Abstract
Importance
Cerebrospinal fluid phosphorylated tau (p-tau) 181, p-tau217, and p-tau231 are associated with neuropathological outcomes, but a comparison of these p-tau isoforms in blood samples is needed.Objective
To conduct a head-to-head comparison of plasma p-tau181 and p-tau231 measured on the single-molecule array (Simoa) platform and p-tau181 and p-tau217 measured on the Meso Scale Discovery (MSD) platform on amyloid and tau positron emission tomography (PET) measures, neurodegeneration, vascular pathology, and cognitive outcomes.Design, setting, and participants
This study included data from the Mayo Clinic Study on Aging collected from March 1, 2015, to September 30, 2017, and analyzed between December 15, 2020, and May 17, 2021. Associations between the 4 plasma p-tau measures and dichotomous amyloid PET, metaregion of interest tau PET, and entorhinal cortex tau PET were analyzed using logistic regression models; the predictive accuracy was summarized using area under the receiver operating characteristic curve (AUROC) statistic. Of 1329 participants without dementia and with p-tau181 and p-tau217 on MSD, 200 participants with plasma p-tau181 and p-tau231 on Simoa and magnetic resonance imaging and amyloid and tau PET data at the same study visit were eligible.Main outcomes and measures
Primary outcomes included amyloid (greater than 1.48 standardized uptake value ratio) and tau PET, white matter hyperintensities, white matter microstructural integrity (fractional anisotropy genu of corpus callosum and hippocampal cingulum bundle), and cognition.Results
Of 200 included participants, 101 (50.5%) were male, and the median (interquartile range [IQR]) age was 79.5 (71.1-84.1) years. A total of 177 were cognitively unimpaired (CU) and 23 had mild cognitive impairment. Compared with amyloid-negative CU participants, among amyloid-positive CU participants, the median (IQR) Simoa p-tau181 measure was 49% higher (2.58 [2.00-3.72] vs 1.73 [1.45-2.13] pg/mL), MSD p-tau181 measure was 53% higher (1.22 [0.91-1.56] vs 0.80 [0.66-0.97] pg/mL), MSD p-tau217 measure was 77% higher (0.23 [0.17-0.34] vs 0.13 [0.09-0.18] pg/mL), and Simoa p-tau231 measure was 49% higher (20.21 [15.60-25.41] vs 14.27 [11.27-18.10] pg/mL). There were no differences between the p-tau species for amyloid PET and tau PET metaregions of interest. However, among CU participants, both MSD p-tau181 and MSD p-tau217 more accurately predicted abnormal entorhinal cortex tau PET than Simoa p-tau181 (MSD p-tau181: AUROC, 0.80 vs 0.70; P = .046; MSD p-tau217: AUROC, 0.81 vs 0.70; P = .04). MSD p-tau181 and p-tau217 and Simoa p-tau181, but not p-tau231, were associated with greater white matter hyperintensity volume and lower white matter microstructural integrity.Conclusions and relevance
In this largely presymptomatic population, these results suggest subtle differences across plasma p-tau species and platforms for the prediction of amyloid and tau PET and magnetic resonance imaging measures of cerebrovascular and Alzheimer-related pathology.Free full text

Comparison of Plasma Phosphorylated Tau Species With Amyloid and Tau Positron Emission Tomography, Neurodegeneration, Vascular Pathology, and Cognitive Outcomes
Key Points
Question
How do plasma phosphorylated tau (p-tau) 181 and p-tau231 as measured on the single-molecule array (Simoa) platform and p-tau181 and p-tau217 as measured on the Meso Scale Discovery (MSD) platform compare with regards to amyloid and tau positron emission tomography and magnetic resonance imaging measures as well as cognition?
Findings
In this cross-sectional study including 200 participants, MSD p-tau181 and p-tau217 predicted entorhinal cortex tau positron emission tomography measures significantly better than Simoa p-tau181 among cognitively unimpaired participants. MSD p-tau181 and p-tau217 and Simoa p-tau181, but not p-tau231, were associated with magnetic resonance imaging measures of cerebrovascular pathology, and MSD p-tau181 and p-tau217 were most strongly associated with magnetic resonance imaging measures of Alzheimer disease pathology.
Meaning
Results from this study suggest subtle differences across plasma p-tau species and platforms.
Abstract
Importance
Cerebrospinal fluid phosphorylated tau (p-tau) 181, p-tau217, and p-tau231 are associated with neuropathological outcomes, but a comparison of these p-tau isoforms in blood samples is needed.
Objective
To conduct a head-to-head comparison of plasma p-tau181 and p-tau231 measured on the single-molecule array (Simoa) platform and p-tau181 and p-tau217 measured on the Meso Scale Discovery (MSD) platform on amyloid and tau positron emission tomography (PET) measures, neurodegeneration, vascular pathology, and cognitive outcomes.
Design, Setting, and Participants
This study included data from the Mayo Clinic Study on Aging collected from March 1, 2015, to September 30, 2017, and analyzed between December 15, 2020, and May 17, 2021. Associations between the 4 plasma p-tau measures and dichotomous amyloid PET, metaregion of interest tau PET, and entorhinal cortex tau PET were analyzed using logistic regression models; the predictive accuracy was summarized using area under the receiver operating characteristic curve (AUROC) statistic. Of 1329 participants without dementia and with p-tau181 and p-tau217 on MSD, 200 participants with plasma p-tau181 and p-tau231 on Simoa and magnetic resonance imaging and amyloid and tau PET data at the same study visit were eligible.
