Abstract
Aims
To consolidate evidence to determine (i) the association between cardiovascular risk factors and health outcomes with coronavirus 2019 (COVID-19); and (ii) the impact of COVID-19 on cardiovascular health.Methods and results
An umbrella review of systematic reviews was conducted. Fourteen medical databases and pre-print servers were searched from 1 January 2020 to 5 November 2020. The review focused on reviews rated as moderate or high-quality using the AMSTAR 2 tool. Eighty-four reviews were identified; 31 reviews were assessed as moderate quality and one was high-quality. The following risk factors were associated with higher mortality and severe COVID-19: renal disease [odds ratio (OR) (95% confidence interval) for mortality 3.07 (2.43-3.88)], diabetes mellitus [OR 2.09 (1.80-2.42)], hypertension [OR 2.50 (2.02-3.11)], smoking history [risk ratio (RR) 1.26 (1.20-1.32)], cerebrovascular disease [RR 2.75 (1.54-4.89)], and cardiovascular disease [OR 2.65 (1.86-3.78)]. Liver disease was associated with higher odds of mortality [OR 2.81 (1.31-6.01)], but not severe COVID-19. Current smoking was associated with a higher risk of severe COVID-19 [RR 1.80 (1.14-2.85)], but not mortality. Obesity associated with higher odds of mortality [OR 2.18 (1.10-4.34)], but there was an absence of evidence for severe COVID-19. In patients hospitalized with COVID-19, the following incident cardiovascular complications were identified: acute heart failure (2%), myocardial infarction (4%), deep vein thrombosis (7%), myocardial injury (10%), angina (10%), arrhythmias (18%), pulmonary embolism (19%), and venous thromboembolism (25%).Conclusion
Many of the risk factors identified as associated with adverse outcomes with COVID-19 are potentially modifiable. Primary and secondary prevention strategies that target cardiovascular risk factors may improve outcomes for people following COVID-19.Free full text

Cardiovascular risk factors, cardiovascular disease, and COVID-19: an umbrella review of systematic reviews
Abstract
Aims
To consolidate evidence to determine (i) the association between cardiovascular risk factors and health outcomes with coronavirus 2019 (COVID-19); and (ii) the impact of COVID-19 on cardiovascular health.
Methods and results
An umbrella review of systematic reviews was conducted. Fourteen medical databases and pre-print servers were searched from 1 January 2020 to 5 November 2020. The review focused on reviews rated as moderate or high-quality using the AMSTAR 2 tool. Eighty-four reviews were identified; 31 reviews were assessed as moderate quality and one was high-quality. The following risk factors were associated with higher mortality and severe COVID-19: renal disease [odds ratio (OR) (95% confidence interval) for mortality 3.07 (2.43–3.88)], diabetes mellitus [OR 2.09 (1.80–2.42)], hypertension [OR 2.50 (2.02–3.11)], smoking history [risk ratio (RR) 1.26 (1.20–1.32)], cerebrovascular disease [RR 2.75 (1.54–4.89)], and cardiovascular disease [OR 2.65 (1.86–3.78)]. Liver disease was associated with higher odds of mortality [OR 2.81 (1.31–6.01)], but not severe COVID-19. Current smoking was associated with a higher risk of severe COVID-19 [RR 1.80 (1.14–2.85)], but not mortality. Obesity associated with higher odds of mortality [OR 2.18 (1.10–4.34)], but there was an absence of evidence for severe COVID-19. In patients hospitalized with COVID-19, the following incident cardiovascular complications were identified: acute heart failure (2%), myocardial infarction (4%), deep vein thrombosis (7%), myocardial injury (10%), angina (10%), arrhythmias (18%), pulmonary embolism (19%), and venous thromboembolism (25%).
Conclusion
Many of the risk factors identified as associated with adverse outcomes with COVID-19 are potentially modifiable. Primary and secondary prevention strategies that target cardiovascular risk factors may improve outcomes for people following COVID-19.
Introduction
The coronavirus 2019 (COVID-19) pandemic has caused global health, social, and economic system challenges. As of 14 February 2021, the global cumulative cases of COVID-19 surpassed 108 million; with over 2.3 million related deaths since the start of the pandemic in December 2019.1 Early in the COVID-19 pandemic, evidence emerged suggesting that adults with cardiovascular disease (CVD) may be at a higher risk of in-hospital mortality with COVID-19.2 In addition, patients hospitalized with COVID-19 demonstrated a propensity to thrombosis.3
A rapidly emerging evidence base of COVID-19 research has suggested the following may all increase risk of severe COVID-19 or mortality with COVID-19: higher age, male sex, black or African American or other ethnic minority backgrounds, and underlying health conditions, including CVD and cardiovascular risk factors (e.g. hypertension, diabetes mellitus, and chronic kidney disease).4–12 In the UK, a prospective observational cohort study of 20 133 patients hospitalized with COVID-19 suggested that the risk of mortality was higher among patients with cardiac, pulmonary, kidney and liver diseases, as well as cancer, dementia, and obesity (hazard ratios from 1.16 to 1.51).13
More severe cases of COVID-19 have been associated with new-onset cardiovascular conditions. Although, the hallmark of COVID-19 is respiratory involvement, ranging from mild upper respiratory symptoms to acute respiratory distress syndrome,14 severe COVID-19 has been implicated in multi-organ involvement, with several observational case series showing a significant proportion of cardiac involvement among hospitalized patients.9,15,16 COVID-19 appears to associate with a wide spectrum of cardiovascular sequelae, including acute-onset heart failure, arrhythmias, acute coronary syndrome, myocarditis, and cardiac arrest. Moreover, the acute cardiac injury seems to be significantly correlated with increased in-hospital mortality in COVID-19 patients.9
Despite our current understanding of CVD and outcomes with COVID-19, the rapid expanse of research during the pandemic has resulted in substantial variability of risk estimates and duplication of patient data. Indeed, this is particularly problematic when focusing on systematic reviews and meta-analyses, arguably the gold standard of scientific evidence, which have included overlapping patient populations and represent a variety of evidence quality.
