Abstract
Free full text

Mucormycosis associated with COVID‐19 in two kidney transplant patients
Abstract
Coronavirus disease 2019 (COVID‐19) predisposes patients to bacterial and fungal superinfections due to the impairment of the immunological system. Among the associated opportunistic fungal infections, mucormycosis is one of the least frequent but with the highest mortality. We describe two cases of mucormycosis in two kidney transplant recipients, while they were hospitalized for SARS‐CoV‐2 pneumonia, with rhinosinusal and musculoskeletal involvement, respectively.
1. INTRODUCTION
Since the beginning of the coronavirus disease 2019 (COVID‐19) pandemic, there were alarming reports of secondary invasive fungal infections. 1 Although the incidence of fungal infection in COVID‐19 patients is generally low, multiple cases have been reported globally, mainly pulmonary aspergillosis. Among the hypotheses raised to explain this connection, there is the pro‐inflammatory state of the patient and the impairment of cellular immunity, in part due to the immunosuppressive regimens used in patients with severe COVID‐19. 2
Mucormycosis is an angioinvasive fungal infection associated with a high mortality, especially among immunosuppressed patients. 3 Diabetes mellitus is the main underlying disease associated with mucormycosis. Nevertheless, transplantation and hematological malignancies are also associated with this infection. A few case reports of COVID‐19‐associated mucormycosis have recently been published. Here, we present the first two cases of COVID‐19 associated mucormycosis infection in kidney recipients.
2. CASE REPORT
2.1. First case
A 62‐year‐old man with past history of type 2 diabetes mellitus was treated with insulin with non‐optimal control (last HbAc1 9.6%), and kidney transplantation 4 years prior, on immunosuppressive treatment with tacrolimus and prednisone with baseline creatinine of 1.4 mg/dL (Table 1). The patient also had history of disseminated cryptococcosis in 2018 for which he was treated with fluconazole for the first 12 months followed by isavuconazole for an additional 12 months. Three months after finishing the antifungal treatment, he was admitted to the hospital for respiratory failure due to confirmed SARS‐CoV‐2 pneumonia (November 30, 2020).
TABLE 1
Characteristics of the two kidney transplant patients with mucormycosis
| Case 1 | Case 2 | |
|---|---|---|
| Gender/age | M/62 y | M/48 y |
| Underlying diseases |
Arterial hypertension Diabetes mellitus II (HbAc1 9.6%) End‐stage renal disease with kidney transplant (IS: tacrolimus, prednisone) Disseminated cryptococcosis (2019) Ischemic heart disease |
Arterial hypertension End‐stage renal disease with 4 kidney transplant (IS: prednisone, mycophenolate and tacrolimus). Hypothyroidism |
| COVID‐19 severity | Severe (bilateral pneumonia requiring non‐invasive mechanical ventilation) |
Moderate (FiO2 28%) |
| Systemic corticosteroid therapy for COVID‐19 | Dexametasone 6 mg daily for 10 d | Prednisone 20 mg daily (administered as immunosuppressant) |
| Concomitant treatment |
Ceftriaxone Azithromycin |
Hydroxychloroquine Azithromycin Lopinavir/ritonavir Tocilizumab |
| Time between diagnosis of COVID‐19 and mucormycosis | 1 wk | 3 wk |
| Mucormycosis associated risk factors | Diabetes, previous fungal disease, immunosuppression, steroid therapy | Immunosuppression, steroid therapy |
| Presentation | Rhinosinusal | Musculoskeletal |
| Diagnostics | Culture from the necrotic tissue | Culture from the necrotic tissue |
| Specie aisled | Rizopus oryzae | Lichtheimia ramose |
| Antifungal treatment | Liposomal amphotericin B, isavuconazole and subsequently posaconazole | Liposomal amphrotericin B and isavuconazol |
| Surgical debridement | 7 times | 3 times |
| Outcome | Alive | Alive |
The patient presented severe respiratory failure and he was transferred to the intensive care unit (ICU) requiring non‐invasive mechanical ventilation for 6 days and received treatment with intravenous (IV) dexamethasone 6 mg daily for 10 days and antibiotic empirical therapy with ceftriaxone 1 gr q24h + azithromycin 500 mg q24h. He had initial favorable clinical and radiological evolution. All bacterial cultures were negative. During admission, he did not present diabetic ketoacidosis.
After an initial clinical improvement and after being discharged from the ICU, a week later, he presented fever, headache, and left malar region swelling. A facial computed tomography (CT) was performed showing left maxillary sinusitis and empirical treatment with piperacillin/tazobactam 4.5 mg q6h IV was initiated, without any clinical improvement. A new CT scan showed progression, with a greater occupation of the left maxillary sinus with progressive cellulitis, subperiosteal abscess in the lateral wall of the left maxillary sinus and involvement of intraorbital adipose tissue (Figure 1A).
