Abstract
Introduction
Recurrent focal and segmental glomerulosclerosis (FSGS) in kidney transplant recipients is associated with lower graft survival and increased morbidity. There are limited data to guide the decision to re-transplant patients with transplant failure due to FSGS recurrence. We aimed to evaluate outcomes in patients re-transplanted after having initial graft failure due to recurrent FSGS and to study physician attitudes and practice patterns.Methods
Retrospective data from 10 centers were collected on 20 patients transplanted between January 1997 and September 2018. A survey was sent to nephrologist members of the Pediatric Nephrology Research Consortium.Results
Mean patient age (years) was 9.8 ± 4.8 at first transplant and 15.9 ± 4.9 at re-transplantation. Pre-transplant plasmapheresis was used in 1 (5.3%) primary transplant vs. 7 (38.9%) re-transplants (p = .03). Nephrotic syndrome recurred in 14 patients (70%) after re-transplantation and was severe in 21.1% vs. 64.7% after first transplant (p = .04). Graft survival was significantly higher in the second transplant (p .009) with 70% having functioning grafts at a median of 25.2 months. Thirty-one physicians from 21 centers completed the survey, 94% indicated they would re-transplant such patients, 44.4% preferred a minimum waiting period before re-transplantation, 36.4% preferred living donors, and 22.2% indicated having protocols for re-transplantation at their centers.Conclusions
Consideration for re-transplantation is high among pediatric nephrologists. Pre-transplant plasmapheresis was more frequent in re-transplanted patients. Nephrotic syndrome recurrence was less severe, with better graft survival. More data and a larger population are necessary to further evaluate outcome determinants and best practices in this special population.Free full text

Re-transplantation in pediatric patients with failure of primary transplant due to recurrent focal segmental glomerulosclerosis: A pediatric nephrology research consortium study
Abstract
Introduction:
Recurrent focal and segmental glomerulosclerosis (FSGS) in kidney transplant recipients is associated with lower graft survival and increased morbidity. There are limited data to guide the decision to re-transplant patients with transplant failure due to FSGS recurrence. We aimed to evaluate outcomes in patients re-transplanted after having initial graft failure due to recurrent FSGS and to study physician attitudes and practice patterns.
Methods:
Retrospective data from 10 centers were collected on 20 patients transplanted between January 1997 and September 2018. A survey was sent to nephrologist members of the Pediatric Nephrology Research Consortium.
Results:
Mean patient age (years) was 9.8 ± 4.8 at first transplant and 15.9 ± 4.9 at re-transplantation. Pre-transplant plasmapheresis was used in 1 (5.3%) primary transplant vs. 7 (38.9%) re-transplants (p = .03). Nephrotic syndrome recurred in 14 patients (70%) after re-transplantation and was severe in 21.1% vs. 64.7% after first transplant (p = .04). Graft survival was significantly higher in the second transplant (p .009) with 70% having functioning grafts at a median of 25.2 months. Thirty-one physicians from 21 centers completed the survey, 94% indicated they would re-transplant such patients, 44.4% preferred a minimum waiting period before re-transplantation, 36.4% preferred living donors, and 22.2% indicated having protocols for re-transplantation at their centers.
Conclusions:
Consideration for re-transplantation is high among pediatric nephrologists. Pre-transplant plasmapheresis was more frequent in re-transplanted patients. Nephrotic syndrome recurrence was less severe, with better graft survival. More data and a larger population are necessary to further evaluate outcome determinants and best practices in this special population.
1 |. INTRODUCTION
Focal segmental glomerulosclerosis (FSGS) is a common cause of kidney failure in children; specifically, it was found in a recent report by NAPRTCS (North American Pediatric Renal Trials and Collaborative Studies) registry to be the cause of kidney failure in 15.1% of children transplanted between 2002 and 2016.1 Recurrent nephrotic syndrome occurs in up to 60% of transplanted pediatric patients with idiopathic FSGS2,3 with inferior graft survival compared to transplanted patients with other glomerular disease and non-glomerular causes of kidney failure.4–6 Limited data suggest that kidney transplant recipients with graft failure secondary to FSGS recurrence are at an increased risk of recurrence and graft failure after a subsequent transplant.7
Recurrent FSGS leading to graft failure often leads to consideration for re-transplantation. However, FSGS recurrence and graft failure after re-transplantation are a significant concern.1,7 Jungraithmyar et al. reported nephrotic syndrome recurrence rates of 36%, 48%, and 100% in first, second, and third transplants, respectively, in those with an idiopathic form of FSGS, compared to 0% recurrence in patients with genetic forms of FSGS. In their series of 38 patients with idiopathic FSGS, 40% of those with recurrent nephrotic syndrome in the first graft lost their grafts due to recurrence, and all of them had recurrent nephrotic syndrome after the second transplant.7 The recent NAPRTCS study also reports an increased risk of graft failure in FSGS patients who had a prior transplant that failed due to recurrent nephrotic syndrome OR 1.58 (1.10–2.27), p = .013.1 Given this unfavorable data, many physicians and pediatric kidney transplant centers may be reluctant to re-transplant patients due to concerns of recurrence of FSGS and graft loss, increased morbidity, poor outcomes, and lack of sufficient data regarding mitigation strategies and therapies to prevent or minimize the impact of recurrence of FSGS after re-transplantation.
The purpose of this study was to evaluate outcomes in pediatric kidney transplant recipients with FSGS who had first graft failure due to recurrent nephrotic syndrome and received a second transplant and to evaluate potential factors affecting outcome. In addition, this study surveyed factors influencing physicians’ decisions to re-transplant or not and institution practices throughout North America.