Main Outcomes And Measures
Primary outcomes included amyloid (greater than 1.48 standardized uptake value ratio) and tau PET, white matter hyperintensities, white matter microstructural integrity (fractional anisotropy genu of corpus callosum and hippocampal cingulum bundle), and cognition.
Results
Of 200 included participants, 101 (50.5%) were male, and the median (interquartile range [IQR]) age was 79.5 (71.1-84.1) years. A total of 177 were cognitively unimpaired (CU) and 23 had mild cognitive impairment. Compared with amyloid-negative CU participants, among amyloid-positive CU participants, the median (IQR) Simoa p-tau181 measure was 49% higher (2.58 [2.00-3.72] vs 1.73 [1.45-2.13] pg/mL), MSD p-tau181 measure was 53% higher (1.22 [0.91-1.56] vs 0.80 [0.66-0.97] pg/mL), MSD p-tau217 measure was 77% higher (0.23 [0.17-0.34] vs 0.13 [0.09-0.18] pg/mL), and Simoa p-tau231 measure was 49% higher (20.21 [15.60-25.41] vs 14.27 [11.27-18.10] pg/mL). There were no differences between the p-tau species for amyloid PET and tau PET metaregions of interest. However, among CU participants, both MSD p-tau181 and MSD p-tau217 more accurately predicted abnormal entorhinal cortex tau PET than Simoa p-tau181 (MSD p-tau181: AUROC, 0.80 vs 0.70; P =
= .046; MSD p-tau217: AUROC, 0.81 vs 0.70; P
.046; MSD p-tau217: AUROC, 0.81 vs 0.70; P =
= .04). MSD p-tau181 and p-tau217 and Simoa p-tau181, but not p-tau231, were associated with greater white matter hyperintensity volume and lower white matter microstructural integrity.
.04). MSD p-tau181 and p-tau217 and Simoa p-tau181, but not p-tau231, were associated with greater white matter hyperintensity volume and lower white matter microstructural integrity.
Conclusions and Relevance
In this largely presymptomatic population, these results suggest subtle differences across plasma p-tau species and platforms for the prediction of amyloid and tau PET and magnetic resonance imaging measures of cerebrovascular and Alzheimer-related pathology.
Introduction
Neurofibrillary tangles, composed of intraneuronal hyperphosphorylated tau, are one of the hallmark pathological characteristics of Alzheimer disease (AD). Immunoassays to measure cerebrospinal fluid (CSF) tau phosphorylated at threonine 181 (p-tau181) have been developed as a biomarker of neurofibrillary tangles to support the clinical diagnosis of AD dementia and as a prognostic marker to predict progression from cognitively unimpaired (CU) to mild cognitive impairment (MCI).1,2,3 However, there are numerous tau phosphorylation sites that show promise as CSF AD biomarkers, including p-tau181, p-tau217, and p-tau231.4,5,6,7,8 Some of these p-tau species may be differentially enriched in the CSF compared with the brain or blood, but species with the strongest plasma-CSF correlations have been shown to be driven by amyloid and are more indicative of AD pathology.9,10
Over the past 2 years, several studies have demonstrated that plasma p-tau181, p-tau217, and p-tau231 are indicators of both amyloid and tau pathology across the clinical AD spectrum and can differentiate AD dementia from other neurodegenerative diseases.10,11,12,13,14,15,16,17,18,19,20,21,22,23 Some studies suggest that plasma p-tau217 has better discriminative accuracy for AD dementia than p-tau181.10,16 While p-tau181, p-tau217, and p-tau231 all correlate tightly in CSF,7 a comparison of these p-tau species or platforms on which they are measured, specifically Meso Scale Discovery (MSD; Meso Scale Diagnostics) and single-molecule array (Simoa; Quanterix Corporation), in blood samples is needed. Therefore, we conducted a head-to-head comparison of plasma p-tau181 and p-tau231, measured on the Simoa platform, and p-tau181 and p-tau217, measured on the MSD platform, among participants without dementia enrolled in the Mayo Clinic Study on Aging (MCSA). Outcomes included amyloid positron emission tomography (PET), tau PET, magnetic resonance imaging (MRI) measures of neurodegeneration and cerebrovascular pathology, and global and domain-specific cognition.
Methods
Study Participants
The MCSA is a prospective population-based study examining the epidemiology of MCI among residents of Olmsted County, Minnesota.24 In 2004, Olmsted County residents between ages of 70 and 89 years were enumerated using the Rochester Epidemiology Project medical records linkage system in an age-stratified and sex-stratified random sampling design.25 The study was extended to include those 50 years and older in 2012. MCSA visits include an interview by a study coordinator, physician examination, and neuropsychological testing. Clinical diagnoses were determined by a consensus committee. Additional details can be found in the eMethods in the Supplement.
The present analysis included the 200 participants who had concurrent measures of plasma p-tau181 and p-tau231 on Simoa, plasma p-tau181 and p-tau217 on MSD, MRI, and both amyloid and tau PET; 164 also had measures of plasma p-tau231. The study was approved by the Mayo Clinic and Olmsted Medical Center institutional review boards. Written informed consent was obtained from all participants. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Plasma P-tau Assays
Blood was collected in clinic after an overnight fast, centrifuged, aliquoted, and stored at −80 °C. Plasma p-tau181 was measured on the HD-X Analyzer (Quanterix Corporation) using the Simoa pTau-181 Advantage kit version 2 (Quanterix Corporation) per manufacturer’s instructions.13 Plasma p-tau231 was measured using an in-house Simoa method.23 Additionally, both p-tau181 and p-tau217 were measured on the MSD platform using proprietary assays developed by Lilly Research Laboratories.26 Further description of each assay is provided in the eMethods in the Supplement.
