Abstract
Free full text

Growth hormone increases DNA damage in ovarian follicles and macrophage infiltration in the ovaries
Abstract
Evidence points to an important role of the growth hormone (GH) in the aging process and longevity. GH-deficient mice are smaller, live longer than normal littermates, and females have an increased ovarian reserve. The aim of the study was to evaluate the role of GH in the ovarian reserve by evaluating DNA damage, macrophage infiltration, and granulosa cell number in primordial and primary follicles. Experiment 1 used GH-deficient Ames dwarf mice (df/df, n =
= 12) and their normal littermates (N/df, n
12) and their normal littermates (N/df, n =
= 12), receiving GH or saline injections. Experiment 2 included transgenic mice overexpressing bovine GH (bGH) (n
12), receiving GH or saline injections. Experiment 2 included transgenic mice overexpressing bovine GH (bGH) (n =
= 6) and normal mice (N, n
6) and normal mice (N, n =
= 6). DNA damage (anti-γH2AX) and macrophage counting (anti-CD68) were evaluated by immunofluorescence. Female df/df mice had lower γH2AX foci intensity in both oocytes and granulosa cells of primordial and primary follicles (p
6). DNA damage (anti-γH2AX) and macrophage counting (anti-CD68) were evaluated by immunofluorescence. Female df/df mice had lower γH2AX foci intensity in both oocytes and granulosa cells of primordial and primary follicles (p <
< 0.05), indicating fewer DNA double-strand breaks (DSBs). GH treatment increased DSBs in both df/df and N/df mice. Inversely, bGH mice had a higher quantity of DSBs in both oocytes and granulosa cells of primordial and primary follicles (p
0.05), indicating fewer DNA double-strand breaks (DSBs). GH treatment increased DSBs in both df/df and N/df mice. Inversely, bGH mice had a higher quantity of DSBs in both oocytes and granulosa cells of primordial and primary follicles (p <
< 0.05). Df/df mice showed ovarian tissue with less macrophage infiltration than N/df mice (p
0.05). Df/df mice showed ovarian tissue with less macrophage infiltration than N/df mice (p <
< 0.05) and GH treatment increased macrophage infiltration (p
0.05) and GH treatment increased macrophage infiltration (p <
< 0.05). In contrast, bGH mice had ovarian tissue with more macrophage infiltration compared to normal mice (p
0.05). In contrast, bGH mice had ovarian tissue with more macrophage infiltration compared to normal mice (p <
< 0.05). The current study shows that GH increases DNA DSBs in oocytes and granulosa cells and raises macrophage infiltration in the ovaries, pointing to the role of the GH/IGF-I axis in maintenance of oocyte DNA integrity and ovarian macrophage infiltration in mice.
0.05). The current study shows that GH increases DNA DSBs in oocytes and granulosa cells and raises macrophage infiltration in the ovaries, pointing to the role of the GH/IGF-I axis in maintenance of oocyte DNA integrity and ovarian macrophage infiltration in mice.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-021-00380-8.
Introduction
Ames dwarf mice (df/df) have a defective Prophet of Pit-1 (Prop1) gene [1]. This mutation impairs the development of anterior pituitary gland and results in growth hormone (GH), prolactin, and TSH deficiency [1]. In consequence df/df mice have severely decreased insulin-like growth factor-1 (IGF-I) serum levels, reduced body size, and live 30 to 75% longer than normal littermates (N/df) [2]. In contrast, mice overexpressing bovine GH (bGH) have higher GH secretion, IGF-I plasma levels, and adult body size [3]. In contrast to long-lived df/df mice, bGH mice lifespan is 50% shorter than for normal (N) littermates [4]. Therefore, the GH/IGF-I axis has a central role in determining lifespan.
Menopause marks the end of reproductive cycles in women. This is the consequence of the gradual decline with aging of the ovarian primordial follicle reserve [5]. In addition to decreasing follicle numbers, the quality of the oocytes enclosed in these aged follicles is also compromised [5]. Reduced gamete quality along with reduced follicle number results in an increase in chromosomally abnormal conceptions and a decline in fertility [6]. Increased oocyte chromosome mis-segregations, karyotypic imbalances, and aneuploidies increase susceptibility to miscarriages with age, resulting in infertility [7]. Most karyotypic defects result in spontaneous abortion during the first trimester and thereby lead to a high rate of pregnancy failure during this time window [8]. Nevertheless, some complications may manifest in later pregnancy, such as miscarriage, placenta praevia, pre-eclampsia, late fetal and perinatal death, preterm and extreme preterm birth, stillbirth, and low birth weight [9].