2 |. MATERIALS AND METHODS
2.1 |. Pediatric nephrology research consortium
This was a multi-center study conducted by investigators who are members of the Pediatric Nephrology Research Consortium (PNRC); formerly the Midwest Pediatric Nephrology Consortium (MWPNC). PNRC is a multi-center international research cohort which conducts retrospective and prospective clinical and translational research within the pediatric nephrology community. The study was approved by the local Institutional Review Board (IRB) at each of the participating centers. This was a two-part study: a retrospective chart review and a physician survey.
2.2 |. Patient outcomes of re-transplantation after FSGS recurrence
For the retrospective chart review, kidney transplant recipients with failed grafts secondary to FSGS recurrence who were then re-transplanted were the population of interest. Inclusion criteria included age 1–21 years at the time of the first transplant, biopsy-confirmed diagnosis of FSGS, transplantation between January 1, 1997, and September 1, 2018 (for both primary and second transplants), and follow-up time of at least 6 months after re-transplantation.
Data collected for each recipient’s first and second transplants included the following: age at first FSGS diagnosis, age at kidney failure diagnosis, age at the time of transplants, time on dialysis, years of transplants, donor type (deceased vs. living), immunosuppressive therapy (induction and maintenance), pre- and post-transplant plasmapheresis use, and use of anti-CD20 therapy (e.g.,, rituximab).
The outcomes examined in this study were FSGS recurrence rate, time to recurrence, severity of recurrence, graft survival, graft function, and acute rejection rate. Severity of recurrence was defined for study purposes as severe: nephrotic-range proteinuria (random urine protein/creatinine ratio of ≥2 mg/mg), serum albumin <2.5 g/dl, and presence of edema; moderate: nephrotic-range proteinuria, serum albumin ≥2.5 g/dl, and with or without edema; and mild: sub-nephrotic proteinuria (urine protein/creatinine ratio 0.5–<2 mg/mg). Full remission and no recurrence were defined by urine protein/creatinine <0.5 mg/mg. Additionally, graft failure data were collected, with graft failure defined as return to dialysis or re-transplantation. Graft function was assessed by serum creatinine and estimated glomerular filtration rate (eGFR) using modified Schwartz formula for patients 18 years and younger and CKD-EPI 2009 formula for patients older than 18 years of age.8,9
2.3 |. Physician practice patterns survey
The survey was sent via email to 174 physician members of PNRC, and informed consent to answer the survey was obtained. The survey sought data regarding the number of transplanted patients at each center, number of patients with FSGS who received transplants, and number of transplanted patients with FSGS who developed graft failure due to disease recurrence.
The survey included questions on physician preferences on re-transplantation. The first question was “Would you re-transplant a patient with FSGS and graft failure due to recurrent disease?” with a “Yes or No” response. Follow-up questions required ranking factors impacting the decision to not re-transplant on a Likert scale of 1–10 with 1 as the most important. Choices for factors impacting the re-transplant decision were as follows: risk of recurrence, risk of graft failure, risk of poor center outcome and regulatory audit, refusal by other team members, refusal by surgeon, high sensitization, cost, lack of resources, patient/family refusal, and other. If the initial question response was “yes,” the follow-up questions were regarding center practices, specifically if a written protocol existed, if decisions were made on individual bases, and if a waiting period was required prior to re-transplantation and opinions in the form of an additional Likert scale of 1–5 on most effective therapies.
2.4 |. Statistical analysis
Descriptive statistics including means, medians, standard deviations, frequencies, and proportions were used to describe demographic and clinical characteristics when analyzing patient outcomes and survey results. For patient outcomes, demographics and clinical characteristics of the patients were compared between first and second transplants using McNemar’s analysis and paired sample t-tests. Kaplan-Meier plots with log-rank test compared graft survival between first and second transplants. Cox regression was performed to assess differences in hazard ratio of graft failure. A p-value of < .05 was considered statistically significant. SPSS (IBM SPSS) statistical software was used to perform all analyses.
3 |. RESULTS
3.1 |. Patient outcomes after FSGS recurrence
Twenty patients from 10 centers were enrolled, with one center enrolling 6 subjects, 2 centers enrolling 3 subjects each, 1 center enrolling 2 subjects, and 6 centers enrolling 1 subject each. Fifty percent of patients were male, 37% were white, 12.5% were black, and 50% were of other races. The mean age was 5.9 ± 4.8 years at initial FSGS diagnosis, 8.8 ± 5.3 years at diagnosis of kidney failure, 9.8 ± 4.8 years at first transplant, and 15.9 ± 4.9 years at re-transplant. Initial transplants took place between 1998 and 2013, and re-transplants took place between 2006 and 2018. Fifty percent of primary transplant patients and 50% of re-transplanted patients received a kidney from a deceased donor. Among the 20 pediatric kidney transplant recipients whose primary transplant failed due to recurrent FSGS, more patients underwent a pre-transplant nephrectomy prior to the initial transplant as compared to allograft nephrectomy prior to the second transplant (47.4% vs. 9.1%, p = 0.05) (Table 1).