Amyloid and Tau PET Imaging
β-Amyloid Pittsburgh Compound B PET and [18F]flortaucipir PET images were acquired using a PET/computed tomography scanner (GE 690 XT [GE Healthcare], GE Discovery MI [GE Healthcare], and Siemens Vision 600 [Siemens Healthineers]) operating in 3-dimensional mode.27,28,29 Quantitative image analysis for Pittsburgh Compound B and [18F]flortaucipir was done using an in-house fully automated image processing pipeline.30 Additional details are described in the eMethods in the Supplement. We dichotomized participants as amyloid positive based on a cutoff value of more than 1.48 standard uptake value ratio (SUVR).31 Participants were dichotomized as tau positive based on a tau PET temporal metaregion of interest (ROI) greater than 1.29 SUVR or tau PET entorhinal cortex (ERC) greater than 1.27 SUVR.32
Structural MRI Outcomes
Structural MRI was acquired using standardized magnetization-prepared rapid gradient echo (MPRAGE) sequences on 3-Tesla GE scanners (GE Medical Systems). FreeSurfer version 5.3 was run on the MPRAGE scans; a temporal meta-ROI using a cortical thickness was computed.31 Diffusion tensor imaging sequences were processed and analyzed for fractional anisotropy of the genu of the corpus callosum (FA-Genu) and hippocampal cingulum bundle (FA-HCB).33,34,35 White matter hyperintensities (WMH) on standard 2-dimensional fluid-attenuated inversion recovery MRI were segmented and edited using a semiautomated method.36
Statistical Analysis
There were 16 MSD p-tau217 values (8%) below the detection limit, including 14 from CU participants and 2 from participants with MCI (13 amyloid negative and 3 amyloid positive). These values were set to the lowest detectable limit. None of the other p-tau measures in this analysis had levels below the detection limit. Data distributions were compared across clinical diagnosis and abnormal amyloid PET using χ2 and Fisher exact tests for categorical data and Kruskal-Wallis tests for continuous data. Spearman correlations and scatterplots between p-tau measures were generated. Bootstrap techniques were used to formally compare correlation measures. Boxplots were generated comparing p-tau measures by amyloid and tau status. A Kruskal-Wallis test was used to determine significance. Pairwise comparisons between participants who were amyloid and tau negative, tau positive, amyloid positive, and amyloid and tau positive were performed using Wilcoxon rank-sum tests. Associations between the 4 plasma p-tau measures and dichotomous amyloid PET, meta-ROI tau PET, and ERC tau PET were analyzed using logistic regression models. The predictive accuracy of each model was summarized using the area under the receiver operating characteristic curve (AUROC) statistic. Baseline models including age; age and sex; and age, sex, and APOE were used for comparison with models with the individual biomarkers. We ran 4 models for each biomarker: biomarker alone; biomarker and age; biomarker, age, and sex; and biomarker, age, sex, and APOE. Finally, we also ran models on all patients and then subset by clinical diagnosis to CU only and MCI only.
Associations between continuous z score p-tau biomarkers and neuroimaging and cognitive z scores were analyzed using unadjusted and multivariable linear regression models. The z scores for each p-tau biomarker were calculated using just the amyloid-negative distribution. The continuous neuroimaging and cognitive outcomes included cortical thickness, WMH volume, FA-Genu, FA-HCB, amyloid PET, global z score, memory z score, attention z score, language z score, and visual spatial z score. Covariables included baseline age, sex, APOE, education (in years), body mass index, and chronic kidney disease. The ability of the p-tau measures to predict the variability in each outcome was measured using the adjusted R2. The adjusted R2 from a model containing just the adjustment variables without the p-tau measure was included for comparison.
In sensitivity analyses, we reran the previous models and restricted the analyses to the 164 participants with plasma p-tau231. All analyses were completed using SAS version 9.4 (SAS Institute) and R version 3.6.2 (The R Foundation). Direct comparisons between AUROC measures were performed using the concordance function from the survival package in R.37 A 2-sided P value less than .05 was considered statistically significant.
Results
Participant Characteristics
The median (interquartile range [IQR]) age of the 200 participants was 79.5 (71.1-84.1) years, 101 (50.5%) were male, 60 (30.0%) had an APOE ε4 allele, 101 (50.5%) were amyloid positive, and 44 (22.0%) were tau positive based on the tau PET meta-ROI (Table 1). There were 177 CU participants and 23 participants with MCI. Based on the tau PET ERC ROI, 36 (18.0%) were tau positive, of which 29 (81%) were amyloid positive. Of the 200 participants, 164 (82.0%) had a concurrent measure of p-tau231. There were no differences between those with vs without p-tau231 measurements with regards to demographic characteristics, amyloid or tau PET SUVR, or plasma p-tau181 and p-tau217 levels.