Commissioned by Public Health England, the aim of this umbrella review of systematic reviews was to consolidate evidence which addressed the following two research questions: (i) What is the association between cardiovascular risk factors or CVD and health outcomes, hospitalization, ventilation, and mortality caused by COVID-19? and (ii) What is the impact of COVID-19 on cardiovascular health?
Methods
This umbrella review was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.17 Although there was no published protocol, the research questions, search strategy, and inclusion/exclusion criteria were independently developed by Public Health England prior to the commencement of the review.
Objectives
To determine (i) the association between cardiovascular risk factors or CVD and health outcomes with COVID-19; and (ii) the impact of COVID-19 on cardiovascular health.
Population, exposure, comparator, and outcomes
The population was people with COVID-19. Reviews which were focused on children (aged <18 years) were not eligible for inclusion. For the first objective, the exposures were cardiovascular risk factors, CVD, or cerebrovascular disease. Cardiovascular risk factors pre-defined as eligible for inclusion were smoking, hypertension, obesity, sedentary behaviour/physical inactivity, alcohol use, diet, cholesterol, familial hypercholesterolaemia, hyperlipoproteinaemia type II, hyperglycaemia, prediabetic state, diabetes, atrial fibrillation, renal insufficiency, kidney diseases, liver diseases, fibrosis, and dementia. The comparator group included individuals with COVID-19 without CVD or the risk factor of interest. The outcomes were any health outcomes with COVID-19 including hospitalization, ventilation, and mortality, or composite measures of these. No exclusions were placed on methods used to diagnose COVID-19, CVD, or cardiovascular risk factors. For the second objective, the exposure was COVID-19 and a comparator group was not needed. The outcomes were any incident cardiovascular or cerebrovascular events following a diagnosis of COVID-19.
years) were not eligible for inclusion. For the first objective, the exposures were cardiovascular risk factors, CVD, or cerebrovascular disease. Cardiovascular risk factors pre-defined as eligible for inclusion were smoking, hypertension, obesity, sedentary behaviour/physical inactivity, alcohol use, diet, cholesterol, familial hypercholesterolaemia, hyperlipoproteinaemia type II, hyperglycaemia, prediabetic state, diabetes, atrial fibrillation, renal insufficiency, kidney diseases, liver diseases, fibrosis, and dementia. The comparator group included individuals with COVID-19 without CVD or the risk factor of interest. The outcomes were any health outcomes with COVID-19 including hospitalization, ventilation, and mortality, or composite measures of these. No exclusions were placed on methods used to diagnose COVID-19, CVD, or cardiovascular risk factors. For the second objective, the exposure was COVID-19 and a comparator group was not needed. The outcomes were any incident cardiovascular or cerebrovascular events following a diagnosis of COVID-19.
Study design
Systematic reviews or meta-analyses were eligible for inclusion. In accordance with the Database of Abstracts of Reviews of Effects (DARE) criteria, to be included, the reviews needed to have detailed the inclusion and exclusion criteria, conducted an adequate search, assessed the quality of included studies, synthesized the results of the included studies and provided sufficient details of the characteristics of the included studies.18 Pre-prints, grey literature, or peer-reviewed publications were eligible for inclusion. Where a pre-print and a peer-reviewed publication of the same systematic review were found, only the peer-reviewed publication was included. Only reviews published in the English language were eligible for inclusion.
Search strategy
The search was conducted in early November 2020, and the following electronic databases were searched from 1 January 2020 to 5 November 2020: Cochrane Library, Ovid Medline, Ovid Emcare, Embase, Epistemonikos COVID-19, EPPI Living Map, Evidence Aid, Global Health, LENUS, medRxiv, Norwegian Institute of Public Health, PROSPERO, PubMed, and the World Health Organisation. Exploded Medical subject headings (MesH) terms were combined with appropriate free-text terms for CVD, cardiovascular risk factors, and COVID-19. These were mapped across different databases. The Medline systematic review search filter was applied to the search to limit the number of results to this type of review. The search strategy conducted in Medline is shown in Supplementary material online, Table S1.
Study selection
The results from the different electronic databases were exported into EndNote X9 and duplicates were removed. Two reviewers (S.L.H. and B.J.R.B.) completed title and abstract screening independently and in duplicate. Of the potentially included reviews, full-texts were retrieved and also independently screened in duplicate by the same two reviewers to identify reviews for inclusion. Disagreements were resolved through discussion to reach a consensus.
Data extraction
A data extraction form was pre-defined in Microsoft Excel with the following information: first author, review search dates, number of included studies, countries of included studies, study designs of included studies, number of patients, population inclusion criteria, exposures examined, outcomes examined, whether a meta-analysis was performed (yes/no), methods if a meta-analysis was performed (e.g. random-effects or fixed-effects model), results for each exposure and outcome of interest (and number of studies and patients for each analysis if different from the total study sample), quality assessment results, conclusions, and reported limitations. The principal summary measures were odds ratios, risk ratios, or hazard ratios. Two reviewers (S.L.H. and B.J.R.B.) independently completed the data extraction in duplicate for 10 of the reviews (12%) and achieved good agreement (≥80%). Data extraction for the remaining included reviews was completed by one reviewer (S.L.H. or B.J.R.B.). This approach is in line with the AMSTAR 2 checklist.19
Quality assessment
Two reviewers (J.M.R.-C. and J.Z.) independently critically assessed the quality of 10 included reviews (12%) using the AMSTAR 2, which is a critical appraisal tool for systematic reviews that include randomized or non-randomized studies of healthcare interventions.19 The reviewers discussed any disagreement until optimal agreement was achieved (100%), and the quality assessment of the remaining included reviews was completed by one reviewer (J.M.R.-C. or J.Z.). The AMSTAR 2 includes 16 items, and as the AMSTAR 2 is designed for reviews of interventions, we modified the items which referred to ‘interventions’ to refer to ‘exposures’ in the included reviews. Using the AMSTAR 2 checklist, each included review was given an overall confidence rating of ‘critically low’ (more than one critical flaw with or without non-critical weaknesses), ‘low’ (one critical flaw with or without non-critical weaknesses), ‘moderate’ (more than one non-critical weakness) or ‘high’ (no or one non-critical weakness).