The GH/IGF-I axis plays a key role in regulating ovary functions [10]. Past experiments in our laboratory have shown that mid-aged and old GH-deficient df/df mice have increased number of primordial follicles in the ovarian reserve compared to N/df mice [11, 12]. This suggests a strong link between longevity and fertility. bGH mice, on the other hand, have lower numbers of primordial follicles in the ovarian reserve compared to N mice [13, 14], further confirming the GH/IGF-I axis role in the reproductive lifespan. Despite the evidence for the role of GH/IGF-I in the preservation of the ovarian reserve, little is known regarding its impact on other markers of ovarian aging.
There are several factors that reduce fertility during aging. The adverse effects of DNA damage on somatic cells are well known [15]. Primordial follicle oocytes are arrested in prophase I of meiosis and can remain in this state for several decades [16]. In the long dormancy period, accumulation of DNA damage is increased, as shown in primordial follicles oocytes of older female mice and humans [17]. If protective cellular responses are not triggered, this damage can result in miscarriage or development of defective embryos that are unable to result in full-term pregnancy [18]. One of the cellular responses to DNA double-strand breaks (DSBs) is the recruitment of kinases that phosphorylate histone 2Ax (γH2AX) on serine 139 [19]. This provides a platform for the ataxia-telangiectasia mutated (ATM)-mediated DNA damage signaling pathways to regulate DNA DSBs repair [20], which can be easily measured and can indicate the levels of DNA damage.
In addition to DNA damage, inflammation also often increases with aging and can have a negative impact on fertility [21]. Mice with a knockout for the interleukin-1 gene have increased primordial follicle numbers and increased pregnancy rates at advanced ages [22]. RNASeq analysis showed that older df/df mice had more than 150 downregulated gene ontology terms linked to the inflammatory response, relative to N/df mice [23], suggesting that increased inflammation during ovarian aging is reduced in df/df mice. Levels of pro-inflammatory cytokines in adipose tissue and blood, such as interleukin-6 and tumor necrosis factor alpha (TNF-α), have been shown to be decreased in df/df mice [24]. Reduced inflammatory response is believed to be one of the key factors for the improved survival of df/df mice [25]. Given this context, macrophages are known to be present in large numbers in the ovary [26]. Inflammation and oxidative stress are frequently interlinked, and decreased cellular antioxidant ability can impair membrane and cellular function [27]. In addition, excess free radicals can increase DNA damage, which contributes to cellular decay with age [28].
Primordial oocytes are surrounded by a flattened layer of granulosa cells [29, 30]. To regulate optimal reproductive lifespan, oocytes are retained in this quiescent condition within primordial follicles [31]. However, the surrounding granulosa cells play a key role in triggering the growth of primordial follicles [31]. Therefore, the number of granulosa cells may be a valuable marker for follicular activation and growth [32]. In order to control the development of dormant oocytes, several molecular networks mediate interaction between somatic and germ cells [31, 33]. Despite this, the associations between the number of granulosa cells in primordial and primary follicles and reproductive longevity are poorly understood.
Based on this evidence, and the fact that we have shown before that GH regulates the activation of the primordial follicle reserve in mice [13], the aim of the present study was to evaluate the DNA damage, macrophage infiltration, and granulosa cell number in primordial and primary follicles in the ovaries of aged, long-living df/df, GH-deficient mice and short-lived bGH transgenic mice.
Materials and methods
Animals and treatment
Experiment 1 was run with 4 groups of Ames dwarf mice (Prop-1df, df/df, n =
= 12) and their normal littermates (N/df, n
12) and their normal littermates (N/df, n =
= 12), between 16 and 18-month-old, that received GH (n
12), between 16 and 18-month-old, that received GH (n =
= 6 for df/df, and n
6 for df/df, and n =
= 6 for N/df mice) or saline injections (n
6 for N/df mice) or saline injections (n =
= 6 for df/df, and n
6 for df/df, and n =
= 6 for N/df mice). Experiment 2 consisted in 2 groups of 10 to 12-month-old bGH (n
6 for N/df mice). Experiment 2 consisted in 2 groups of 10 to 12-month-old bGH (n =
= 6) and normal mice (N, n
6) and normal mice (N, n =
= 6) (Supplemental Fig. 1). Mice were maintained under temperature (22
6) (Supplemental Fig. 1). Mice were maintained under temperature (22 ±
± 2 °C) and humidity (40–60%) controlled conditions. Mice treated with GH received recombinant porcine GH (pGH) subcutaneous injections (4 µg/g of body weight; Alpharma, Inc., Victoria, Australia) twice daily for 6 weeks, beginning at 14 months of age. Control mice received saline injections on the same schedule as GH treated mice. After 6 weeks, the treatment was interrupted; the mice were kept under the same conditions for two more weeks, then euthanized. The GH treatment used in this study was proven effective for increasing serum IGF-I concentrations in previously study using the same dose and treatment length in young (3-week-old) and old mice (16-month-old) [34]. Our previously study measured body weights before the first GH injection and at the end of the 6-week treatment, confirming effectiveness of the treatment [12]. All experiments were approved by the Ethics Committee for Animal Experimentation from the University of Southern Illinois, IL, USA.