TABLE 1
Patient demographics
| First transplant (N = 20) | Second transplant (N = 20) | p-Value | |
|---|---|---|---|
| Sex (male) | 50% | ||
| Race | |||
 White White | 37% | ||
 Black Black | 12.50% | ||
 Other Other | 50% | ||
| Mean age at FSGS diagnosis (years) | 5.9 ± 4.8 | ||
| Mean age at diagnosis of kidney failure (years) | 8.8 ± 5.3 | ||
| Mean age at transplant (years) | 9.8 ± 4.8 | 15.9 ± 4.9 | |
| Transplant period | 1998–2003 | 2006–2018 | |
| Deceased donor transplant (%) | 50% | 50% | 1 |
| Pre-transplant nephrectomy (%) | 47.4%a (9/19) | 9.1%b (1/11) | .05 |
Thymoglobulin was more commonly used for induction therapy in re-transplants compared to primary transplants (47.4% vs. 10.5%, p = .02) (Table 2). Tacrolimus was the primary calcineurin inhibitor (CNI) used in 63.2% and 74.8% of primary and re-transplants, respectively (p= NS). CNI switch between tacrolimus and cyclosporine occurred in 29.4% of first transplants and 15.8% of re-transplants (p = .43). Pre-transplant and post-transplant use of anti-CD20 therapy was not statistically significant between primary and re-transplants. Pre-transplant plasmapheresis was used in 7 re-transplanted patients (38.9%) vs. only 1 primary transplant patient (5.3%) (p = .03). Post-transplant plasmapheresis was similar in both transplants (57.9% vs. 63.2%, p = 1.00) (Table 2).
TABLE 2
Immunosuppression and plasmapheresis
| First transplant (N = 20) | Second transplant (N = 20) | p-Value | |
|---|---|---|---|
| Induction immunosuppression a | |||
 Basiliximab Basiliximab | (8/19) 42.1% | (6/19) 31.6% | 1 |
 Thymoglobulin Thymoglobulin | (2/19) 10.5% | (9/19) 47.4% | 0.02 |
 Alemtuzumab Alemtuzumab | (3/19) 15.8% | (4/19) 21.1% | 1 |
 None None | (2/19) 10.5% | (0/19) 0% | |
| Maintenance immunosuppression | |||
 Initial CNI—tacrolimus Initial CNI—tacrolimus | (12/17) 70.6% | (15/19) 78.9% | .29 |
 Initial CNI—cyclosporine Initial CNI—cyclosporine | (5/17) 29.4% | (4/19) 21.1% | 1 |
 Switch in CNI Switch in CNI | (5/17) 29.4% | (3/19) 15.8% | .43 |
| Plasmapheresis and anti-CD20 therapy | |||
 Pre-transplant plasmapheresis Pre-transplant plasmapheresis | (1/19) 5.3% | (7/18) 38.9% | .03 |
 Mean (range) # of sessions per patient Mean (range) # of sessions per patient | 6 | 3.9 (1–10)b | |
 Post-transplant plasmapheresis Post-transplant plasmapheresis | (11/19) 57.9% | (12/19) 63.2% | 1 |
 Mean # of sessions per patient Mean # of sessions per patient | 19.4 (2–60) | 10.2 (8–24) | |
 Pre-transplant anti-CD20 therapy Pre-transplant anti-CD20 therapy | (7/19) 36.8% | (6/19) 31.6% | 1 |
 Post-transplant anti-CD20 therapyc Post-transplant anti-CD20 therapyc | (5/19) 26.3% | (6/19) 31.6% | 1 |
Nephrotic syndrome recurred in 70% of children after the second transplant and was less severe in the second transplant (p = .03) (odds ratio for severe recurrence in second transplant 0.19, 95CI 0.047, 0.81, p = .04). Recurrence after the second transplant was diagnosed a median of 6 days post-transplant compared to 12 days after the primary transplant, p = .01. The nadir protein/creatinine ratio (mg/mg) was statistically significantly lower after the second transplant compared to the first (0.73 vs. 3.75, p < .001), and peak values were higher in first transplants but not significant (37.4 and 12.0, for first and second transplants, respectively, p = .06) (Table 3). One month after re-transplantation, 68.4% were no longer nephrotic, and 42% were in complete remission. At 1 year, 68.4% were in complete remission, 10.5% had proteinuria in the sub-nephrotic range, and only 20.1% continued to have nephrotic-range proteinuria (2 had moderate recurrence, and 2 had severe recurrence), with an overall remission (complete and partial) rate of 78.9%. At last follow-up, 66.7% were still in complete remission, 11.1% had sub-nephrotic proteinuria, and only 16.7% were still with nephrotic-range proteinuria, with an overall remission rate of 77.8% (Table 3). There were no statistically significant differences in delayed graft function, serum creatinine values, or eGFR at one month or one year post-transplant (Table 4), and eGFR was significantly higher at last follow-up in the second transplant (p < .001). Recurrent nephrotic syndrome resulted in graft loss in 30% of those who underwent a second transplant compared to 100% after the first transplant (p < .001), with no difference in the median follow-up time or median time to graft failure. Of the 6 patients who had graft failure, 2 patients had severe recurrence and 4 patients had moderate recurrence. The biopsy findings did not show any statistically significant differences between the first and second transplants. Rates of acute tubular necrosis, acute cell-mediated rejection, and FSGS lesion seen on biopsy were numerically higher in the primary transplants (Table 4). Kaplan-Meier graft survival showed significantly better graft survival in re-transplanted patients (p = .009) (Figure 1).