Overlapping reviews
Reviews overlapped if they examined associations between the same exposure and outcome. It was noted that duplication of primary studies within reviews which examined the same exposure and outcome was extensive; therefore, incorporating results from reviews which examined the same exposure and outcomes was likely to lead to the inclusion of the same primary studies more than once. More recent reviews tended to include larger numbers of patients, greater numbers of cohort studies, and data from a wider variety of countries. Therefore, for each risk factor included in this umbrella review, we have highlighted the findings from the largest moderate- or high-quality systematic review.
Data synthesis
A narrative synthesis of the included systematic reviews and meta-analyses was conducted. Summary tables describe review characteristics and findings of meta-analyses are presented using forest plots.
Results
Screening
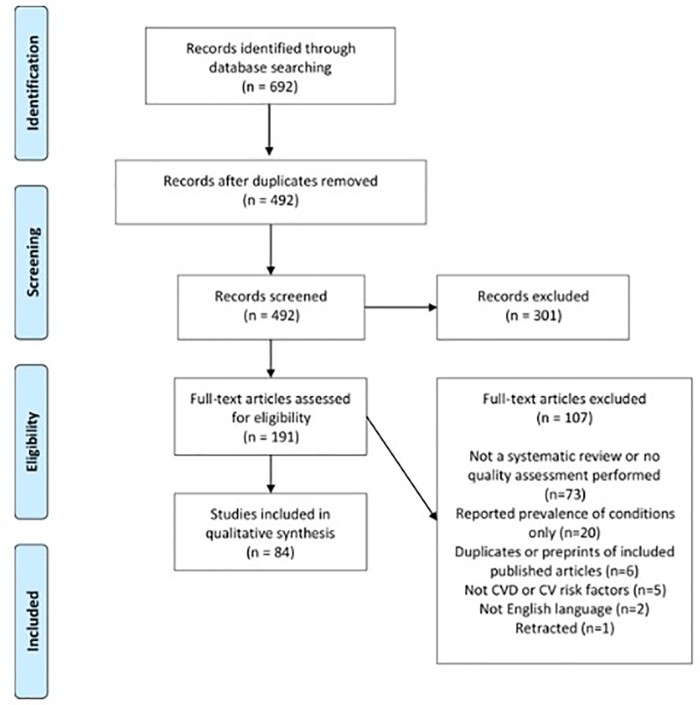
The searches resulted in 692 studies identified and after the removal of duplicates, 492 studies were screened at the title and abstract stage (Figure 1). After reviewing the title and abstracts, 301 (61.2%) were removed, and the full-texts were retrieved for 191 studies and subsequently assessed for eligibility. At the full-text screening stage, 107 articles were excluded and the reasons are listed in the PRISMA flow diagram. Attempts were made to contact the authors of one of the included reviews for further information, but no response was received. After the full-text screening, 84 systematic reviews or meta-analyses which addressed the research questions were identified.
Assessment of the quality of the included reviews
Of the 84 included reviews, according to the AMSTAR 2 rating, 33% (n =
= 28 reviews) were assessed as critically low quality; 29% (n
28 reviews) were assessed as critically low quality; 29% (n =
= 24) were assessed as low quality, 37% (n
24) were assessed as low quality, 37% (n =
= 31) were moderate quality,20–50 and only one review, which reported associations between smoking and outcomes with COVID-19, was assessed as high-quality.51 The full AMSTAR 2 assessments and references to the critically low and low-quality studies are provided in Supplementary material online, Table S2.
31) were moderate quality,20–50 and only one review, which reported associations between smoking and outcomes with COVID-19, was assessed as high-quality.51 The full AMSTAR 2 assessments and references to the critically low and low-quality studies are provided in Supplementary material online, Table S2.
The AMSTAR 2 criteria which were often met by the included reviews were: (i) outlining the research questions and inclusion criteria including the elements of participants, intervention, comparator group, and outcomes (AMSTAR 2 criterion #1), (ii) explaining the selection of study designs for inclusion (AMSTAR 2 criterion #3), (iii) performing duplicate screening and duplicate data extraction (AMSTAR 2 criteria #5 and #6), and (iv) declaring potential conflicts of interest and sources of funding (AMSTAR 2 criterion #16). Most of the reviews achieved ‘Partially Yes’ in the following sections: assessing selection bias and confounding factors in the risk of bias assessment (AMSTAR 2 criterion #9), and providing sufficient explanation on the method prior to its conduction (AMSTAR 2 criterion #2), although limited studies reported clear plans to investigate causes of heterogeneity. Although most reviews mentioned publication bias or planned for its assessment (AMSTAR 2 criterion #15), some of the reviews did not perform an assessment of this bias or report this bias either due to small sample size, or no explanation was provided.
The AMSTAR 2 criteria which were not reported in the majority of the reviews were (i) a list of excluded studies and justifications for exclusion (AMSTAR 2 criterion #7) and (ii) reporting sources of funding for studies included in the review (AMSTAR 2 criterion #10). Further, AMSTAR 2 criteria which were often not fulfilled in the included reviews were justification of restrictions of the search, adjustment for confounding factors in meta-analyses, assessment on the impact of risk of bias for individual studies on the results of the meta-analysis, and sufficient discussion and interpretation of the results with the impact of individual risk of bias.
Characteristics of the moderate and high-quality reviews
The number of studies in the included 32 moderate or high-quality reviews ranged from 345 to 75.38 The earliest search date was to 2 March 2020,26 and the most recent search date was to 11 August 2020.39 Three reviews only included studies from China,22,26,46 two reviews did not report the country of the studies24,36 and 27 reviews included studies from multiple countries. The reviews included observational studies such as case reports, case series, cross-sectional studies, and retrospective and prospective cohort studies. Supplementary material online, Table S3 summarizes the characteristics and results of all 84 reviews identified.