Table 1.
| Characteristic | Median (IQR) | P value | ||||
|---|---|---|---|---|---|---|
Total (N = = 200) 200) | Amyloid-negative CU (n = = 89) 89) | Amyloid-positive CU (n = = 88) 88) | Amyloid-negative MCI (n = = 10) 10) | Amyloid-positive MCI (n = = 13) 13) | ||
| Demographic characteristics | ||||||
| Age, y | 79.5 (71.1-84.1) | 76.4 (69.1-81.5) | 81.8 (76.2-84.8) | 71.6 (69.2-80.0) | 84.8 (82.0-88.4) | <.001 |
| Male, No. (%) | 101 (50.5) | 50 (56.2) | 39 (44.3) | 7 (70.0) | 5 (38.5) | .19 |
| Education, y | 14 (12-16) | 16 (12-16) | 13.5 (12-16) | 12.5 (12-15) | 14 (12-15) | .02 |
| Body mass indexa | 27.0 (24.2-29.5) | 26.4 (24.2-28.9) | 27.6 (24.7-30.3) | 28.5 (24.2-29.2) | 22.6 (21.4-27.2) | .03 |
Presence of APOE  4 allele, No. (%) 4 allele, No. (%) | 60 (30.0) | 13 (14.6) | 40 (45.5) | 2 (20.0) | 5 (38.5) | <.001 |
| Race, No. (%) | ||||||
| White | 198 (99.0) | 87 (97.8) | 88 (100) | 10 (100) | 13 (100) | .87 |
| Multiracial | 2 (1.0) | 2 (2.2) | 0 | 0 | 0 | |
| Plasma measures, pg/mL | ||||||
| Simoa p-tau181 | 2.13 (1.68-3.01) | 1.73 (1.45-2.13) | 2.58 (2.00-3.72) | 2.06 (1.10-2.29) | 3.15 (2.34-4.83) | <.001 |
| MSD p-tau181 | 0.94 (0.75-1.32) | 0.80 (0.66-0.97) | 1.22 (0.91-1.56) | 0.87 (0.63-0.94) | 1.38 (0.94-2.02) | <.001 |
| MSD p-tau217 | 0.17 (0.12-0.25) | 0.13 (0.09-0.18) | 0.23 (0.17-0.34) | 0.15 (0.13-0.16) | 0.25 (0.22-0.43) | <.001 |
| Simoa p-tau231b | 16.51 (13.52-23.05) | 14.27 (11.27-18.10) | 20.21 (15.60-25.41) | 15.06 (14.46-18.07) | 25.05 (14.96-37.22) | <.001 |
| Neuroimaging | ||||||
| Amyloid PET SUVR | 1.46 (1.36-1.76) | 1.36 (1.32-1.42) | 1.77 (1.54-2.20) | 1.36 (1.28-1.39) | 2.05 (1.69-2.60) | <.001 |
| Meta-ROI tau PET SUVR | 1.21 (1.14-1.27) | 1.20 (1.12-1.25) | 1.23 (1.17-1.30) | 1.15 (1.11-1.21) | 1.29 (1.24-1.37) | <.001 |
| ERC tau PET SUVR | 1.11 (1.04-1.20) | 1.08 (1.00-1.15) | 1.15 (1.08-2.28) | 1.05 (1.01-1.07) | 1.17 (1.10-1.35) | <.001 |
| Temporal thickness, mm | 2.66 (2.56-2.75) | 2.69 (2.60-2.77) | 2.63 (2.52-2.71) | 2.64 (2.56-2.67) | 2.56 (2.46-2.65) | <.001 |
| WMH volume, cm3 | 13.62 (7.62-24.17) | 11.88 (6.59-15.99) | 18.15 (9.44-33.64) | 14.60 (7.52-33.19) | 34.97 (20.44-70.54) | <.001 |
| Presence of ≥2 microbleeds, No. (%) | 22 (11.1) | 2 (2.3) | 15 (17.2) | 1 (10.0) | 4 (30.8) | .002 |
| DTI: FA-Genu | 0.59 (0.55-0.61) | 0.59 (0.57-0.62) | 0.58 (0.54-0.60) | 0.58 (0.55-0.63) | 0.56 (0.54-0.61) | .04 |
| DTI: FA-HCB | 0.45 (0.42-0.47) | 0.46 (0.43-0.48) | 0.45 (0.42-0.47) | 0.46 (0.44-0.47) | 0.44 (0.40-0.46) | .02 |
Abbreviations: CU, cognitively unimpaired; DTI, diffusion tensor imaging; ERC, entorhinal cortex; FA, fractional anisotropy; Genu, genu of corpus callosum; HCB, hippocampal cingulum bundle; IQR, interquartile range; MCI, mild cognitive impairment; MSD, Meso Scale Discovery; ROI, region of interest; Simoa, single-molecule array; SUVR, standardized uptake value ratio; WMH, white matter hyperintensities.