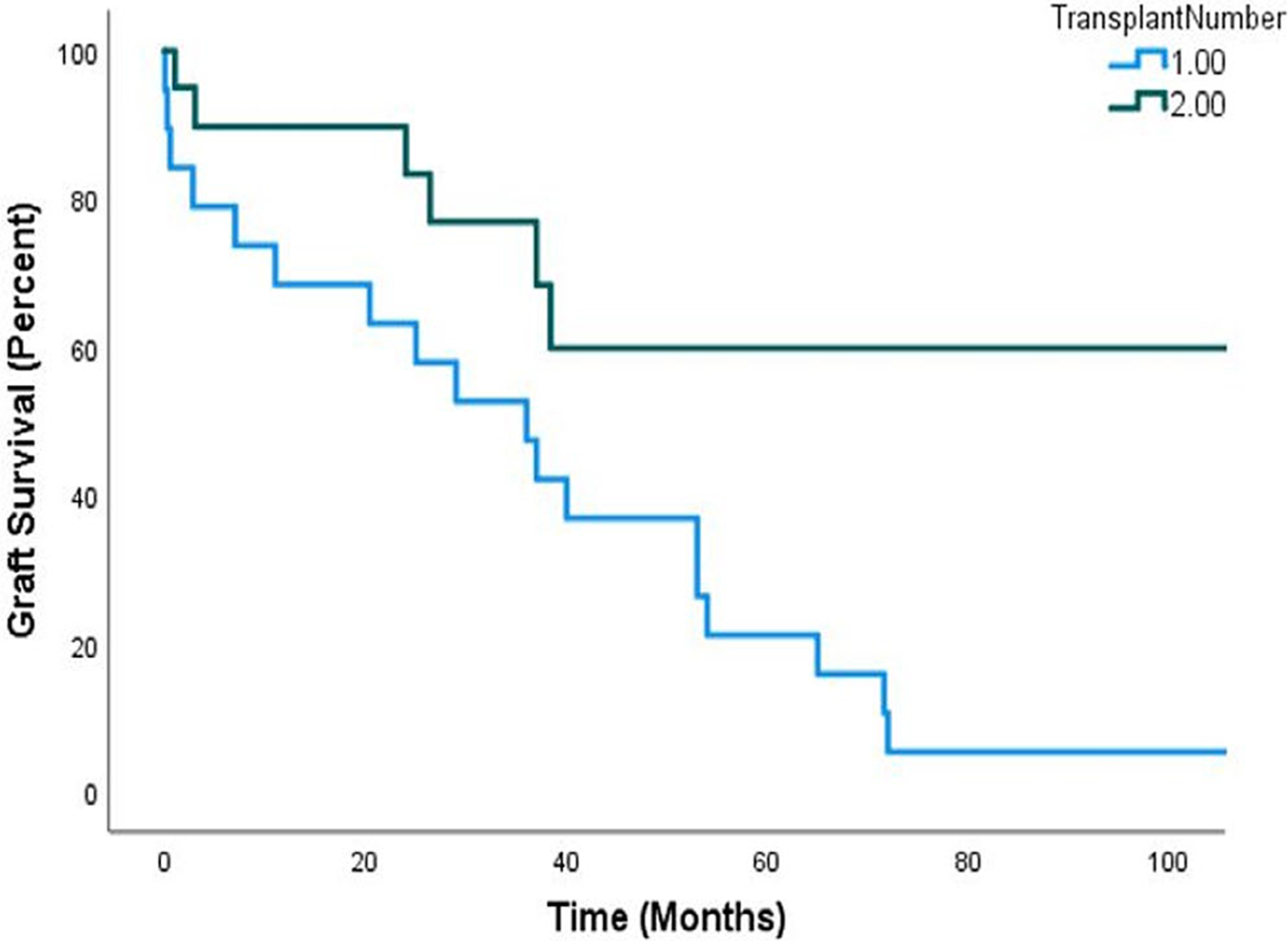
Kaplan Meier survival analysis. Graft survival in first and second transplants, Long-rank test p = .009
TABLE 3
Recurrence of FSGS and proteinuria post-transplant
| First transplant (N = 20) | Second transplant (N = 20) | p-Value | |
|---|---|---|---|
| Recurrence rate | 20 (100%) | 14 (70%) | .01 |
| Median time to recurrence (days) | 12.0 (IQR 1, 467) (n = 16) | 6.0 (IQR 1, 51) (n = 11) | .01 |
| Severity of post-transplant recurrence | |||
 Mild Mild | 1/17 (5.9%) | 5/19 (26.3%) | .03 |
 Moderate* Moderate* | 5/17 (29.4%) | 4/19 (21.1%) | |
 Severe* Severe* | 11/17 (64.7%) | 5/19 (26.3%) | |
 Odds ratio for severe recurrence Odds ratio for severe recurrence | 1 | 0.19 (0.047, 0.81) | .04 |
| Urine protein/creatinine ratio (mg/mg) | |||
 Peak Peak | 37.4 IQR (11.6, 30.6) | 12.0 IQR (2.0, 12.0) | .06 |
 Nadir Nadir | 3.75 IQR (0.2, 5.6) | 0.73 IQR (0.2, 0.8) | <.001 |
 1 month 1 month | 17.6 IQR (0.1, 30.3) | 2.03 IQR (0.26, 2.29) | .04 |
 1 year 1 year | 10.8 IQR (1.88, 12.7) | 1.40 IQR (0.14, 1.54) | .04 |
 Last follow-up Last follow-up | 11.8 IQR (6.3, 18.0) | 0.55 IQR (0.10, 0.65) | .16 |
| Remission rates after second transplant a | NA | 1 year (n = 19) | Last follow-up (n = 18) |
 Complete remission Complete remission | 68.40% | 66.70% | |
 Sub-nephrotic proteinuria Sub-nephrotic proteinuria | 10.50% | 11.10% | |
 Nephrotic-range proteinuria Nephrotic-range proteinuria | 21.10% | 16.70% | |
 Overall remission Overall remission | 78.90% | 77.80% |
TABLE 4
Graft function and biopsy data post-transplant
| First transplant (N = 20) | Second transplant (N = 20) | p-Value | |
|---|---|---|---|
| Delayed graft function | 21% (4/19) | 15.8% (3/19) | 1 |
| Serum creatinine post-transplant (mg/dl) | |||
 1 month 1 month | 1.22 (IQR 0.7, 1.46) | 1.24 (IQR 0.7, 1.42) | .28 |
 1 year 1 year | 1.31 (IQR 0.77, 1.70) | 1.07 (IQR 0.7, 1.44) | .28 |
 Last follow-up Last follow-up | 9.89 (IQR 6.15, 10.60) | 1.56 (IQR 0.79, 1.90) | .25 |
| Estimated glomerular filtration rate (ml/min/1.73 m2) | |||
 1 month 1 month | 107 (IQR 68, 121) (n = 9) | 99 (IQR 59, 123) (n = 16) | .79 |
 1 year 1 year | 106 (IQR 64, 117) (n = 8) | 112 (IQR 71, 139) (n = 14) | .83 |
 Last follow-up Last follow-up | 10 (IQR 6, 11) (n = 9) | 87 (IQR 53, 124) (n = 16) | <.001 |
| CKD stage | |||
 1 month 1 month | n = 8 | n = 15 | |
  Stage 1 Stage 1 | 50% | 53.30% | |
  Stage 2 Stage 2 | 25% | 20% | |
  Stage 3 Stage 3 | 12.50% | 13.30% | |
  Stage 4 Stage 4 | 12.50% | 6.70% | |
  Stage 5 Stage 5 | 0 | 6.70% | |
 1 year 1 year | n = 7 | n = 13 | |
  Stage 1 Stage 1 | 57.10% | 61.50% | |
  Stage 2 Stage 2 | 14.30% | 23.10% | |
  Stage 3 Stage 3 | 14.30% | 15.40% | |
  Stage 4 Stage 4 | 14.30% | 0 | |