Cardiovascular disease or cardiovascular risk factors and outcomes with COVID-19
Figures 2 and and33 provide a summary of the main findings of moderate or high-quality reviews which examined associations between CVD or cardiovascular risk factors and outcomes with COVID-19. Figures 2 and 33 focus on the outcomes which were most consistently reported in the included reviews (mortality and severe COVID-19). However, there was variation in how severe COVID-19 was defined (Supplementary material online, Table S3).
33 focus on the outcomes which were most consistently reported in the included reviews (mortality and severe COVID-19). However, there was variation in how severe COVID-19 was defined (Supplementary material online, Table S3).
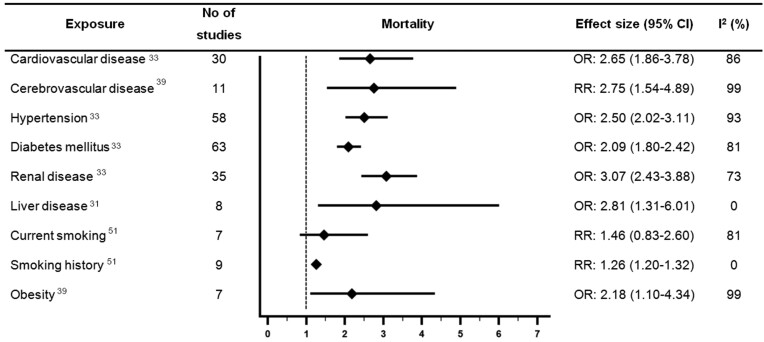
Forest plot showing results of meta-analyses from reviews which investigated associations between cardiovascular disease or cardiovascular risk factors and mortality with COVID-19. Largest moderate- or high-quality review included, according to assessment with the AMSTAR 2 criteria. CI, confidence interval; OR, odds ratio; RR, relative risk.
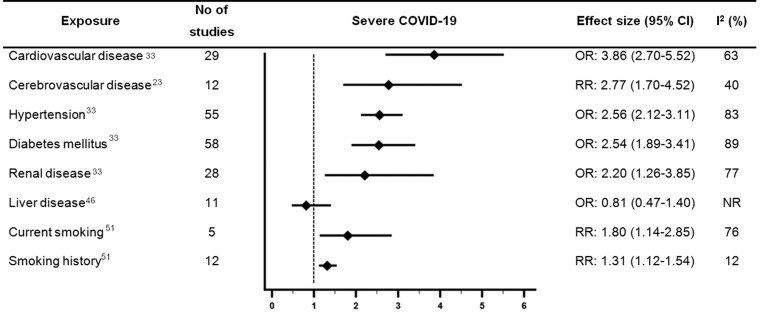
Forest plot showing results of meta-analyses from reviews which investigated associations between cardiovascular disease or cardiovascular risk factors and severe COVID-19. Largest moderate- or high-quality review included, according to assessment with the AMSTAR 2 criteria. CI, confidence interval; NR, not reported; OR, odds ratio; RR, relative risk.
Cardiovascular disease
Ten moderate quality systematic reviews examined associations between CVD and outcomes with COVID-19.20,21,23,31,33,36,38,39,44,49 The largest of which (Luo et al.,) suggested CVD was associated with 2.65 times higher odds of mortality with COVID-19 [pooled odds ratio (OR) 2.65, 95% confidence interval (CI) 1.86–3.78, n =
= 30 studies, considerable heterogeneity (I2 = 86%)].33
30 studies, considerable heterogeneity (I2 = 86%)].33
Five reviews examined the association between CVD and severe COVID-19 and were rated as moderate quality.23,24,33,35,48 However, the definition of severe COVID-19 was inconsistent across the reviews. The largest of which (Luo et al.,) suggested CVD was associated with 3.86 times higher odds of severe COVID-19 [pooled OR 3.86 (2.70–5.52), n =
= 29 studies, substantial heterogeneity (I2 = 63%)].33
29 studies, substantial heterogeneity (I2 = 63%)].33
Three reviews examined associations between coronary heart disease (CHD) and outcomes with COVID-19 and were rated moderate quality.20,23,39 The largest review included 11 studies and suggested CHD was significantly associated with 3.63 times higher odds of mortality with COVID-19 [pooled OR 3.63 (1.52–8.65), considerable heterogeneity (I2 = 100%)].39 One review which was rated moderate quality reported CHD was associated with 2 times higher odds of severe COVID-19 [pooled OR 2.03 (1.39–2.97), moderate heterogeneity (I2 = 44%)].23
Cerebrovascular disease
Nine reviews which were rated as moderate quality examined associations between cerebrovascular disease and outcomes with COVID-19.21,23,25,31,38,39,44,46,48 The moderate quality review with the largest number of studies reported cerebrovascular disease was associated with a significant 2.75 times higher risk of mortality [pooled relative risk (RR) 2.75 (1.54–4.89), n =
= 11 studies, considerable heterogeneity (I2 = 99%)].39 The review by Fang et al.,23 was also rated moderate quality and included the largest number of studies examining the association between cerebrovascular disease and severe COVID-19. Fang et al.,23 reported that cerebrovascular disease was associated with 2.77 times higher risk of severe COVID-19 [pooled RR 2.77 (1.70–4.52), n
11 studies, considerable heterogeneity (I2 = 99%)].39 The review by Fang et al.,23 was also rated moderate quality and included the largest number of studies examining the association between cerebrovascular disease and severe COVID-19. Fang et al.,23 reported that cerebrovascular disease was associated with 2.77 times higher risk of severe COVID-19 [pooled RR 2.77 (1.70–4.52), n =
= 12 studies, moderate heterogeneity (I2 = 40%)].
12 studies, moderate heterogeneity (I2 = 40%)].
Two moderate quality reviews examined the association between cerebrovascular disease and intensive care unit (ICU) admission with COVID-19. The largest of these reviews did not find a significant association between cerebrovascular disease and risk of ICU admission with COVID-19 [pooled RR 1.9 (0.9–4.0), n =
= 4 studies, considerable heterogeneity (I2 = 92%)].38
4 studies, considerable heterogeneity (I2 = 92%)].38
It was unclear in the majority of the reviews if the stroke occurred prior to or following a COVID-19 diagnosis.