2 °C) and humidity (40–60%) controlled conditions. Mice treated with GH received recombinant porcine GH (pGH) subcutaneous injections (4 µg/g of body weight; Alpharma, Inc., Victoria, Australia) twice daily for 6 weeks, beginning at 14 months of age. Control mice received saline injections on the same schedule as GH treated mice. After 6 weeks, the treatment was interrupted; the mice were kept under the same conditions for two more weeks, then euthanized. The GH treatment used in this study was proven effective for increasing serum IGF-I concentrations in previously study using the same dose and treatment length in young (3-week-old) and old mice (16-month-old) [34]. Our previously study measured body weights before the first GH injection and at the end of the 6-week treatment, confirming effectiveness of the treatment [12]. All experiments were approved by the Ethics Committee for Animal Experimentation from the University of Southern Illinois, IL, USA.
Tissue collection and processing
The mice were anesthetized after fasting for 12 h. The ovaries were collected, cleared of surrounding adipose tissue, and embedded in 10% formalin buffered solution. The ovaries were removed from the formalin solution, dehydrated an alcohol, cleared in xylene, and embedded in Paraplast Plus (Sigma Chemical Company®, St. Louis, MO, USA). A semi-automated microtome (RM2245, Leica Biosystems, San Diego, CA, USA) was used to serially section, at 5 µm one ovary from each pair. The sections were randomly selected for immunohistochemistry analysis using slides impregnated with 3% organosilane (Sigma Chemical Company®, St. Louis, MO, USA) in ethanol.
Immunofluorescence
The ovarian samples were deparaffinized with xylene and rehydrated with graded alcohols for immunofluorescence analysis. The antibodies were obtained from Abcam (Abcam Plc, Cambridge, UK) and diluted in 1.5% bovine serum albumin (BSA) solution. The primary monoclonal antibody anti-gamma H2AX (γH2AX) phospho S139 (ab11174), used to indicate DNA damage [17], or anti-CD68 antibody (ab955), to indicate the presence of macrophages [35], were used at a final dilution of 1:500. Hydrogen peroxide was used to block the endogenous peroxidase activity. Antigen recovery was performed in humidified heat with a citrate solution at pH 6.0 for 3 min. To reduce background non-specific staining, tissue sections received BSA and goat serum. Next, slides were incubated with the primary antibody in a humid chamber at 4 °C overnight. The slides with anti-γH2AX and anti-CD68 antibodies were incubated with secondary Alexa Fluor® 488 (ab150113) for 1 h and Hoechst (ab228550) during 15 min for nuclei staining. Thereafter, slides were mounted with a drop of mounting medium under coverslips. A confocal microscope (Olympus FluoView™ 1000) was used to capture images of the follicles. The quantification for γH2AX fluorescence intensity and macrophage counting was performed by image analysis software ImageJ® [36]. γH2AX fluorescence intensity and granulosa cell number were evaluated in 3 follicles/class/mouse. Macrophage numbers were evaluated as the total number of macrophages per section. For each mouse, four random sections from the center of the ovary were counted. Granulosa cells were counted as visible nuclei using the Hoechst (ab228550) for nuclei staining.
Statistical analyses
All statistical analyses were performed using SAS® 9.4 (SAS Institute Inc., Cary, NC). The data was analyzed using the mixed procedure to compare the fluorescence intensity, number of granulosa cells, and macrophage number of df/df and N/df mice (effect of the genotype, treatment with GH, and the interaction). Within animal replicates were accounted in the model. A p value lower than 0.05 was considered as statistically significant.