  Stage 5 Stage 5 | 0 | 0 | |
 Last follow-up Last follow-up | n = 8 | n = 15 | |
  Stage 1 Stage 1 | 0 | 46.70% | |
  Stage 2 Stage 2 | 0 | 20% | |
  Stage 3 Stage 3 | 0 | 13.30% | |
  Stage 4 Stage 4 | 25% | 20% | |
  Stage 5 Stage 5 | 75% | 0 | |
| Graft failure | |||
 Graft failure rate Graft failure rate | 100% (n=20) | 30% (n=6) | <.001 |
 Median time to failure (months) Median time to failure (months) | 36.0 (IQR 7, 54) (n = 19) | 25.2 (IQR 2.5, 37.4) (n = 6) | .23 |
 Median follow-up (months) Median follow-up (months) | 66.0 (IQR 36.5, 76.2) (n = 15) | 40.0 (IQR 19.0, 68.7) (n = 17) | .23 |
| Graft biopsy (at any point) | |||
 Cell-mediated acute rejection Cell-mediated acute rejection | 10 (50%) | 4 (20%) | .11 |
 Acute tubular necrosis Acute tubular necrosis | 11 (55%) | 6 (30%) | .3 |
 Light microscopy changes of FSGS Light microscopy changes of FSGS | 12 (60%) | 9 (45%) | .51 |
3.1.1 |. Physician practice patterns survey
Thirty-one pediatric nephrologists among 174 surveyed from 21 PNRC centers responded to the survey (17.8% response rate). Of the respondents, 94% reported they would re-transplant patients with graft failure due to FSGS recurrence. Within those who would re-transplant patients, 44.4% indicated they would require a minimum waiting period before re-transplanting patients, 25% of those chose 6 months as a minimum waiting period, 66.7% chose 1 year, 8.3% chose >1–3 years, and 55.6% responded they have no requirement for a waiting period.
Some physicians preferred a living donor (36.4%), and 22.2% of physicians indicated having a protocol for re-transplanting patients after FSGS recurrence at their centers. Most physicians reported deciding on re-transplantation on an individual patient basis (92.6%). For respondents who answered they would not re-transplant such patients, the most important factors influencing the decision not to re-transplant, on a 10-point scale with 1 being most important, were risk of recurrence (2.83) and risk of graft failure (3.16), followed by family refusal (4.12), risk of poor center outcomes and regulatory audit (4.33), refusal by other team members (4.7), refusal by surgeon (5.17), high sensitization (6.29), cost (6.75), and lack of resources (6.75) (breakdown of responses is shown in Figure 2). For the most effective therapy when considering re-transplantation in order of priority on a 5-point scale, nephrologists reported plasmapheresis (1.41) as the most effective therapy, followed by anti-CD20 therapy (2.52), CNI (3.04), and steroids (3.52) (breakdown is shown in Figure 3). The Likert graphs show the various limiting factors influencing decisions regarding re-transplantation, and the therapies viewed as most effective when considering re-transplantation.