Correlations of Plasma P-tau Species With Amyloid and Tau PET
Spearman correlations between each of the plasma p-tau measures are shown in eFigure 1 in the Supplement. Correlations between p-tau species measured on the same platform were significantly higher (MSD p-tau181 vs p-tau217: ρ =
= 0.86; P
0.86; P <
< .001; Simoa p-tau181 vs p-tau231: ρ
.001; Simoa p-tau181 vs p-tau231: ρ =
= 0.77; P
0.77; P <
< .001). Correlations across platforms were lower, with MSD p-tau217 and Simoa p-tau231 being lowest (ρ
.001). Correlations across platforms were lower, with MSD p-tau217 and Simoa p-tau231 being lowest (ρ =
= 0.51; P
0.51; P <
< .001). This correlation was significantly lower than all correlations, with the exception of the correlation between MSD p-tau217 and Simoa p-tau181 (ρ
.001). This correlation was significantly lower than all correlations, with the exception of the correlation between MSD p-tau217 and Simoa p-tau181 (ρ =
= 0.52; P
0.52; P <
< .001). Spearman correlations between each of the plasma p-tau measures and amyloid and tau PET are shown in Figure 1. MSD p-tau217 (ρ
.001). Spearman correlations between each of the plasma p-tau measures and amyloid and tau PET are shown in Figure 1. MSD p-tau217 (ρ =
= 0.58; P
0.58; P <
< .001) had the highest correlation and p-tau231 (ρ
.001) had the highest correlation and p-tau231 (ρ =
= 0.39; P
0.39; P <
< .001) the lowest with amyloid PET. In direct comparisons, the correlation between Simoa p-tau 231 and amyloid PET (ρ
.001) the lowest with amyloid PET. In direct comparisons, the correlation between Simoa p-tau 231 and amyloid PET (ρ =
= 0.39) was significantly lower than the correlations between MSD p-tau181 and amyloid PET (ρ
0.39) was significantly lower than the correlations between MSD p-tau181 and amyloid PET (ρ =
= 0.54) and MSD p-tau 217 and amyloid PET (ρ
0.54) and MSD p-tau 217 and amyloid PET (ρ =
= 0.58), while all other correlation comparisons did not significantly differ. Correlations between each of the plasma p-tau measures and tau PET meta-ROI were insignificantly different, with the highest for Simoa p-tau181 (ρ
0.58), while all other correlation comparisons did not significantly differ. Correlations between each of the plasma p-tau measures and tau PET meta-ROI were insignificantly different, with the highest for Simoa p-tau181 (ρ =
= 0.28; P
0.28; P <
< .001) and the lowest for p-tau231 (ρ
.001) and the lowest for p-tau231 (ρ =
= 0.21; P
0.21; P =
= .008). Correlations between the plasma p-tau measures and tau PET ERC were higher than with the meta-ROI; highest for MSD p-tau217 (ρ
.008). Correlations between the plasma p-tau measures and tau PET ERC were higher than with the meta-ROI; highest for MSD p-tau217 (ρ =
= 0.36; P
0.36; P <
< .001) and lowest for p-tau231 (ρ
.001) and lowest for p-tau231 (ρ =
= 0.24; P
0.24; P =
= .002). However, in direct comparisons, none of the correlations between ERC tau PET and p-tau biomarkers were significantly different from one another. All measures correlated with age (Simoa p-tau181: ρ
.002). However, in direct comparisons, none of the correlations between ERC tau PET and p-tau biomarkers were significantly different from one another. All measures correlated with age (Simoa p-tau181: ρ =
= 0.53; P
0.53; P <
< .001; MSD p-tau181: ρ
.001; MSD p-tau181: ρ =
= 0.48; P
0.48; P <
< .001; MSD p-tau217: ρ
.001; MSD p-tau217: ρ =
= 0.48; P
0.48; P <
< .001; Simoa p-tau231: ρ
.001; Simoa p-tau231: ρ =
= 0.46; P
0.46; P <
< .001). None of the p-tau levels differed by sex.
.001). None of the p-tau levels differed by sex.
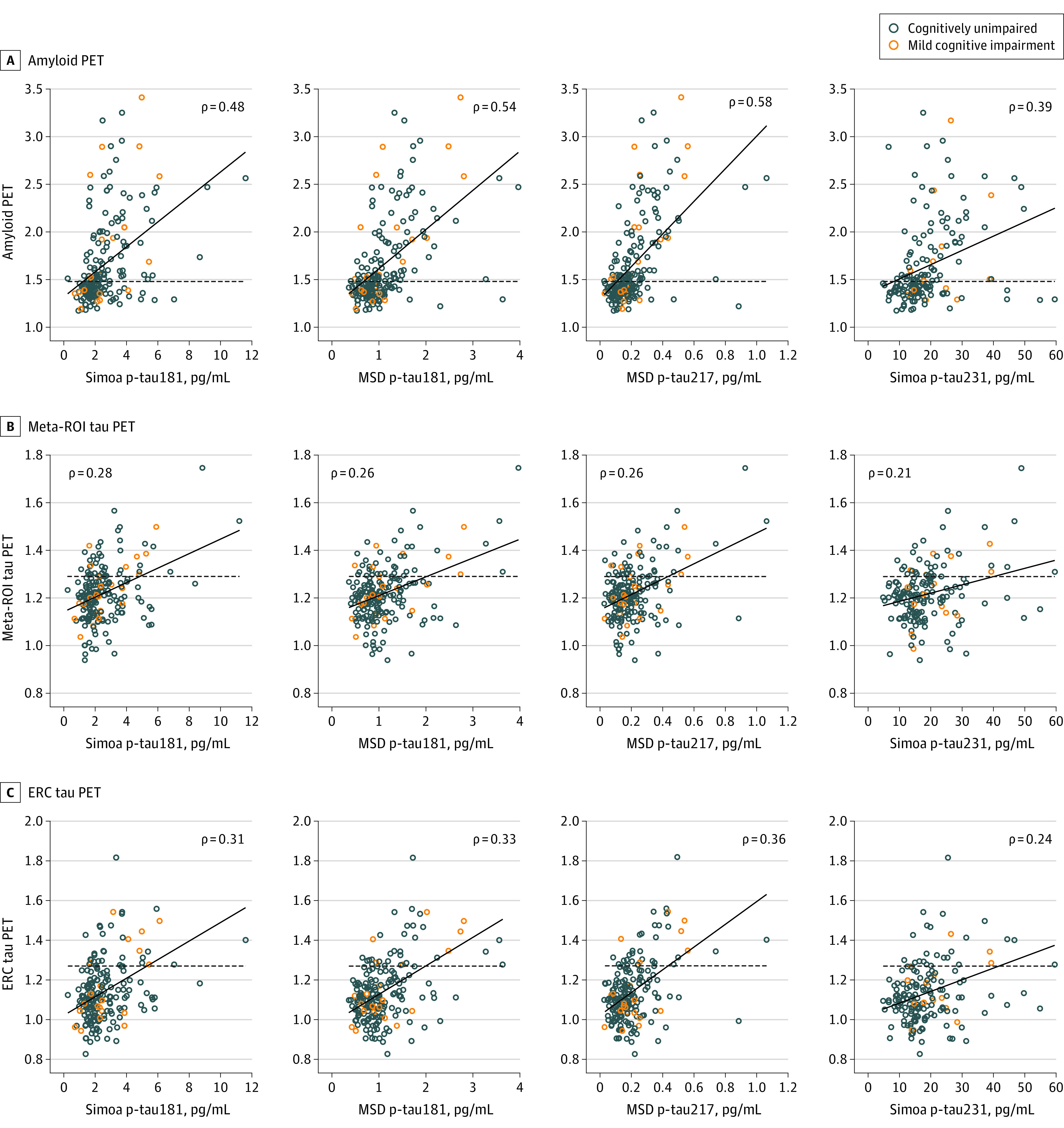
Solid lines indicate the correlation, and dashed lines indicate cut points for determining abnormal neuroimaging results. ERC indicates entorhinal cortex; MSD, Meso Scale Discovery; ROI, region of interest; Simoa, single-molecule array.