Results
Experiment 1—df/df mice treated with exogenous GH
As shown in Fig. 1, female df/df mice had fewer DNA DSBs, as indicated by lower fluorescence for γH2AX in oocytes from primordial (p <
< 0.0001) and primary (p
0.0001) and primary (p <
< 0.0001) follicles, when compared to N/df mice. Granulosa cells of primary (p
0.0001) follicles, when compared to N/df mice. Granulosa cells of primary (p =
= 0.0022) follicles of df/df mice had fewer DNA DSBs. Granulosa cells of primordial follicles tended (p
0.0022) follicles of df/df mice had fewer DNA DSBs. Granulosa cells of primordial follicles tended (p =
= 0.0588) to have fewer DSBs. GH treatment increased (p
0.0588) to have fewer DSBs. GH treatment increased (p <
< 0.0001) DNA DSBs in oocytes and granulosa cells of primordial and primary follicles of both genotypes. Representative images of anti-γH2AX intensity in oocytes and granulosa cells of primordial and primary follicles are shown in Fig. 3. Similar behavior was observed on macrophage infiltration on the ovary. df/df mice displayed reduced macrophage infiltration (p
0.0001) DNA DSBs in oocytes and granulosa cells of primordial and primary follicles of both genotypes. Representative images of anti-γH2AX intensity in oocytes and granulosa cells of primordial and primary follicles are shown in Fig. 3. Similar behavior was observed on macrophage infiltration on the ovary. df/df mice displayed reduced macrophage infiltration (p <
< 0.0001) and GH treatment increased macrophage infiltration (p
0.0001) and GH treatment increased macrophage infiltration (p =
= 0.0001) in both genotypes (Fig. 4a).
0.0001) in both genotypes (Fig. 4a).

Fluorescence intensity of γH2AX immunostaining in nuclei of oocytes. Primordial follicles (n =
= 6, a), primary follicles (n
6, a), primary follicles (n =
= 6, b), and granulosa cell nuclei from primordial (n
6, b), and granulosa cell nuclei from primordial (n =
= 6, c) and primary follicles (n
6, c) and primary follicles (n =
= 6, d) of N/df and df/df mice treated with exogenous GH or saline. Data presented as media
6, d) of N/df and df/df mice treated with exogenous GH or saline. Data presented as media ±
± SEM
SEM

Representative images of immunofluorescence of anti-γH2AX in primordial and primary follicles of N/df and df/df mice—Experiment 1 (a); and normal and bGH mice—Experiment 2 (b). Blue images represent Hoechst (staining genetic material) and green represent anti-γH2AX staining γH2AX protein. Merged images are showed as both Hoechst and anti-γH2AX staining combination. Red arrows represent some granulosa cells and yellow arrow represents oocyte nucleus
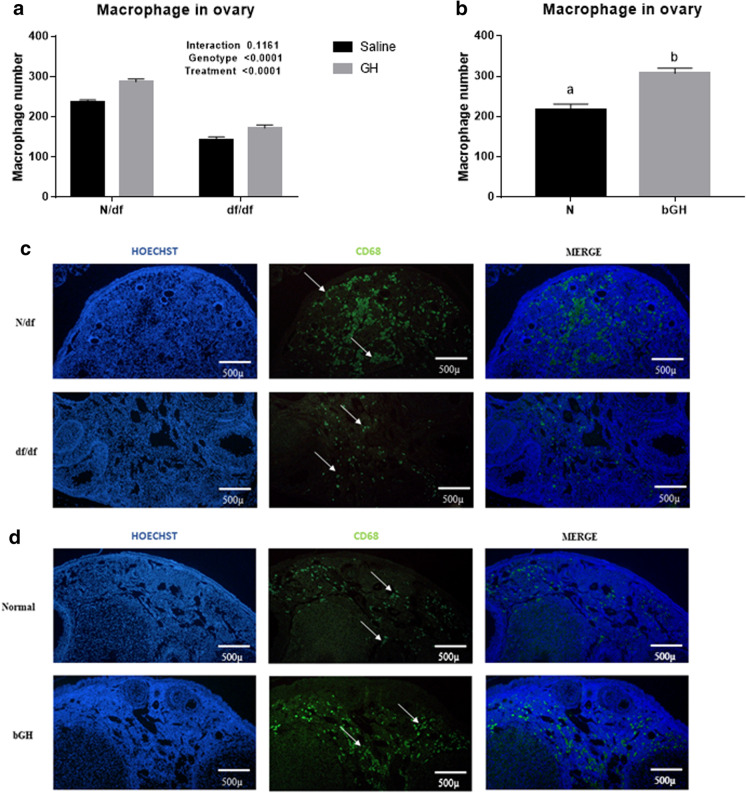
Macrophage number per ovarian section. N/df (n =
= 6) and df/df (n
6) and df/df (n =
= 6) mice treated with exogenous GH or saline (a); and macrophage number on ovarian section of normal (n
6) mice treated with exogenous GH or saline (a); and macrophage number on ovarian section of normal (n =
= 6) and bGH (n
6) and bGH (n =
= 6) transgenic mice (b). Representative images of macrophage infiltration on ovarian section of N/df and df/df (c); normal and bGH transgenic mice (d). White arrow indicates macrophage staining. Different letters indicate significant differences (p
6) transgenic mice (b). Representative images of macrophage infiltration on ovarian section of N/df and df/df (c); normal and bGH transgenic mice (d). White arrow indicates macrophage staining. Different letters indicate significant differences (p <
< 0.05)
0.05)
Df/df mice had fewer granulosa cells on primordial (p =
=
 <
< 0.0001) and primary (p
0.0001) and primary (p <
< 0.0001) follicles than N/df mice, and GH treatment did not affect this parameter in any follicular types (Table (Table1;1; p
0.0001) follicles than N/df mice, and GH treatment did not affect this parameter in any follicular types (Table (Table1;1; p >
> 0.05).