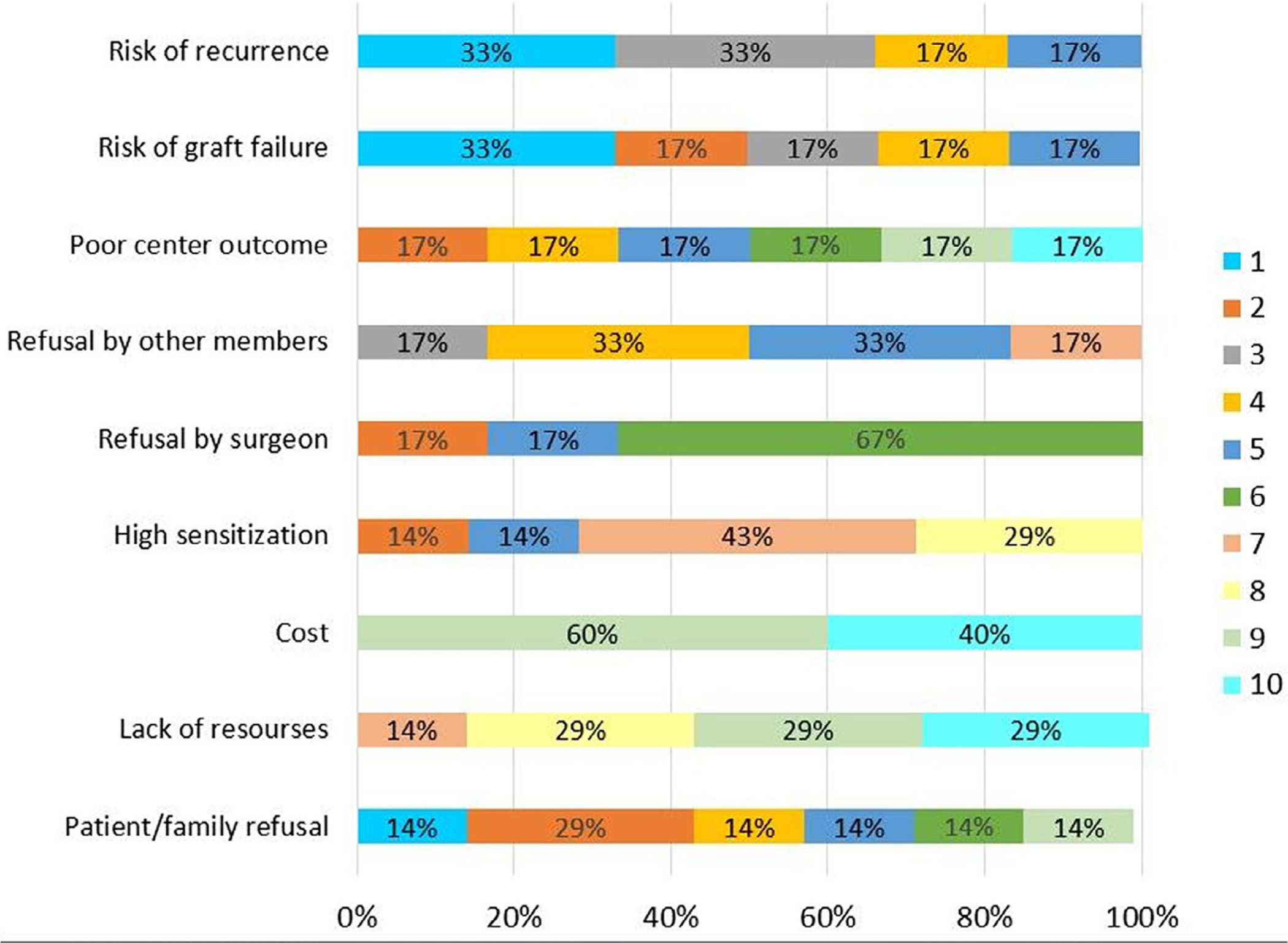
Nephrologist survey responses – factors affecting decision to re-transplant. Numbers reflect priority of response, with the most significant factor assigned the number 1, and least important factor assigned the number 10. Percentages on the horizontal bars reflect the proportion of responses to the specific question
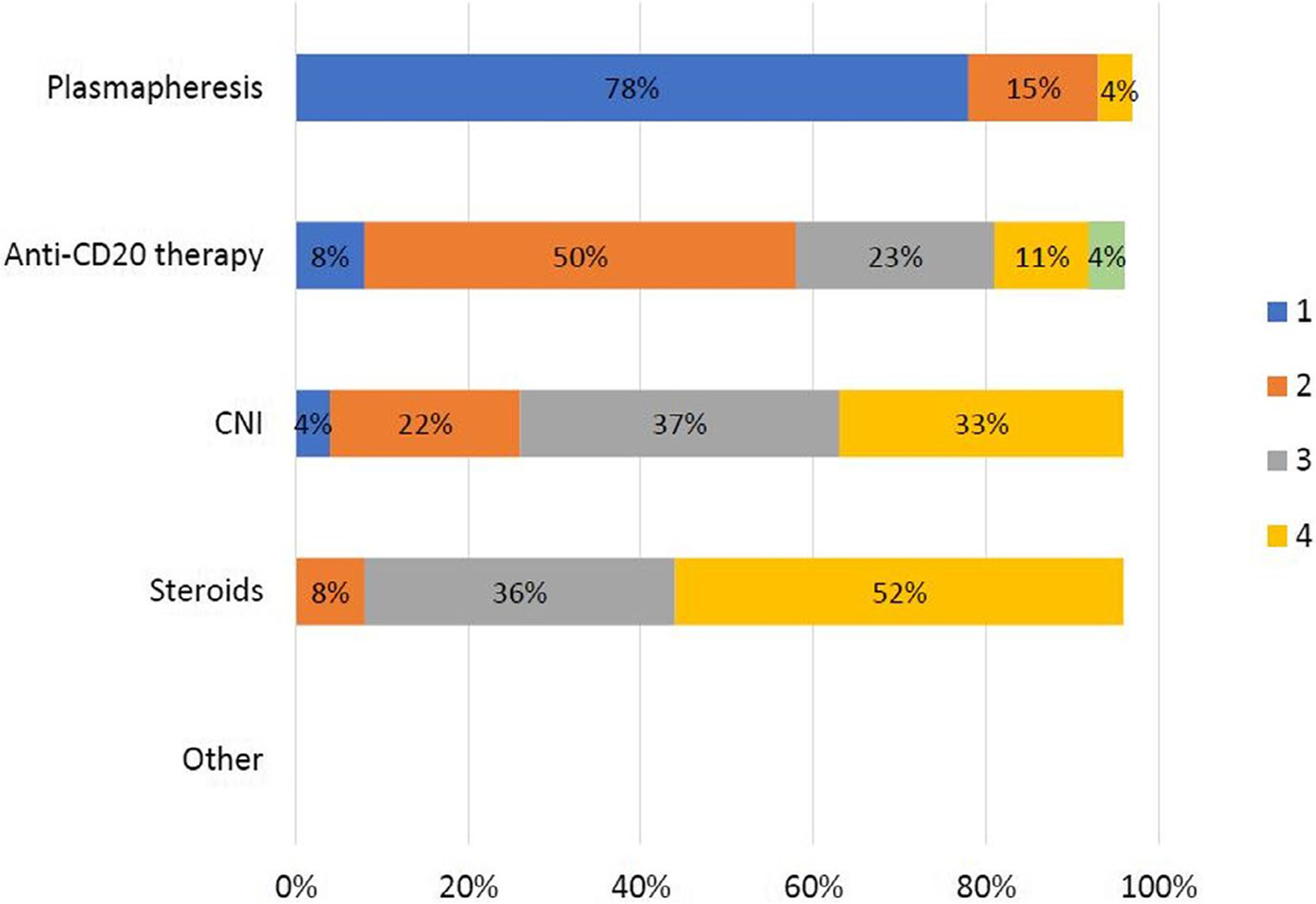
Nephrologist survey responses – most effective therapies. Numbers reflect priority of response, with the therapy viewed as most effective assigned the number 1, and the least effective assigned the number 4 (the number 5 was for other – there were no responses). Percentages on the horizontal bars reflect the proportion of responses to the specific question
4 |. DISCUSSION
We are encouraged by the unique results of this study which show that failure of a first transplant due to recurrent nephrotic syndrome in pediatric kidney transplant recipients with FSGS is not associated with worse outcome after re-transplantation, contrary to many previous reports. In fact, our study found that second transplants are associated with better graft survival compared to the primary transplants, in spite of high recurrence rates, likely because recurrence after the second transplant in our patients was less severe compared to that in the first transplant. The earlier diagnosis of recurrence in the second transplants does not seem to be associated with worse outcome and is likely due to increased vigilance in evaluating for recurrence of nephrotic syndrome in the second transplant. Although the numbers are small and the design is retrospective, the study is unique in that the populations in the first and second transplants were identical, which allows for excellent case-control matching for sex, race, ethnicity, and socioeconomic status. Most studies focus on the incidence of recurrence after re-transplantation, without stratifying for severity of recurrence. In our study, we examined the severity of recurrence. Even though the definition of severity is somewhat arbitrary, it was defined a priori based mostly on the practical experience of the authors. Our study confirms the previously reported high rates of recurrence after re-transplantation7; however, the recurrence was less severe after the second transplant, with significantly lower proteinuria, high rate of complete remission, and improved graft survival. Although our sample size did not allow us to examine the relationship between severity of recurrence and graft failure, proteinuria is a known risk for progression of renal disease, and lower proteinuria is likely associated with improved graft survival. Additionally, many of the re-transplanted children with recurrence achieved remission and were no longer nephrotic and no longer had proteinuria. The small number of patients also limits our ability to analyze the effect of therapies such as plasmapheresis, choice of CNI, and anti-CD20 therapy on outcomes.
Plasmapheresis in our study was used more frequently in re-transplants, with several sessions performed pre-transplant in a few patients. Plasmapheresis has become an essential component of therapy for recurrent nephrosis in transplanted patients with FSGS.10–13 However, the timing and length of therapy vary greatly and are not well established. The role of prophylactic or pre-emptive plasmapheresis is still in question. Verghese et al. reported no difference in the incidence or timing of recurrent nephrosis or graft survival in 57 patients who received pre-emptive plasmapheresis including