Comparison of P-tau Isoforms by Clinical Diagnosis and Amyloid and Tau PET Status
Differences in the p-tau measures by clinical diagnosis and amyloid PET status are shown in Table 1 and eFigure 2 in the Supplement. Median levels of all plasma p-tau measures were higher for amyloid-positive participants compared with amyloid-negative participants. Compared with amyloid-negative CU participants, among amyloid-positive CU participants, the median (IQR) Simoa p-tau181 measure was 49% higher (2.58 [2.00-3.72] vs 1.73 [1.45-2.13] pg/mL), MSD p-tau181 measure was 53% higher (1.22 [0.91-1.56] vs 0.80 [0.66-0.97] pg/mL), MSD p-tau217 measure was 77% higher (0.23 [0.17-0.34] vs 0.13 [0.09-0.18] pg/mL), and Simoa p-tau231 measure was 49% higher (20.21 [15.60-25.41] vs 14.27 [11.27-18.10] pg/mL). Similarly, compared with amyloid-negative participants with MCI, among amyloid-positive participants with MCI, the median (IQR) Simoa p-tau181, MSD p-tau181, MSD p-tau217, and Simoa p-tau231 measures were higher by 52% (3.15 [2.34-4.83] vs 2.06 [1.10-2.29] pg/mL), 58% (1.38 [0.94-2.02] vs 0.87 [0.63-0.94] pg/mL), 67% (0.25 [0.22-0.43] vs 0.15 [0.13-0.16] pg/mL), and 66% (25.05 [14.96-37.22] vs 15.06 [14.46-18.07] pg/mL), respectively.
Overall, there were significant differences among all p-tau measures by amyloid and tau status (eFigure 3 in the Supplement). In amyloid- and tau-positive participants, compared with amyloid-positive participants, the median Simoa p-tau181 was 40% higher, MSD p-tau181 was 43% higher, MSD p-tau217 was 57% higher, and Simoa p-tau231 was 25% higher. However, there were no differences in any of the p-tau measures between amyloid- and tau-negative participants compared with tau-positive participants. We further compared the median plasma p-tau measures by amyloid and tau PET status, defining abnormal tau PET based on ERC ROI (eFigure 4 in the Supplement). Tau-positive participants had significantly higher p-tau measures compared with amyloid- and tau-negative participants for MSD p-tau181, MSD p-tau217, and Simoa p-tau231. Each of the 4 p-tau measure was significantly higher in amyloid- and tau-positive participants than amyloid-positive participants.
The accuracy of the continuous plasma p-tau measures for abnormal amyloid PET, tau PET, and ERC tau PET are shown in Figure 2, Figure 3, and eFigure 5 and eTables 1 to 3 in the Supplement. The 4 p-tau measures did not differ in terms of accuracy for abnormal amyloid PET or tau PET meta-ROI. In contrast, among CU participants, both MSD p-tau181 and MSD p-tau217 more accurately predicted abnormal ERC tau PET than Simoa p-tau181 (MSD p-tau181: AUROC, 0.80 vs 0.70; P =
= .046; MSD p-tau217: AUROC, 0.81 vs 0.70; P
.046; MSD p-tau217: AUROC, 0.81 vs 0.70; P =
= .04). There were no differences in AUROC between the p-tau measures among participants with MCI. In all analyses, the addition of age, sex, and APOE did not enhance the AUROC beyond that obtained by the p-tau measure alone.
.04). There were no differences in AUROC between the p-tau measures among participants with MCI. In all analyses, the addition of age, sex, and APOE did not enhance the AUROC beyond that obtained by the p-tau measure alone.