0.05).
Table 1
Number of granulosa cells of df/df and N/df mice treated with exogenous GH (Experiment 1) and bGH transgenic mice (Experiment 2)
| Follicle class | Granulosa cell number (n = = 6) (SEM) 6) (SEM) | p valuea | |||||
|---|---|---|---|---|---|---|---|
| Genot.1 | Treat.2 | Genot   Treat3 Treat3 | |||||
| N/df | df/df | ||||||
| Saline | GH | Saline | GH | ||||
| Primordial follicles | 6.0 (± 0.2) 0.2) | 6.2 (± 0.2) 0.2) | 4.8 (± 0.2) 0.2) | 5.4 (± 0.2) 0.2) | 0.0001* | 0.0794 | 0.3372 |
| Primary follicles | 10.5 (± 0.4) 0.4) | 11.2 (± 0.4) 0.4) | 8.8 (± 0.4) 0.4) | 8.8 (± 0.4) 0.4) |  < < 0.0001* 0.0001* | 0.3148 | 0.3269 |
| Normal | bGH | p valueb | |||||
| Primordial follicles | 5.7 (± 0.2) 0.2) | 8.2 (± 0.2) 0.2) |  < < 0.0001* 0.0001* | ||||
| Primary follicles | 9.6 (± 0.8) 0.8) | 18.4 (± 0.8) 0.8) |  < < 0.0001* 0.0001* | ||||
aTwo-way ANOVA test; bt-test; *Significant p value—p <
< 0.05
0.05
1Genotype; 2Treatment; 3Genotype

 Treatment
Treatment
Experiment 2—bGH mice
Following a pattern similar to the results from Experiment 1, mice with higher GH levels (bGH) had more DNA DSBs in oocytes from primordial (p =
= 0.0001) and primary (p
0.0001) and primary (p =
= 0.0044) follicles (Fig. 2). The granulosa cells from primordial follicles (p
0.0044) follicles (Fig. 2). The granulosa cells from primordial follicles (p <
< 0.0001) had more DNA DSBs in bGH mice than in N mice (Fig. 2c and andd).d). For granulosa cells from primary follicles, no significant effects were observed (p
0.0001) had more DNA DSBs in bGH mice than in N mice (Fig. 2c and andd).d). For granulosa cells from primary follicles, no significant effects were observed (p =
= 0.0691). Representative images of anti-γH2AX intensity in oocyte and granulosa cells of primordial and primary follicles are shown in Fig. 3. Increased GH levels (bGH) also increased macrophage infiltration (p
0.0691). Representative images of anti-γH2AX intensity in oocyte and granulosa cells of primordial and primary follicles are shown in Fig. 3. Increased GH levels (bGH) also increased macrophage infiltration (p =
= 0.0002) in the ovary (Fig. 4b and andd)d) and increased the number of granulosa cells in primordial (p
0.0002) in the ovary (Fig. 4b and andd)d) and increased the number of granulosa cells in primordial (p <
< 0.0001) and primary (p
0.0001) and primary (p <
< 0.0001) follicles, when compared to N mice (Table (Table11).
0.0001) follicles, when compared to N mice (Table (Table11).