Hypertension
Of the reviews which examined hypertension, 15 were rated as moderate quality.20,21,23,24,30–33,35,36,38,39,44,46,48 Of the moderate quality reviews, Luo et al.,33 included the largest number of studies and suggested hypertension was associated with 2.5 times higher odds of mortality [pooled OR 2.50, (2.02–3.11), n =
= 58 studies, considerable heterogeneity (I2 = 93%)]. Luo et al. also reported a significant association between hypertension and higher odds of severe COVID-19 [pooled OR 2.56 (2.12–3.11), n
58 studies, considerable heterogeneity (I2 = 93%)]. Luo et al. also reported a significant association between hypertension and higher odds of severe COVID-19 [pooled OR 2.56 (2.12–3.11), n =
= 55 studies, considerable heterogeneity (I2 = 83%)]. One moderate quality review reported a significant association between hypertension and higher odds of a composite adverse outcome of mortality, mechanical ventilation, or severe COVID-19 [pooled OR 3.15 (2.26–4.41), n
55 studies, considerable heterogeneity (I2 = 83%)]. One moderate quality review reported a significant association between hypertension and higher odds of a composite adverse outcome of mortality, mechanical ventilation, or severe COVID-19 [pooled OR 3.15 (2.26–4.41), n =
= 38 studies, moderate heterogeneity (I2 = 40%)].24 Two moderate quality reviews examined the association between hypertension and risk of ICU admission,23,38 with the largest, more recent review reporting a pooled RR of 1.4 (1.1–1.7), n
38 studies, moderate heterogeneity (I2 = 40%)].24 Two moderate quality reviews examined the association between hypertension and risk of ICU admission,23,38 with the largest, more recent review reporting a pooled RR of 1.4 (1.1–1.7), n =
= 9 studies, and substantial heterogeneity (I2 = 53%).38 Associations between antihypertensive medication use and outcomes with COVID-19 were not examined in this umbrella review.
9 studies, and substantial heterogeneity (I2 = 53%).38 Associations between antihypertensive medication use and outcomes with COVID-19 were not examined in this umbrella review.
Diabetes mellitus
Of the reviews which examined diabetes mellitus, 18 were rated as moderate quality.20,21,23,24,30–36,38–40,44,46–48 Luo et al., conducted the largest moderate quality review and reported significant associations between diabetes mellitus and higher odds of mortality [pooled OR 2.09 (1.80–2.42), n =
= 63 studies, considerable heterogeneity (I2 = 81%)], and severe COVID-19 [pooled OR 2.54 (1.89–3.41), n
63 studies, considerable heterogeneity (I2 = 81%)], and severe COVID-19 [pooled OR 2.54 (1.89–3.41), n =
= 58 studies, considerable heterogeneity (I2 = 89%)].33 One moderate quality review reported a significant association between diabetes mellitus and 2.34 times higher odds of a composite adverse outcome of mortality, mechanical ventilation or severe COVID-19 [pooled OR 2.34 (1.64–3.33), n
58 studies, considerable heterogeneity (I2 = 89%)].33 One moderate quality review reported a significant association between diabetes mellitus and 2.34 times higher odds of a composite adverse outcome of mortality, mechanical ventilation or severe COVID-19 [pooled OR 2.34 (1.64–3.33), n =
= 34 studies, substantial heterogeneity (I2 = 80%)].24 Two moderate quality reviews examined the association between diabetes and risk of ICU admission,23,38 and the largest, more recent review reported a pooled RR of 1.9 (1.4–2.6), n
34 studies, substantial heterogeneity (I2 = 80%)].24 Two moderate quality reviews examined the association between diabetes and risk of ICU admission,23,38 and the largest, more recent review reported a pooled RR of 1.9 (1.4–2.6), n =
= 12 studies, and considerable heterogeneity (I2 = 90%)].38 The association between diabetes and mortality with COVID-19 was stratified by age group in one moderate quality review of nine studies, and the association only remained statistically significant for patients aged <70
12 studies, and considerable heterogeneity (I2 = 90%)].38 The association between diabetes and mortality with COVID-19 was stratified by age group in one moderate quality review of nine studies, and the association only remained statistically significant for patients aged <70 years [pooled OR 2.05 (1.44–2.94), moderate heterogeneity (I2 = 32%)].47
years [pooled OR 2.05 (1.44–2.94), moderate heterogeneity (I2 = 32%)].47
Renal disease
All reviews compared renal/kidney disease/disorder or chronic kidney disease to no renal disease/disorder. No reviews examined the impact of different stages of renal disease on outcomes with COVID-19. Eight reviews rated as moderate quality examined associations between renal disease and outcomes with COVID-19.20,23,31,33,38,39,44,48 The largest moderate quality review reported a significant association between renal disease and higher odds of mortality [pooled OR 3.07 (2.43–3.88), n =
= 35 studies, substantial heterogeneity (I2 = 73%)],33 and severe COVID-19 [pooled OR 2.20 (1.26–3.85), n
35 studies, substantial heterogeneity (I2 = 73%)],33 and severe COVID-19 [pooled OR 2.20 (1.26–3.85), n =
= 28 studies, considerable heterogeneity (I2 = 77%)).33
28 studies, considerable heterogeneity (I2 = 77%)).33
Liver disease
Six reviews rated as moderate quality examined associations between liver disease and outcomes with COVID-19. Some of the reviews referred to ‘chronic liver disease’ whilst others only specified ‘liver disease’, and no distinctions were made for the severity of liver disease. Four moderate quality reviews examined the association between liver disease and mortality with COVID-19.31,38,39,44 Islam et al.31 was the largest review and reported a significant association between liver disease and 2.81 times higher odds of mortality [pooled OR 2.81 (1.31–6.01), n =
= 8 studies, no heterogeneity (I2 = 0%)]. The largest moderate quality review which examined the association between liver disease and severe COVID-19 did not find a significant association [pooled OR 0.81 (0.47–1.40), n
8 studies, no heterogeneity (I2 = 0%)]. The largest moderate quality review which examined the association between liver disease and severe COVID-19 did not find a significant association [pooled OR 0.81 (0.47–1.40), n =
= 11 studies, I2 not reported], but this review was relatively older (searches until April 2020).46
11 studies, I2 not reported], but this review was relatively older (searches until April 2020).46
Obesity
Three reviews rated as moderate quality examined associations between obesity or body mass index (BMI) and outcomes with COVID-19.39,45,46 The largest moderate quality review reported a statistically significant association between obesity and mortality [pooled OR 2.18, (1.10–4.34), n =
= 7 studies considerable heterogeneity (I2 = 99%)].39 One further moderate quality review also suggested obesity was associated with increased risk of in-hospital critical care with COVID-19, but a meta-analysis was not performed.45 One moderate quality review including only four studies of 221 patients reported no statistically significant association between BMI and severe COVID-19.46