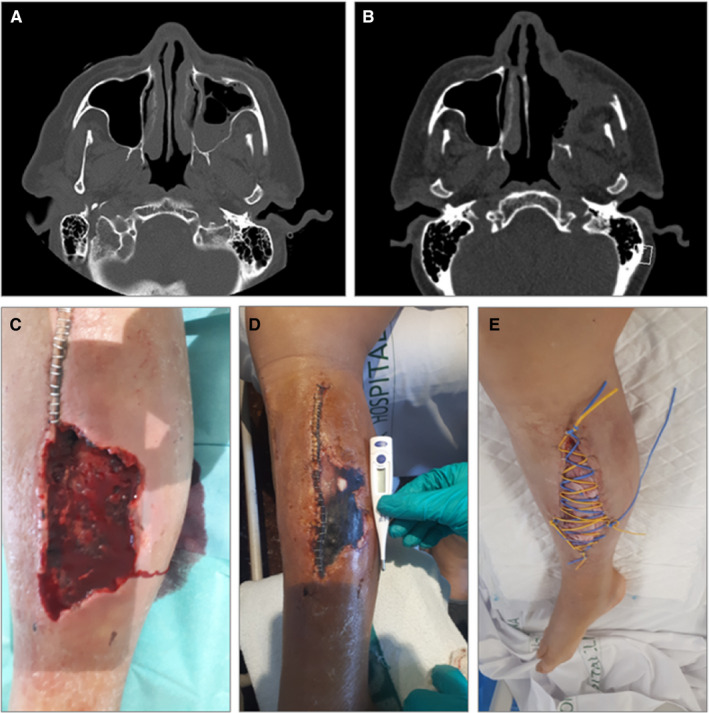
A, Case 1: occupation of the left maxillary sinus and osteomeatal complex, with trabeculation of the left premaxillary facial adipose tissue. B, Case 1: post‐surgical left maxillary, frontal, ethmoidal and sphenoid rhinosinusitis, with collections underlying the anterior and lateral wall of the left maxillary sinus and intraorbital. C–E, Case 2: lower right limb with dorsal hematoma and subsequent compartment syndrome with tissue necrosis, with posterior superinfection by Lichtheimia ramose, which required various debridement surgeries and prolonged antifungal treatment
An endoscopic evaluation and a first ENT debridement were performed. The swab culture showed Rhizopus oryzae resistant to voriconazole and caspofungin, sensitive to isavuconazole and posaconazole. A palate biopsy confirmed the diagnosis. Treatment with amphotericin B and an azole (initially isavuconazole and subsequently posaconazole) was initiated. Although isavuconazole and posaconazole could be used in this case equally, in order to avoid generating resistance to mucor (since the patient had a history of prolonged exposure to isavuconazol, and because it was easier to monitor serum levels of both tacrolimus and posaconazol to control possible interactions), the change from isavuconazol to posaconazol was performed. Within the following two months the patient underwent six subsequent ENT surgical debridement procedures, including a total left maxillectomy (Figure 1B). Prednisone dose was decreased to 2.5 mg, and tacrolimus levels were maintained between 6‐8 ng/mL. As adjuvant treatment, the patient received hyperbaric chamber therapy. Control CT showed no recurrence after 5 months of antifungal therapy.
2.2. Second case
The second case was a 48‐year‐old man with a history of chronic kidney disease due to reflux nephropathy. He received a fourth renal transplant from a cadaveric donor 5 years prior, and was on immunosuppressive treatment with prednisone, mycophenolate and tacrolimus (Table 1). He had chronic graft dysfunction due to transplant glomerulopathy, with basal creatinine of 2.8 mg/dL.
He was admitted to the hospital on April 14, 2020 with bilateral SARS‐CoV‐2 pneumonia. The patient was started on hydroxychloroquine, azithromycin and lopinavir/ritonavir and anticoagulation with prophylactic low weight heparin according to our hospital protocol at that time. Due to worsening respiratory failure, a unique dose of tocilizumab 400 mg was administered during the hospitalization. Tacrolimus and mycophenolate were stopped, and prednisone was maintained at a dose of 20 mg/d. During admission, he did not present diabetic ketoacidosis. The patient had a favorable respiratory outcome.
Three weeks after admission, the patient presented with pain and an increase of lower right limb diameter and was diagnosed with a hematoma in the dorsal area with compartment syndrome, probably secondary to anticoagulation. A fasciotomy and surgical debridement were performed, and surgical findings showed necrotic muscle tissue (Figure 1C‐E). A culture from the necrotic tissue smears showed Lichtheimia ramosa and the patient was diagnosed with musculoskeletal mucormycosis infection and treatment was started with liposomal amphotericin B 5 mg/kg q24h together with isavuconazole 200 mg/8 h for 24 days. Two other surgical debridements were performed, with negative surgical cultures. The patient received a total of 3 months of isavuconazole, with favorable outcome.