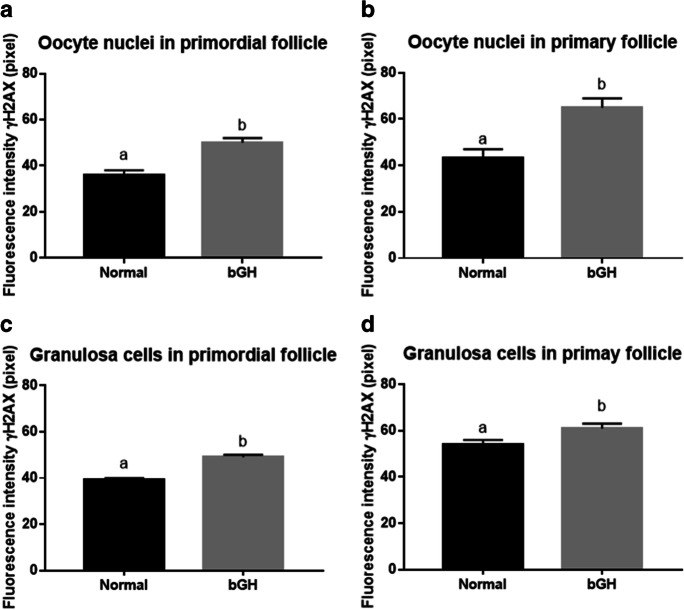
Fluorescence intensity of γH2AX immunostaining in nuclei of oocytes. Primordial follicle (n =
= 6, a), primary follicle (n
6, a), primary follicle (n =
= 6, b), and granulosa cell nuclei from primordial (n
6, b), and granulosa cell nuclei from primordial (n =
= 6, c) and primary follicles (n
6, c) and primary follicles (n =
= 6, d) of normal and bGH transgenic mice. Different letters indicate significant differences (p
6, d) of normal and bGH transgenic mice. Different letters indicate significant differences (p <
< 0.05). Data presented as media
0.05). Data presented as media ±
± SEM
SEM
Discussion
The key novel results of this study indicate that decreased GH/IGF-I, which has a central role in somatic and reproductive aging, can also prevent accumulation of DNA damage in oocytes from the ovarian reserve, further contributing to the younger phenotype observed in GH-deficient mice. GH-deficient df/df mice are known to live longer and be healthier than N/df littermates [2], whereas bGH mice have a short lifespan [4]. Our previous study [12] showed that df/df mice had more primordial follicles than N/df littermates, and treatment with GH increased primordial follicle activation. In addition, we have shown that bGH mice had a reduced primordial follicle reserve. Therefore, our previous observations appear to demonstrate a clear role for GH in primordial follicle activation and depletion of the ovarian reserve. This follow-up study shows a role for GH in increasing oocyte and granulosa DNA DSBs and ovarian inflammation. Taken together, these results suggest that, in addition to preserving the follicular reserve, the GH/IGF-I axis is involved in preventing or repairing the DNA damage that can lead to reduced oocyte quality with age.
DNA DSBs in oocytes and granulosa cells from primordial and primary follicles were reduced in old GH-deficient df/df mice compared to old N/df mice. Exogenous GH treatment increased DNA DSBs accumulation and bGH mice had increased accumulation of DNA DSBs in oocytes and granulosa cells from primordial and primary follicles. DNA damage is a threat to both somatic and germline cells during their lifespan [37]. Cells respond to DSBs by triggering DNA damage checkpoints (DDCs), resulting in cell cycle arrest and allowing repair mechanisms to be activated [38]. Endocrinologically identical to df/df mice, the Snell dwarf mice have deficient GH secretion and increased cellular DNA repair ability and upregulation of many DNA repair-related genes, in contrast to normal littermates [39]. Therefore, our evidence further suggests a role for the GH/IGF-I axis in DNA damage in mouse oocytes and surrounding granulosa cells. Mouse and human oocytes accumulate DNA DSBs with age [17]. Therefore, our data suggests that decreased GH/IGF-I signaling can reduce DNA DSB accumulation, either by preventing it or increasing DNA repair. In addition, granulosa cells are important for oocyte growth and development and display increased, age-related DNA DSBs in monkeys [40]. Our study also shows that somatic, granulosa cell DNA DSBs are reduced by GH deficiency. Expression of breast cancer 1 protein (BRCA1) is decreased with age in human oocytes, leading to the accumulation of DNA DSBs [17]. We have previously reported a three-fold decrease of Brca1 gene expression with aging in N mice, while no change with aging was observed in df/df mice [23]. Overall, this study demonstrates that GH/IGF-I deficiency, in addition to preserving the ovarian reserve for longer periods, also reduced oocyte reserve DNA damage, further increasing the odds of successful pregnancies in older mice.