pre- and post-transplant.14 However, post-transplant plasmapheresis therapy was limited per protocol to only 5 sessions of plasmapheresis, including in those with recurrence, and only 2 patients with recurrence received rituximab therapy. Several studies including a meta-analysis showed plasmapheresis to be associated with high rates of complete or partial remission post-transplantation,15–17 with early initiation of therapy associated with higher likelihood of achieving remission. A recent consensus statement from the CERTAIN (Cooperative European Pediatric Renal Transplant Initiative) study group recommends the early use of plasmapheresis or immunoadsorption in the management of kidney transplant patients with FSGS and a history of previous graft failure due to recurrent nephrotic syndrome.18 In our study, there were more patients receiving pre-emptive plasmapheresis therapy before the second transplant compared to the first transplant (38.9% vs. 5.3%, p = .03) with the number of plasmapheresis sessions ranging from 1 to 10 sessions. The hypothesis behind pre-transplant plasmapheresis is that it may prevent or decrease the severity of recurrence and improve proteinuria and graft survival by removing circulating protein permeability factors associated with recurrence.19–22 The recent NAPRTCS report shows improved graft survival in a recent cohort of transplanted patients with FSGS; however, the causes for improvement were not determined, but were likely multifactorial.1
In our study, the frequency of anti-CD20 therapy utilization was not different between primary and re-transplants. Several studies examining the efficacy of anti-CD20 therapy suggest a positive effect in terms of inducing remission and preventing recurrence although it was most frequently used in conjunction with plasmapheresis.23–25 The mechanisms of action of anti-CD20 therapy in treating glomerular disease including FSGS and recurrent post-transplant nephrosis are not fully understood. Modulating B- and T-cell interaction has been proposed, and more recently, rituximab has been shown to act in a manner that affects the podocyte cytoskeleton and function by direct modulation of sphingomyelinase and sphingomyelin-phosphodiesterase-acid-like-3b (SMPDL-3b).24,26
The effect of donor type (living vs deceased) could not be analyzed in this study as 50% of donors were living donors in both primary and second transplants. Although a survival benefit from living donation could not be assessed in this study,1,27 living donation is associated with less delayed graft function. Delayed graft function often delays the introduction and optimization of CNI use, which is felt to be critical in managing nephrosis post-transplant. In addition, delayed graft function has been shown to be independently associated with greater risk of graft failure in transplanted FSGS patients.1 Therefore, living donation may be beneficial by lowering the risk of delayed graft function and by allowing patients the ability to receive pre-transplant therapies such as plasmapheresis and anti-CD20 therapies before recurrent disease is well established and potentially modifying the risk and severity of recurrence post-transplant. Conversely, graft failure after living donation can be an even more traumatic and emotional experience, both for the donor and for the recipient. Although living donation as an independent factor has not been shown to be associated with lower recurrence or survival benefit,1 it would be interesting to evaluate living donor effect in the context of pre-transplant plasmapheresis.