7 studies considerable heterogeneity (I2 = 99%)].39 One further moderate quality review also suggested obesity was associated with increased risk of in-hospital critical care with COVID-19, but a meta-analysis was not performed.45 One moderate quality review including only four studies of 221 patients reported no statistically significant association between BMI and severe COVID-19.46
Smoking
One of the reviews which examined smoking and outcomes with COVID-19 was rated high-quality,51 and six reviews were rated moderate quality.21,28,35,39,41,46 There were differences in the comparisons made in the reviews which examined smoking (e.g. current/former smoker vs. never smoker and current smoking vs. not current smoking). The high-quality review by Reddy et al.,51 reported a statistically significant association between current smoking and 1.80 times higher risk of severe COVID-19 [pooled RR 1.80 (1.14–2.85), n =
= 5 studies, considerable heterogeneity (I2 = 76%)], but no significant association between current smoking and disease progression, ICU admission, mechanical ventilation, or mortality. One moderate quality review which examined smoking had a more recent search date than the high-quality review and also did not find a significant association between current smoking and mortality with COVID-19.39
5 studies, considerable heterogeneity (I2 = 76%)], but no significant association between current smoking and disease progression, ICU admission, mechanical ventilation, or mortality. One moderate quality review which examined smoking had a more recent search date than the high-quality review and also did not find a significant association between current smoking and mortality with COVID-19.39
In the same high-quality review, smoking history vs. never smoking was associated with severe COVID-19 [pooled RR 1.31 (1.12–1.54), n =
= 12 studies, low heterogeneity (I2 = 12%)], disease progression [pooled RR 2.18 (1.06–4.49), n
12 studies, low heterogeneity (I2 = 12%)], disease progression [pooled RR 2.18 (1.06–4.49), n =
= 5 studies, substantial heterogeneity (I2 = 69%)], mechanical ventilation [pooled RR 1.20 (1.01–1.42), n
5 studies, substantial heterogeneity (I2 = 69%)], mechanical ventilation [pooled RR 1.20 (1.01–1.42), n =
= 4 studies, no heterogeneity (I2 = 0%)] and mortality [pooled RR 1.26 (1.20–1.32), n
4 studies, no heterogeneity (I2 = 0%)] and mortality [pooled RR 1.26 (1.20–1.32), n =
= 9 studies, no heterogeneity (I2 = 0%)], but not ICU admission.51
9 studies, no heterogeneity (I2 = 0%)], but not ICU admission.51
Alcohol
One moderate quality review was identified which examined associations between alcohol consumption and severe COVID-19.46 The review only identified one study which included 30 patients with COVID-19 and did not find a statistically significant association between alcohol use and severe COVID-19 [OR 1.86 (0.40–8.69)].
Multiple cardiovascular risk factors
One moderate quality review examining 21 studies with >77 000 patients reported a significant association between increasing numbers of cardiovascular co-morbidities or cardiovascular risk factors and higher mortality was significantly associated with COVID-19 case fatality rate (regression coefficient 0.001, 95% CI 0.003–0.005, P <
< 0.001).42
0.001).42
The impact of COVID-19 on cardiovascular health
All of the reviews which examined the impact of COVID-19 on cardiovascular health were completed in the acute phase, and no reviews were found which examined the impact of COVID-19 on longer-term cardiovascular outcomes.
Acute cardiac injury
Five reviews rated as moderate quality reported the pooled incidence of acute cardiac injury in patients with COVID-19.27,30,36,37,42 Of these reviews, only two further defined acute cardiac injury.27,37 One of the reviews defined acute cardiac injury as ‘serum levels of troponin or CK-MB above the 99th percentile upper reference limit, regardless of new abnormalities in electrocardiography and echocardiography’,27 and one review defined acute cardiac injury as ‘troponin levels >28 pg/mL’.37 Amongst these reviews the incidence of acute cardiac injury ranged from 6%30 to 25%.36 The largest moderate quality review included over 77 000 participants and suggested the incidence of acute myocardial injury was 10.3%.42
pg/mL’.37 Amongst these reviews the incidence of acute cardiac injury ranged from 6%30 to 25%.36 The largest moderate quality review included over 77 000 participants and suggested the incidence of acute myocardial injury was 10.3%.42
Four reviews rated as moderate quality examined the association between acute cardiac injury and outcomes with COVID-19.24,33,36,48 The largest, most recent moderate quality review reported an association between acute cardiac injury and 17 times higher odds of mortality with COVID-19 [pooled OR 16.97 (7.87–36.57), n =
= 14 studies, considerable heterogeneity (I2 = 89%)].33
14 studies, considerable heterogeneity (I2 = 89%)].33
One moderate quality review reported a significant association between acute cardiac injury and a composite adverse outcome of mortality, mechanical ventilation, or severe COVID-19 [pooled OR 10.58 (5.00–22.40), n =
= 12 studies, substantial heterogeneity (I2 = 59%)].24 Two moderate quality reviews examined the association between acute cardiac injury and severe COVID-19.33,48 The largest, more recent moderate quality review reported a significant association between acute cardiac injury and severe COVID-19 [pooled OR 6.57 (3.70–11.65), n
12 studies, substantial heterogeneity (I2 = 59%)].24 Two moderate quality reviews examined the association between acute cardiac injury and severe COVID-19.33,48 The largest, more recent moderate quality review reported a significant association between acute cardiac injury and severe COVID-19 [pooled OR 6.57 (3.70–11.65), n =
= 11 studies, considerable heterogeneity (I2 = 75%)].33
11 studies, considerable heterogeneity (I2 = 75%)].33
Incident venous thromboembolism, pulmonary embolism, and deep vein thrombosis
One moderate quality meta-analysis of 17 studies estimated the incidence of venous thromboembolism (VTE), pulmonary embolism, and deep vein thrombosis as 25% (95% CI: 19%-31%), 19% (13%-25%) and 7% (4%–10%), respectively for patients hospitalized with COVID-19. All of these estimates were shown to have considerable heterogeneity. A higher incidence of VTE was observed in severe compared to non-severe patients (pooled RR 4.76 (2.66–8.50), moderate heterogeneity (I2 = 47%)).50
Incident arrhythmia, cardiac failure, and cerebrovascular disease
Two reviews rated moderate quality examined the incidence of arrhythmia developed during hospitalization with COVID-19.29,42 The larger of these reported incidence of arrhythmias as 18.4% (95% CI 7.8–32.3%).42 One moderate quality review of four studies (245 patients) estimated the incidence of acute cardiac failure with COVID-19 as 6.5% (2.2%–12.2%), considerable heterogeneity (I2 = 78%).26 The incidence of acute cerebrovascular disease was not examined in any reviews rated as moderate quality.