Old df/df mice had reduced ovarian macrophage infiltration, compared to N/df mice. Treatment with GH increased the number of macrophages in the ovary and bGH mice also displayed increased macrophage infiltration. This evidence suggests that the GH/IGF-I axis is essential to the control of ovarian macrophage infiltration. Long-lived df/df, GH receptor knockout (GHRKO), and calorie restricted mice have all been thoroughly described as having a reduced pro-inflammatory profile, which could be one of the main mechanisms that promotes increased insulin sensitivity and extended survival in these mice [25]. Ovarian macrophages promote cell proliferation and follicle development by secreting cytokines and growth factors [41], including hepatocyte growth factor (HGF), basic fibroblast growth factor (bFGF), TNFα and β, and IGF-I [42]. In addition, extended exposure to a high-fat diet (both short-term and long-term) in young adult female mice decreases primordial follicle numbers, compromises fertility, increases systemic pro-inflammatory cytokines, and increases ovarian macrophage infiltration in the ovarian stroma [35]. Reduced macrophage infiltration reduces inflammatory potential, and interleukin-1 knockout mice have increased numbers of primordial follicles [43]. Exposure to lipopolysaccharide also reduces the primordial follicle pool, mediated by toll-like receptor 4 in mice [44]. This evidence suggests a significant role of inflammation in preservation of the ovarian reserve. Our previous ovarian transcriptome study demonstrated that the top 150 downregulated genes, between old df/df and old N/df mice, were linked to inflammatory responses [23]. In addition, among the top downregulated biological processes in old df/df, compared to old N/df mice, were macrophage chemotaxis, macrophage activation, and macrophage differentiation. As we observed reduced macrophage infiltration in this same group of mice in the current study, this suggests that active regulation of these pathways reduced migration of macrophages to the ovary. Previous studies had shown that in vivo GH administration increases monocyte migration to tissues [45]. We observed that treatment of macrophages in vitro with GH increases pathways with a role in macrophage migration and adhesion [46]. Such findings demonstrate the connection between the GH/IGF-I axis and inflammation in the ovaries of aged df/df mice. This reduced macrophage migration can be driving less DNA damage, or even less DNA damage can be driving reduced migration of macrophages. More functional studies are needed to access that association. In bGH mice, there is little data regarding ovarian inflammation. However, these transgenic mice have an increased pro-inflammatory profile in kidney [47] and age-related increases in blood pro-inflammatory markers [48]. Overall, our data provides an important connection between ovarian longevity and macrophage population, coordinated by the GH/IGF-I axis. However, further studies are needed to understand the specific role of macrophages in ovarian aging.
It is well established that the number of primordial follicles decreases as female mice age; however, there is no clear menopausal transition defined in this species. The number of primordial follicles is reduced to half at 10 months of age and at 18 months of age the reserve is reduced approximately 10 times in mice [49]. Breeding studies had shown that female mice stop producing litters around 18 months of age [50], which would be close to the age were follicular reserves become critically depleted. We have observed that 18-month-old N and df/df mice still have a significant number of primordial follicles in the ovary, despite more follicles are present in df/df mice [12]. Previous studies in GHRKO mice, phenotypically very similar to df/df mice, indicate that while 24-month-old N mice had no visible follicles in the ovary, GHRKO mice had all classes of follicles [51] and could even become pregnant. Based on these observations, the df/df mice used in our study still had primordial follicles in the ovary but N mice were likely close to deplete the ovarian reserve. There is no temporal data on ovarian reserve for bGH mice; however, life expectancy of female bGH mice is approximately half that of N mice [4]. As our bGH mice were 10 to 12-month-old, this would be equivalent to 20 to 24-month-old N mice, which according to previous studies would be on the verge of exhausting follicular reserves and close to the 18-month-old df/df mice used for our experiments.
The microenvironment within follicles and ovaries may also be influenced by GH/IGF-I deficiency. Our research indicates that df/df mice had fewer granulosa cells surrounding oocytes than N/df mice in both primordial and primary follicles, supporting the late activation of primordial follicles observed in df/df mice in a previous study [12]. Moreover, bGH mice had more granulosa cells compared to N mice, suggesting that granulosa cell number is a contributing factor to successful follicle activation and development, since bGH mice presented fewer primordial follicles than N mice. Granulosa cells are strongly connected with oocytes and play a crucial role in oocyte growth, follicle activation, and oocyte quality [52]. In order to control the development of dormant oocytes, several molecular networks mediate the crosstalk between somatic granulosa cells and oocytes [31, 33]. It is suggested that such mechanisms ensure the gradual stimulation of a limited number of primordial follicles during the reproductive lifespan. Follicular activation is likely to be trigged by molecular and cellular changes in the granulosa cells, followed by awakening of the dormant oocytes [31]. The regulation of granulosa cell number by GH/IGF-I is consistent with our previous research, which shows that the activation of FoxO3a is regulated by the GH/IGF-I axis in mouse primordial and primary oocytes [13]. The regulation of granulosa cell number by GH/IGF-I can, therefore, be a critical point in the determination of ovarian lifespan, providing further evidence for crosstalk with reproductive lifespan.