3. DISCUSSION
Immunosuppressed patients such as solid organ transplant recipients with SARS‐CoV‐2 infection have a high risk of developing severe COVID‐19, 4 and they present high mortality. 5 , 6 About 29% of these patients present diffuse alveolar damage requiring mechanical ventilation and admission to the ICU which prolongs hospital stay and increases the chances of developing fungal co‐infections. 3 , 7
In patients with severe COVID‐19 infection, there have been reports of an impairment of immunity with up balance of proinflammatory cytokines (IL‐1, IL2, IL6, TNF‐α), disturbance in the lymphocyte Th1 and Th2 responses and lower number of CD4 and CD8 cell counts. 8 In addition, these patients usually receive high‐dose corticosteroids and immunomodulatory drugs as part of COVID‐19 treatment. In this context, these patients are especially predisposed to the development of fungal infections, including mucormycosis. Maoorthy et al reported 16 cases of mucormycosis in diabetic patients treated for COVID‐19, but none of them were transplant recipients. The main risk factor in that cohort of patients was poor control of their diabetes and concomitant use of corticosteroids. 9 In our cases, the severity of SARS‐CoV‐2 infection and the specific SARS‐CoV‐2 treatment such as corticosteroids therapy were probably associated with the development of mucormycosis.
Mucormycosis is a rare invasive fungal infection, with an incidence of up to two of 1000 transplanted patients. 10 Commonly described risk factors are diabetes mellitus, re‐transplantation (probably due to multiple periods of induction with immunosuppressive therapies), chronic graft dysfunction, history of invasive fungal disease as well as long‐term use of antifungal, and chronic corticosteroid therapy. 11 On the other hand, a beneficial effect has been described with the use of calcineurin inhibitors (CNI) 12 since the calcineurin pathway plays a key role in the yeast‐hyphal and spore‐size dimorphic transitions that contributes to virulence of this uncommon fungal pathogen. Also, the inhibition of this pathway reduces the antifungal resistance. 13 , 14 An in vitro study reported that tacrolimus increased the effectiveness of posaconazole against Rhizopus oryzae. 15 In our cases, both patients had several risk factors for mucormycosis, and despite being under treatment with CNI, they developed the disease. It has been suggested that the protective effect of CNI was not patent since immunosuppressants are usually stopped or reduced as part of COVID‐19 management. Nevertheless, different mucormycosis mutations that confer resistance against CNI have been identified. 16
Rhinosinusal disease is the most common presentation of mucormycosis in solid organ transplant recipients, 3 , 10 , 17 and similar locations have been reported in COVID‐19‐associated cases. 9 However, pulmonary infection is also frequent. 18 , 19 , 20 , 21 , 22 , 23 In these cases, the differential diagnosis with invasive pulmonary aspergillosis should be considered, since these two entities have similar risk factors and may present similar radiological patterns and clinical course. 24 The diagnosis of mucormycosis is made after isolating the pathogen in cultures obtained after surgery or bronchoalveolar lavage, tissue biopsy, or PCR‐amplification. 3 , 10 The lack of clinical suspicion and the difficulty in isolating the fungus could lead to a delay in diagnosis and treatment initiation.
Currently, liposomal amphotericin B, posaconazole, isavuconazole, and itraconazole, although the latter with limited activity, are the main available therapeutic options. 25 Some studies have reported a lower mortality with the use of a combination of two antifungal compared to monotherapy. 26 More importantly one of the pillars of treatment is the surgical debridement of the necrotic tissue, which often has to be performed several times, as reflected in both of our cases. Hyperbaric chambers have been described as adjuvant therapy; although there are no randomized clinical trials, some case series data have shown a potential benefit. 27 , 28
Transplant physicians must be aware that patients with COVID‐19 are at risk of developing unusual opportunistic infections. Some authors suggest reducing immunosuppressant therapy in these patients, such as reducing or stopping CNI and antimetabolites (mycophenolate or azathioprine) in moderate or severe cases, leaving corticosteroids as monotherapy. However, in case of developing mucormycosis, different strategies other than steroids should be considered. 9 , 10 , 11 , 29
The mortality of mucormycosis is high, with an estimate of around 30%‐50%, 3 , 12 mostly attributed to impossibility to perform surgical debridement. In the published COVID‐19 related cases, an even higher mortality rate was reported, up to even 90% of the patients, and in some, the diagnosis was made post‐mortem. 18 , 19 , 20 , 21 , 22 , 23 , 30 , 31 , 32 , 33 , 34 However, lower rates have also been described in a recent review with an in‐hospital mortality of 49% in a series of 41 cases. 35 Our cases had a more favorable outcome. This is explained in part due to the presentation, since the musculoskeletal forms are associated with a better prognosis, in part due to the accessibility to obtain histological samples allowing an early diagnosis. In both cases, the patients were able to benefit from surgical debridement.