Incident composite cardiovascular complications
Two moderate quality reviews examined the incidence of any cardiovascular complication with COVID-19.42,43 The most recent moderate quality review reported a pooled incidence of 14.1% (10.3–20.2%) for any cardiovascular complication developed in-hospital with COVID-19.42 This review also reported a statistically significant association between cardiovascular complications and COVID-19 case fatality rate (regression coefficient 0.001, 95% CI 0.000–0.003, P =
= 0.038).42
0.038).42
Discussion
Evidence from this umbrella review suggests CVD, hypertension, diabetes mellitus, renal disease, and smoking history associate with a higher likelihood of severe COVID-19 and mortality with COVID-19. Current smoking associated with significantly higher risk of severe COVID-19, but not mortality. Liver disease was associated with significantly higher odds of mortality, but not severe COVID-19. Obesity associated with higher odds of mortality with COVID-19. Although cerebrovascular disease was associated with a higher likelihood of adverse outcomes with COVID-19, it was often unclear if the stroke occurred prior to or following infection. There was insufficient evidence to make conclusions regarding alcohol consumption and outcomes with COVID-19. Furthermore, although an extensive search was conducted, no moderate quality reviews were identified which examined cholesterol levels, arrhythmias, diet, physical activity, or dementia and outcomes with COVID-19. No identified reviews examined the impact of cardiovascular health on long-COVID.
Acute heart failure following COVID-19 was 2%, the incidence of myocardial infarction was 4%, myocardial injury was 10%, angina was 10%, arrhythmias was 18%, and incidence of venous thromboembolism, pulmonary embolism, and deep vein thrombosis was 25%, 19%, and 7%, respectively. No identified reviews examined the impact of COVID-19 on long-term cardiovascular health.
Early in the COVID-19 pandemic, concerns were raised about the potential for COVID-19 to cause new-onset cardiovascular complications or exacerbate existing CVD because of prior knowledge from influenza epidemics and outbreaks of other respiratory viruses.52 Influenza epidemics have been associated with a rise in cardiovascular mortality.53 Emerging evidence has suggested the COVID-19 pandemic may also have resulted in excess deaths in people with CVD, but this may be due to direct effects of infection and indirect effects due to changes in availability of healthcare and behaviour changes.54
The higher proportion of adverse outcomes with COVID-19 for patients with CVD or cardiovascular risk factors may also be due to underlying changes these risk factors have caused on inflammatory pathways, immune function, and/or lung function. First, Influenza and other upper respiratory infections may result in cardiovascular complications by causing a pro-inflammatory state.53,55 Similarly, dysregulation of coagulation and a hyperinflammatory response has been reported in some patients with COVID-19, which may cause a ‘hypercoagulable state’ and a propensity for thrombosis and thrombosis-related complications.3,56 Second, cardiovascular risk factors such as diabetes mellitus may lead to immune function dysregulation, which may in turn increase susceptibility and predispose these patients to severe presentation of COVID-19.57 Third, some cardiovascular risk factors including smoking and obesity may impair pulmonary function resulting in a higher risk of severe outcomes with COVID-19. Both current and former smokers have accelerated lung function decline compared to never-smokers.58 Additionally, obesity may also impair lung function by pushing the diaphragm upward and reducing lung volume.59
Identifying cardiovascular risk factors for worsened COVID-19 prognosis is important to identify high-risk patient groups and targeting of intervention strategies. Many of the risk factors identified as significantly associated with adverse outcomes with COVID-19 are potentially modifiable. Therefore, primary and secondary prevention strategies which target these cardiovascular risk factors and conditions may improve outcomes for people following COVID-19.
Further research should focus on the impact of multiple cardiovascular risk factors and other multi-morbidity associations with COVID-19, as cardiovascular risk factors rarely occur in isolation. Although evidence will be observational, further research should include appropriate adjustment for confounding factors including age, socioeconomic status, and ethnicity, which are established risk factors for COVID-19 severity.
All included reviews which examined the impact of COVID-19 on cardiovascular health observed in-hospital cardiovascular outcomes only, and the impact of COVID-19 on long-term cardiovascular health was not investigated. Future research should determine longer-term outcomes for people with COVID-19, including whether COVID-19 impacts incident cardiovascular and cerebrovascular outcomes post-discharge, and whether CVD associates with a higher risk of long-COVID.