Conclusion
In conclusion, the present study indicates that GH/IGF-I is one of major modulators of oocyte and granulosa cell DNA damage and ovarian macrophage infiltration, adding to our previous evidence that GH/IGF-I are main regulators of primordial follicle activation. Therefore, the current study demonstrates GH/IGF-I deficiency in long-living df/df mice reduces accumulation of DNA damage and inflammation, both factors significantly associated with fertility in older ages. Overall, these observations confirm that the role of GH/IGF-I in the regulation of healthspan and lifespan in these mice is also reflected in ovarian aging.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
TDS performed sample analysis, interpreted data, and wrote the manuscript; MTR, DNG, JP, and LAXC performed sample analysis and data interpretation; RGM, CCB, AB, JBM, MMM, and AS designed the experiments, provided reagents and equipment, and data interpretation; YF and SM performed animal experiments and sample collection. All authors revised and edited the final manuscript draft.
Funding
This work was supported by CAPES, CNPq, FAPERGS, and NIH/NIA grants R15 AG059190, R03 AG059846, and R56 AG061414 (MMM).
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tatiana D. Saccon, Email: moc.liamg@noccasitat.
Monique T. Rovani, Email: moc.liamg@inavortm.
Driele N. Garcia, Email: rb.moc.oohay@eksen_akird.
Jorgea Pradiee, Email: rb.dem.airaniretev@eeidarpj.
Rafael G. Mondadori, Email: moc.liamg@irodadnomgr.
Luis Augusto X. Cruz, Email: moc.liamg@zurcotsugual.
Carlos C. Barros, Email: moc.liamg@lepccsorrab.
Yimin Fang, Email: ude.demuis@gnafy.
Samuel McFadden, Email: ude.demuis@77neddafcms.
Andrzej Bartke, Email: ude.demuis@ektraba.
Michal M. Masternak, Email: [email protected].
Augusto Schneider, Email: [email protected].
References
Articles from GeroScience are provided here courtesy of Springer
Full text links
Read article at publisher's site: https://doi.org/10.1007/s11357-021-00380-8
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9135908
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/105419676
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1007/s11357-021-00380-8
Article citations
Ovarian microenvironment: challenges and opportunities in protecting against chemotherapy-associated ovarian damage.
Hum Reprod Update, 30(5):614-647, 01 Oct 2024
Cited by: 3 articles | PMID: 38942605 | PMCID: PMC11369228
Review Free full text in Europe PMC
Molecular regulation of DNA damage and repair in female infertility: a systematic review.
Reprod Biol Endocrinol, 22(1):103, 14 Aug 2024
Cited by: 0 articles | PMID: 39143547 | PMCID: PMC11323701
Review Free full text in Europe PMC
Editorial: Endocrine regulation of aging: impacts of humoral factors and circulating mediators.
Front Endocrinol (Lausanne), 15:1387435, 06 Mar 2024
Cited by: 1 article | PMID: 38510697 | PMCID: PMC10951053
Roles of immune microenvironment in the female reproductive maintenance and regulation: novel insights into the crosstalk of immune cells.
Front Immunol, 14:1109122, 28 Dec 2023
Cited by: 2 articles | PMID: 38223507 | PMCID: PMC10786641
Review Free full text in Europe PMC
Mechanisms of ovarian aging in women: a review.
J Ovarian Res, 16(1):67, 06 Apr 2023
Cited by: 20 articles | PMID: 37024976 | PMCID: PMC10080932
Review Free full text in Europe PMC
Go to all (8) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Primordial follicle reserve, DNA damage and macrophage infiltration in the ovaries of the long-living Ames dwarf mice.
Exp Gerontol, 132:110851, 25 Jan 2020
Cited by: 13 articles | PMID: 31987917 | PMCID: PMC7025715
Ovarian aging and the activation of the primordial follicle reserve in the long-lived Ames dwarf and the short-lived bGH transgenic mice.
Mol Cell Endocrinol, 455:23-32, 19 Oct 2016
Cited by: 19 articles | PMID: 27771355 | PMCID: PMC5397383
Influence of hypothalamus and ovary on pituitary function in transgenic mice expressing the bovine growth hormone gene and in growth hormone-deficient Ames dwarf mice.
Biol Reprod, 54(5):1002-1008, 01 May 1996
Cited by: 19 articles | PMID: 8722619
Growth hormone in fertility and infertility: Mechanisms of action and clinical applications.
Front Endocrinol (Lausanne), 13:1040503, 14 Nov 2022
Cited by: 17 articles | PMID: 36452322 | PMCID: PMC9701841
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior
Fundação Coordenação de Projetos, Pesquisas e Estudos Tecnológicos
Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul
NIA NIH HHS (4)
Grant ID: R03 AG059846
Grant ID: R15 AG059190
Grant ID: R15 AG061795
Grant ID: R56 AG061414
National Institute on Aging (3)
Grant ID: R15 AG059190
Grant ID: R56 AG061414
Grant ID: R03 AG059846
 3
3