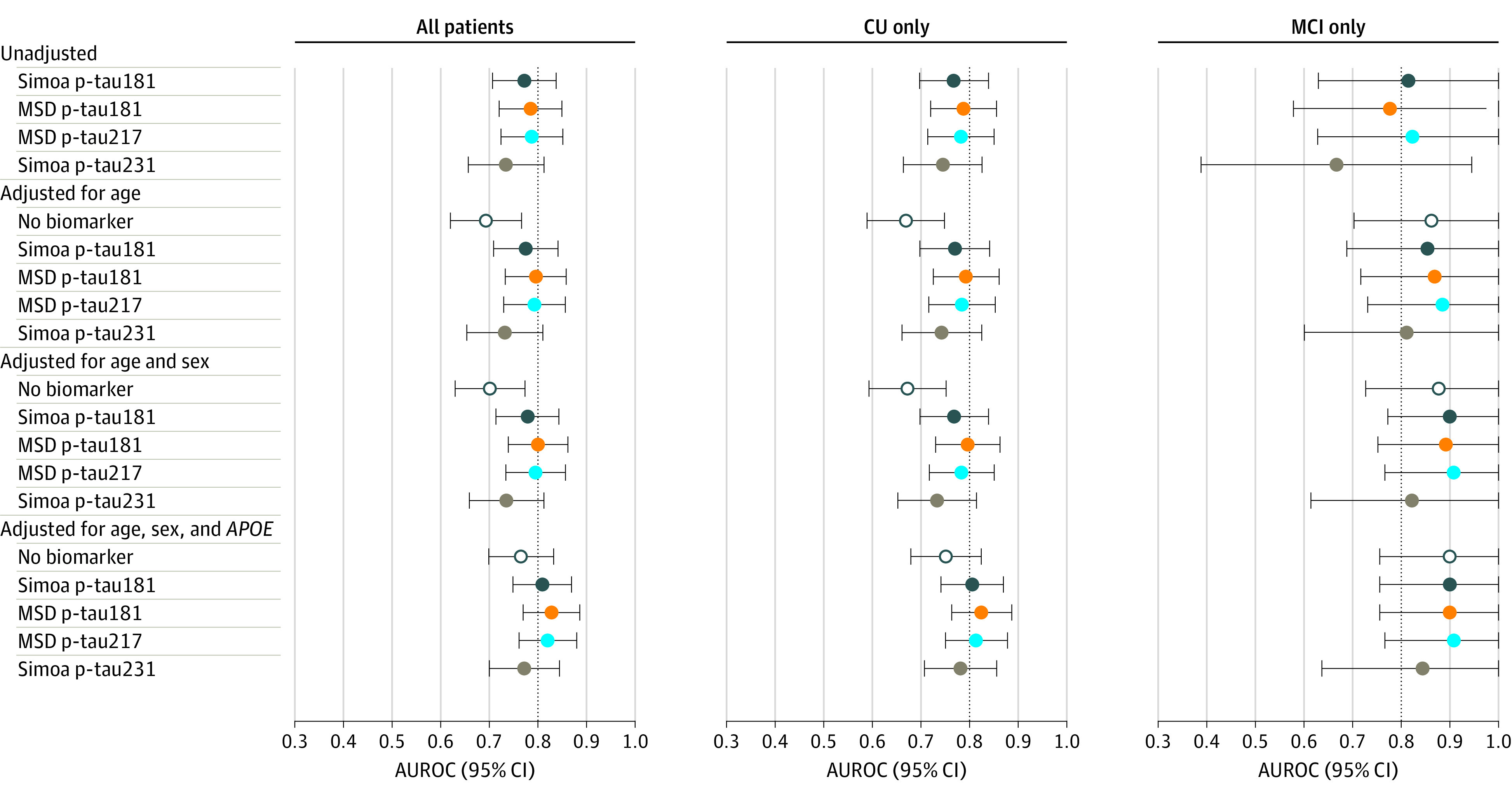
AUROC indicates area under the receiver operating characteristic curve; CU, cognitively unimpaired; MCI, mild cognitive impairment; MSD, Meso Scale Discovery; Simoa, single-molecule array.
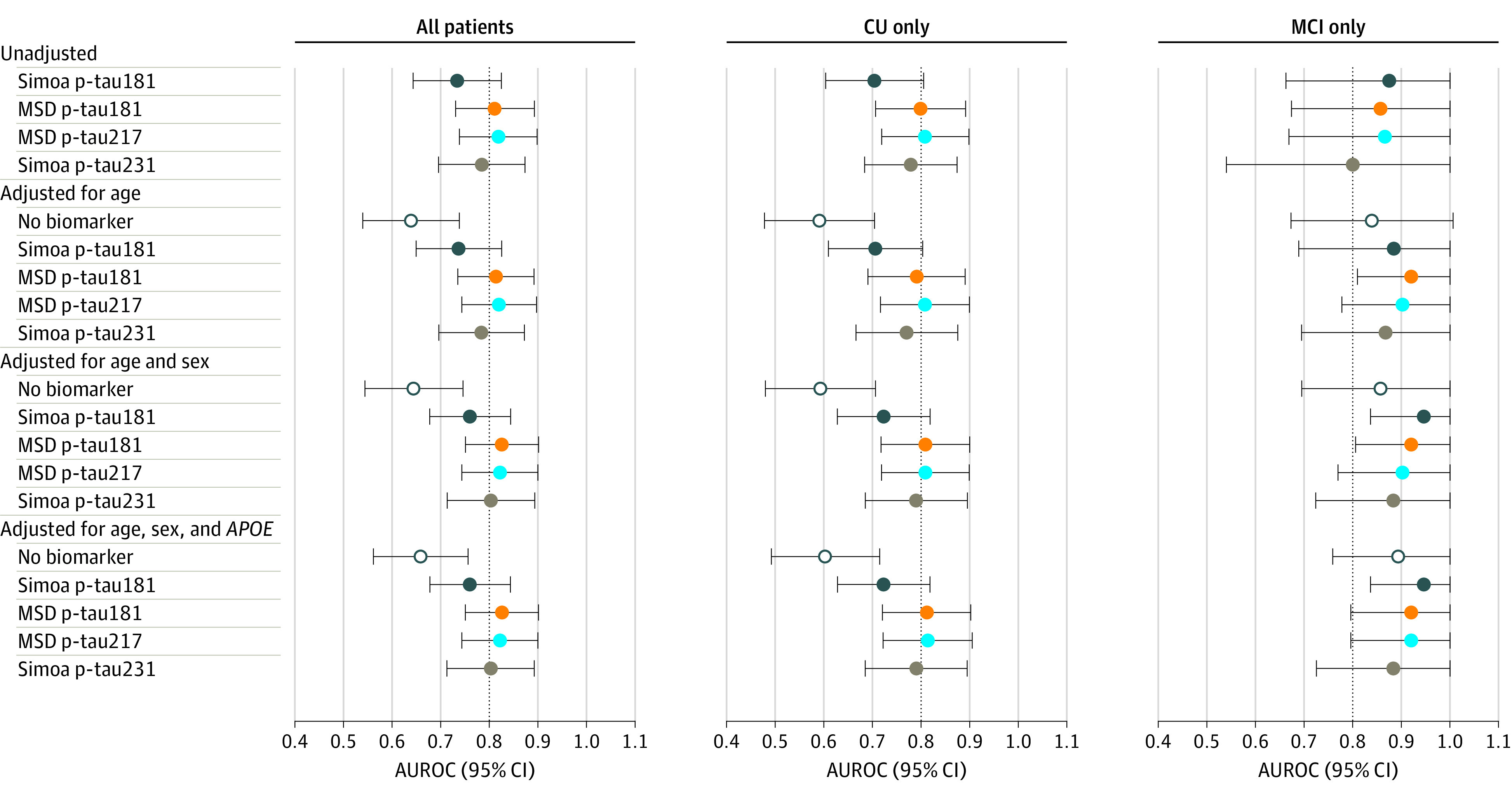
AUROC indicates area under the receiver operating characteristic curve; CU, cognitively unimpaired; MCI, mild cognitive impairment; MSD, Meso Scale Discovery; Simoa, single-molecule array.
Cross-sectional Associations With Imaging Measures of Neurodegeneration and Vascular Pathology and Cognitive z Scores
Univariable models examining the cross-sectional associations of each plasma p-tau measure and neuroimaging outcomes or global and domain-specific cognitive decline are shown in eTable 2 in the Supplement. Multivariable models are shown in Table 2. All 4 p-tau measures were associated with lower temporal lobe cortical thickness in multivariable analyses; the adjusted R2 was highest for Simoa p-tau181. Increasing levels of Simoa p-tau181, MSD p-tau181, and MSD p-tau217, but not Simoa p-tau231, were significantly associated with higher WMH volumes and lower (ie, more abnormal) FA-Genu. MSD p-tau181 and p-tau217 were most strongly associated with amyloid PET SUVR and FA-HCB.
Table 2.
| Measure | Participants, No. | β (95% CI)a | P value | R2 for full model | R2 for covariables only |
|---|---|---|---|---|---|
| Neuroimaging measures | |||||
| Cortical thickness | |||||
| Simoa p-tau181 | 200 | −0.025 (−0.038 to −0.012) | <.001 | 0.318 | 0.270 |