Graft failure due to recurrent FSGS in transplanted patients with FSGS is often a traumatic experience for the patient, family, and the medical team and comes at a high cost, both financially and emotionally. Delayed graft function, edema, infectious complications, frequent procedures, prolonged or frequent hospitalizations, and frequent clinic visits are the hallmarks of this disease, which also has significant psychosocial implications.28–30 The decision to re-transplant after graft failure due to recurrent nephrotic syndrome is often a difficult one, both for the physicians and for the patients and their families. Reluctance to re-transplant is often driven by fear of repeating the traumatic and emotionally charged experience and concern about another recurrence of nephrotic syndrome and subsequent graft loss. This may be justifiable on the grounds of having such past experience resulting in post-traumatic stress and even depression31 and also on the grounds of having data that suggest higher rates of recurrence and graft loss with re-transplantation.7
In our survey of nephrologist members of the Pediatric Nephrology Research Consortium, the majority indicated they would re-transplant their patients, with 44% of surveyed nephrologists requiring a waiting period of 1 year before re-transplantation. Waiting 1 year between graft failure and re-transplantation may be reasonable considering the increased risk of early acute rejection, humoral rejection, graft failure, and overall mortality as well as death with a functioning graft in patients re-transplanted within 6 months of graft failure.32 However, the time lag between graft failure and re-transplantation in our cohort is much longer (median of 37 months) than what surveyed nephrologists reported as a preference and longer than previously reported median waiting time of 1.6 years (IQR 0.5, 3.6) for re-transplanted children in the United States.33 In their report, Van Arendonk et. al. did not find a difference in rates of re-transplantation or waiting times between FSGS patients and other patients. Reasons for the longer waiting times to re-transplantation in our cohort were not examined, but are likely multifactorial. These factors may include morbidities related to nephrotic syndrome, high sensitization status resulting in longer waiting times, changes in waiting times due to changes in the allocation system, psychosocial and socioeconomic factors,33 hesitancy to re-transplant older patients due to reported higher graft failure rates,34,35 and to a lesser extent, center-related factors such as lack of consensus among transplant center members, and center outcome and cost considerations, as reported by surveyed nephrologists in this study. The fact that only 22% of centers have an established protocol for re-transplanting such patients, and that the majority of nephrologists would consider re-transplantation on an individual basis, reflects the difficulty in the decision-making process for this challenging disease and unique patient population.
The small sample size and the retrospective study design limit the generalizability of the results of our study. Moreover, there were some missing data elements, especially with the first transplant as many of these transplants were performed at a different center, and before the wide utilization of electronic medical records, which limits the ability to extract data.
5 |. CONCLUSIONS
Our study provides additional information and outcome data, which should help better inform the pediatric nephrologists and transplant centers when considering re-transplantation after recurrence of nephrotic syndrome in kidney transplant recipients with FSGS. Knowing that re-transplants are not destined to fail and that severe recurrence is less likely in a second transplant may encourage more frequent and more timely re-transplantation in this unique population and improve patient overall survival and quality of life. We believe that a prospective study with carefully designed treatment protocols based on the findings of this study and existing data is warranted to guide management and subsequently improve outcomes in this special and very challenging patient population.
Abbreviations:
| CERTAIN | cooperative European pediatric renal transplant initiative |
| CNI | calcineurin inhibitor |
| eGFR | estimated Glomerular Filtration Rate |
| FSGS | focal and segmental glomerulosclerosis |
| MWPNC | Midwest pediatric nephrology consortium |
| NAPRTCS | north American pediatric renal trials and collaborative studies |
| PNRC | pediatric nephrology research consortium |
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.1111/petr.14085
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8968923
Citations & impact
This article has not been cited yet.
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/109222821
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
European Society of Pediatric Nephrology survey on current practice regarding recurrent focal segmental glomerulosclerosis after pediatric kidney transplantation.
Pediatr Transplant, 23(3):e13385, 01 Mar 2019
Cited by: 6 articles | PMID: 30825259
Mortality and Allograft Loss Trends Among US Pediatric Kidney Transplant Recipients With and Without Focal Segmental Glomerulosclerosis.
Am J Kidney Dis, 71(3):392-398, 23 Dec 2017
Cited by: 4 articles | PMID: 29277509 | PMCID: PMC5828873
Management and long-term outcome of recurrent idiopathic FSGS in pediatric kidney transplant recipients.
Sci Rep, 14(1):25493, 26 Oct 2024
Cited by: 0 articles | PMID: 39461970 | PMCID: PMC11513095
Incidence, risk factors, and outcomes of recurrent focal segmental glomerulosclerosis in pediatric kidney transplant recipients: A systematic review and meta-analysis.
Clin Transplant, 37(11):e15119, 19 Sep 2023
Cited by: 1 article | PMID: 37725070
Review




