frequently reported, the fact that the COVID‐19 vaccines will be extensively administrated worldwide to a high proportion of the population should caution healthcare providers of the potential allergic reactions that may occur in individuals previously sensitized to the components of the vaccines, especially to PEG and PEG analogues as well as to trometamol in the case of mRNA‐1273. 60
4. THERAPEUTIC OPTIONS
This allergy is of particular interest since some of the drugs used to treat anaphylactic reactions, such as antihistamines or injectable corticosteroids, contain PEG or polysorbates. Substances that are cross‐reactive to PEG, that is, polysorbates, 27 are widely distributed and commonly used in bread, pastry, chewing gum, ice cream, etc. They are also present in a high number of vaccines, biologics, and mediations to treat rheumatologic, cardiovascular, hematologic, gastrointestinal, or oncologic diseases, and during diagnostic procedures. It is very likely that hypersensitivity reactions to such agents have been underestimated in the past.
Two doses of the vaccine must be administered to achieve an effect. Sensitization might occur during the administration of the first dose, or individuals may develop allergic reactions to the second dose. Whether the new route of delivery of PEG via intramuscular injection plays a role in its allergenicity has yet to be determined. Since both mRNA‐1273 and BNT162b2 contain PEG‐2000, PEG‐allergic patients, or patients allergic to components that are cross‐reactive to PEG should be vaccinated with alternative COVID‐19 vaccines. Physicians should be aware of this potential risk and should interrogate thoroughly for previous allergic reactions to PEG, PEG analogues, or tromethamine. They should also be trained to respond to potential anaphylactic reactions during vaccination. In these patients, the administration of emergency medications containing PEG should be avoided. Alternatives should be considered, such as clemastine solution for intravenous injection, cetirizine syrup for oral intake, soluble prednisolone or methylprednisolone for oral intake or injection, and, of course, as recommended for all patients with severe anaphylaxis, most importantly, adrenaline. 52
CONFLICT OF INTEREST
Dr. Akdis reports grants from Allergopharma, Idorsia, Swiss National Science Foundation, Christine Kühne—Center for Allergy Research and Education, European Commission's Horison's 2020 Framework Programme, Cure, Novartis Research Institutes, Astra Zeneca, Scibase, advisory role in Sanofi/Regeneron, grants from Glakso Smith‐Kline, advisory role in Scibase. Dr. Bousquet reports personal fees from Chiesi, Cipla, Hikma, Menarini, Mundipharma, Mylan, Novartis, Purina, Sanofi‐Aventis, Takeda, Teva, Uriach, other from KYomed Innov, outside the submitted work. Dr. Jutel reports personal fees from ALK‐Abello, Allergopharma, Stallergenes, Anergis, Allergy Therapeutics, Circassia, Leti, Biomay, HAL, during the conduct of the study; Astra‐Zeneka, GSK, Novartis, Teva, Vectura, UCB, Takeda, Roche, Janssen, Medimmune, Chiesi, outside the submitted work. Dr. Klimek reports grants and/or personal fees from Allergopharma, MEDA/Mylan, HAL Allergie, ALK‐Abelló, from LETI Pharma, Stallergenes, Quintiles, Sanofi, ASIT biotech, Lofarma, Allergy Therapeut., AstraZeneca, GSK, Inmunotk, Cassella med, outside the submitted work; and reports Membership: AeDA, DGHNO, Deutsche Akademie für Allergologie und klinischeImmunologie, HNO‐BV, GPA, EAACI. Dr. Novak reports grants and/or personal fees from ALK‐Abello, HAL Allergy, Stallergenes Geer, Leti Pharma, Novartis, Leo Pharma, Abbvie, Sanofi Genzmye, Lofarma, Bencard Allergy Therapeutics, outside the submitted work. Dr. Cabanillas has nothing to disclose.
5.
TABLE 1
| BNT162b2 vaccine | mRNA−1273 vaccine | |
| Active component: mRNA encoding the viral spike (S) glycoprotein of SARS‐CoV−2 | ↔ | Active component: mRNA encoding the viral spike (S) glycoprotein of SARS‐CoV−2 |
| Excipients | Excipients | |
| ALC‐0315=(4‐hydroxybutyl)azanediyl)bis(hexane‐6,1‐diyl)bis(2‐hexyldecanoate) | SM‐102 | |
| ALC‐0159=2‐[(polyethylene glycol)2000]‐N,N‐ditetradecylacetamide | ↔ | 1,2‐dimyristoyl‐rac‐glycero‐3‐methoxypolyethylen glycol‐2000 [PEG2000‐DMG] |
| 1,2‐distearoyl‐sn‐glycero‐3‐phosphocholin | ↔ | 1,2‐distearoyl‐sn‐glycero‐3‐phosphocholin |
| Cholesterol | ↔ | Cholesterol |
| Potassium chloride | Tromethamine | |
| Potassium dihydrogen phosphate | Tromethamine hydrochloride | |
| Sodium chloride | Acetic acid | |
| Disodium hydrogen phosphate dihydrate | Sodium acetate | |
| Sucrose | ↔ | Sucrose |
| Water for injections |
Notes
Ludger Klimek and Natalija Novak participated equally in this paper.
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.1111/all.14794
Read article for free, from open access legal sources, via Unpaywall:
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/all.14794
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/101331289
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1111/all.14794
Article citations
Occurrence of Myopericarditis Following COVID-19 Vaccination Among Adults in the Eastern Region, Saudi Arabia: A Multicenter Study.
Int J Gen Med, 17:3231-3237, 23 Jul 2024
Cited by: 0 articles | PMID: 39070223 | PMCID: PMC11283260
Medical occurrence and safety of SARS-CoV-2 vaccination outside of the hospital setting.
Intern Emerg Med, 19(6):1593-1604, 23 Jul 2024
Cited by: 0 articles | PMID: 39042210
Incidence and management of the main serious adverse events reported after COVID-19 vaccination.
Pharmacol Res Perspect, 12(3):e1224, 01 Jun 2024
Cited by: 3 articles | PMID: 38864106
Review
Understanding COVID-19 Vaccine Hesitancy in the United States: A Systematic Review.
Vaccines (Basel), 12(7):747, 06 Jul 2024
Cited by: 2 articles | PMID: 39066385 | PMCID: PMC11281578
Review Free full text in Europe PMC
Does COVID-19 vaccination trigger gross hematuria in patients with IgA nephropathy?
Clin Kidney J, 17(7):sfae160, 31 May 2024
Cited by: 0 articles | PMID: 38962251 | PMCID: PMC11217807
Go to all (70) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Anaphylaxis associated with the mRNA COVID-19 vaccines: Approach to allergy investigation.
Clin Immunol, 227:108748, 28 Apr 2021
Cited by: 22 articles | PMID: 33932618 | PMCID: PMC8080508
Basophil reactivity to BNT162b2 is mediated by PEGylated lipid nanoparticles in patients with PEG allergy.
J Allergy Clin Immunol, 148(1):91-95, 12 May 2021
Cited by: 45 articles | PMID: 33991580
Role of anti-polyethylene glycol (PEG) antibodies in the allergic reactions to PEG-containing Covid-19 vaccines: Evidence for immunogenicity of PEG.
Vaccine, 41(31):4561-4570, 05 Jun 2023
Cited by: 15 articles | PMID: 37330369 | PMCID: PMC10239905
COVID-19 mRNA vaccine allergy.
Curr Opin Pediatr, 33(6):610-617, 01 Dec 2021
Cited by: 12 articles | PMID: 34670264 | PMCID: PMC8577302
Review Free full text in Europe PMC

 1
1