Strengths and limitations
This umbrella review included a systematic search strategy to examine a wide-range of cardiovascular risk factors and cardiovascular conditions in relation to outcomes with COVID-19, and the impact of COVID-19 on cardiovascular health. Cardiovascular biomarkers were not included and the impact of treatments for COVID-19 on the observed associations was not examined as this was beyond the scope of the current review. Only reviews available in the English language were included. The quality of the included reviews varied, 52 critically low- and low-quality reviews according to the AMSTAR 2 checklist were included and there was duplication of primary studies within the reviews. However, we have focused on the results of reviews which were rated as moderate and high-quality. Due to the nature of the research questions, only observational evidence was available to address the questions, which typically provides low certainty evidence and cannot infer causality. Confounding factors such as age, sex, and ethnicity may impact the results of reviews, but it was not clear in many of the reviews if the studies included in meta-analyses adjusted for these factors. Furthermore, high levels of heterogeneity were often reported in meta-analyses, which was typically not further investigated. Within the included reviews, there were inconsistencies in definitions used for severe COVID-19. Pre-prints were included because of the rapidly emerging evidence base, but the results reported in these articles may be subject to change following peer-review. However, only one of the reviews included in the forest plots was a pre-print.31
Conclusions
In conclusion, the evidence reported in this umbrella review suggests that CVD and certain cardiovascular risk factors including hypertension, diabetes mellitus, renal disease, liver disease, cerebrovascular disease, obesity, smoking history and current smoking associated with a higher likelihood of severe COVID-19 and/or mortality with COVID-19. Incident cardiovascular complications following hospitalization with COVID-19 may be up to 25% depending on the complication, but further evidence is needed to determine the impact of COVID-19 on long-term cardiovascular health outcomes. Many of the cardiovascular risk factors associated with COVID-19 are modifiable. Clinicians and policy makers should consider that primary and secondary prevention strategies which improve cardiovascular health may also improve outcomes for people following COVID-19.
Supplementary material
Supplementary material is available at European Heart Journal – Quality of Care and Clinical Outcomes online.
Acknowledgements
Joanna Wood, Hannah Sullivan, and Katherine Thompson (Public Health England) are acknowledged for their contributions to the research. Joanna Wood developed the search strategies and completed the searches. Hannah Sullivan and Katherine Thompson developed the research questions and provided feedback on the results.
Funding
This work was funded by Public Health England.
Conflict of interest
S.L.H. reports grants from Public Health England, during the conduct of the study; grants from Bristol Myers-Squibb, outside the submitted work. B.J.R.B. reports grants from Public Health England, during the conduct of the study; grants from Pfizer/Bristol-Myers Squibb (BMS), outside the submitted work. J.M.R.-C. reports grants from Sociedad Española de Trombosis y Hemostasia, grants from European Society of Cardiology Council on Basic Cardiovascular Science, outside the submitted work. J.Z. has nothing to disclose. G.Y.H.L. reports consultancy and speaker fees from Bayer, Bayer/Janssen, BMS/Pfizer, Biotronik, Medtronic, Boehringer Ingelheim, Microlife, Roche, and Daiichi-Sankyo outside the submitted work. No fees received personally.
References
 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ (Clinical Research ed.) 2020;369:m1985. [Europe PMC free article] [Abstract] [Google Scholar]
133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ (Clinical Research ed.) 2020;369:m1985. [Europe PMC free article] [Abstract] [Google Scholar]Articles from European Heart Journal. Quality of Care & Clinical Outcomes are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/ehjqcco/qcab029
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/ehjqcco/article-pdf/7/4/330/39236664/qcab029.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/107376672
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1093/ehjqcco/qcab029
Article citations
Cardiovascular Drug Interactions with Nirmatrelvir/Ritonavir for COVID-19: Considerations for Daily Practice.
Eur Cardiol, 19:e15, 09 Aug 2024
Cited by: 0 articles | PMID: 39220617 | PMCID: PMC11363061
Review Free full text in Europe PMC
Adverse events associated with SARS-CoV-2 neutralizing monoclonal antibodies using the FDA adverse event reporting system database.
Toxicol Res, 40(4):673-682, 30 Jul 2024
Cited by: 0 articles | PMID: 39345748
Pathogenic mechanisms of cardiovascular damage in COVID-19.
Mol Med, 30(1):92, 19 Jun 2024
Cited by: 1 article | PMID: 38898389 | PMCID: PMC11186295
Review Free full text in Europe PMC
Persisting exercise ventilatory inefficiency in subjects recovering from COVID-19. Longitudinal data analysis 34 months post-discharge.
BMC Pulm Med, 24(1):258, 25 May 2024
Cited by: 0 articles | PMID: 38796432 | PMCID: PMC11128102
Risk factors for running-related injuries: An umbrella systematic review.
J Sport Health Sci, 13(6):793-804, 30 Apr 2024
Cited by: 0 articles | PMID: 38697289 | PMCID: PMC11336318
Review Free full text in Europe PMC
Go to all (76) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Kidney health in the COVID-19 pandemic: An umbrella review of meta-analyses and systematic reviews.
Front Public Health, 10:963667, 12 Sep 2022
Cited by: 6 articles | PMID: 36172213 | PMCID: PMC9511113
Review Free full text in Europe PMC
Risk of SARS-CoV-2 infection, severe COVID-19 illness and COVID-19 mortality in people with pre-existing mental disorders: an umbrella review.
BMC Psychiatry, 23(1):181, 20 Mar 2023
Cited by: 10 articles | PMID: 36941591 | PMCID: PMC10026202
Review Free full text in Europe PMC
DASH Dietary Pattern and Cardiometabolic Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses.
Nutrients, 11(2):E338, 05 Feb 2019
Cited by: 189 articles | PMID: 30764511 | PMCID: PMC6413235
Review Free full text in Europe PMC
Hormone therapy for preventing cardiovascular disease in post-menopausal women.
Cochrane Database Syst Rev, (3):CD002229, 10 Mar 2015
Cited by: 141 articles | PMID: 25754617 | PMCID: PMC10183715
Review Free full text in Europe PMC
Funding
Funders who supported this work.
National Institute for Health Research (NIHR) (3)
Grant ID: CL-2020-07-501
Grant ID: NIHR201320
Grant ID: NF-SI-0616-10010