In conclusion, we believe these two cases reflect the importance of suspicion by transplant physicians of opportunistic infections in solid organ transplant recipients, particularly mucormycosis, in patients with severe COVID‐19 infection, where patients have an impaired immune response and are commonly treated with high doses of corticosteroids. It is possible that this entity might be under diagnosed since these patients often have overlapping or non‐specific symptoms, the complexity of the diagnosis, especially in cases with lung involvement.
AUTHOR CONTRIBUTIONS
CA and RC: data collection, data analysis and manuscript writing. MX and SH: conceptualization, data collection, data analysis, and manuscript editing. JC: data collection. AM, MB, FC, FD, and NE: conceptualization and manuscript editing. All authors approved the latest version of the manuscript.
Notes
Arana C, Cuevas Ramírez RE, Xipell M, et al. Mucormycosis associated with COVID‐19 in two kidney transplant patients. Transpl Infect Dis. 2021;23:e13652. 10.1111/tid.13652 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
Carolt Arana and Cuevas Ramírez contributed equally to this manuscript.
Funding information
This work has not received funding.
Contributor Information
Carolt Arana, @AranaCarolt.
Rafael E. Cuevas Ramírez, @emmanuel_cr3.
Marc Xipell, @mxipell.
Joaquim Casals, @QCasals.
Marta Bodro, @MartaBodro.
Fritz Diekmann, Email: tac.cinilc@namkeidf, @FritzDiekmann.
DATA AVAILABILITY STATEMENT
Data partially available on request due to privacy/ethical restrictions.
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.1111/tid.13652
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8209809
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/106468121
Article citations
COVID-19-Associated Rhino-Orbito-Cerebral Mucormycosis: A Single Tertiary Care Center Experience of Imaging Findings With a Special Focus on Intracranial Manifestations and Pathways of Intracranial Spread.
Cureus, 16(4):e57441, 02 Apr 2024
Cited by: 0 articles | PMID: 38699084 | PMCID: PMC11064103
Epidemiology, Clinical Manifestations, and Outcome of Mucormycosis in Solid Organ Transplant Recipients: A Systematic Review of Reported Cases.
Open Forum Infect Dis, 11(6):ofae043, 30 Jan 2024
Cited by: 0 articles | PMID: 38887489 | PMCID: PMC11181195
Review Free full text in Europe PMC
Proven Cutaneous Mucormycosis in a COVID-19 Patient: A Case Report and Literature Review.
Iran J Pathol, 19(2):259-268, 15 Feb 2024
Cited by: 0 articles | PMID: 39118799 | PMCID: PMC11304458
COVID-19-Associated Rhinocerebral Mucormycosis, an Incidental Finding or a Matter of Concern - Mixed-Method Systematic Review.
Infect Drug Resist, 17:387-402, 31 Jan 2024
Cited by: 1 article | PMID: 38312523 | PMCID: PMC10838509
Review Free full text in Europe PMC
Case report: The clinical utility of metagenomic next-generation sequencing in mucormycosis diagnosis caused by fatal Lichtheimia ramosa infection in pediatric neuroblastoma.
Front Pediatr, 11:1130775, 19 Jun 2023
Cited by: 1 article | PMID: 37404554 | PMCID: PMC10315538
Go to all (44) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Risk factors and outcomes of COVID associated mucormycosis in kidney transplant recipients.
Transpl Infect Dis, 24(2):e13777, 31 Jan 2022
Cited by: 5 articles | PMID: 34932870
Mucormycosis as SARS-CoV2 sequelae in kidney transplant recipients: a single-center experience from India.
Int Urol Nephrol, 54(7):1693-1703, 18 Nov 2021
Cited by: 6 articles | PMID: 34792722 | PMCID: PMC8600912
COVID-19-Related Rhino-Orbital-Cerebral Mucormycosis in a Renal Transplant Recipient.
Exp Clin Transplant, 20(2):213-217, 03 Jan 2022
Cited by: 1 article | PMID: 34981710
Mucormycosis and COVID-19 pandemic: Clinical and diagnostic approach.
J Infect Public Health, 15(4):466-479, 18 Feb 2022
Cited by: 16 articles | PMID: 35216920 | PMCID: PMC8855610
Review Free full text in Europe PMC

 2
and
2
and 