| MSD p-tau181 | 200 | −0.020 (−0.032 to −0.007) | .003 | 0.299 | 0.270 |
| MSD p-tau217 | 200 | −0.016 (−0.028 to −0.003) | .02 | 0.288 | 0.270 |
| Simoa p-tau231 | 164 | −0.026 (−0.046 to −0.005) | .02 | 0.297 | 0.270 |
| WMH volume | |||||
| Simoa p-tau181 | 199 | 4.378 (2.488 to 6.268) | <.001 | 0.319 | 0.249 |
| MSD p-tau181 | 199 | 4.772 (2.960 to 6.585) | <.001 | 0.337 | 0.249 |
| MSD p-tau217 | 199 | 5.364 (3.598 to 7.130) | <.001 | 0.363 | 0.249 |
| Simoa p-tau231 | 163 | 1.071 (−1.700 to 3.841) | .45 | 0.218 | 0.249 |
| FA-Genu | |||||
| Simoa p-tau181 | 200 | −0.006 (−0.011 to −0.002) | .007 | 0.256 | 0.231 |
| MSD p-tau181 | 200 | −0.006 (−0.011 to −0.002) | .004 | 0.259 | 0.231 |
| MSD p-tau217 | 200 | −0.008 (−0.013 to −0.004) | <.001 | 0.281 | 0.231 |
| Simoa p-tau231 | 164 | 0.001 (−0.006 to 0.008) | .83 | 0.205 | 0.231 |
| FA-HCB | |||||
| Simoa p-tau181 | 200 | −0.002 (−0.005 to 0.001) | .27 | 0.189 | 0.188 |
| MSD p-tau181 | 200 | −0.003 (−0.006 to 0) | .06 | 0.199 | 0.188 |
| MSD p-tau217 | 200 | −0.004 (−0.007 to 0) | .03 | 0.204 | 0.188 |
| Simoa p-tau231 | 164 | −0.002 (−0.007 to 0.003) | .38 | 0.226 | 0.188 |
| Amyloid PET | |||||
| Simoa p-tau181 | 200 | 0.119 (0.078 to 0.160) | <.001 | 0.290 | 0.173 |
| MSD p-tau181 | 200 | 0.149 (0.111 to 0.187) | <.001 | 0.369 | 0.173 |
| MSD p-tau217 | 200 | 0.154 (0.117 to 0.190) | <.001 | 0.386 | 0.173 |
| Simoa p-tau231 | 164 | 0.116 (0.051 to 0.181) | <.001 | 0.203 | 0.173 |
| Cognitive measures | |||||
| Global z score | |||||
| Simoa p-tau181 | 178 | −0.068 (−0.160 to 0.023) | .15 | 0.259 | 0.254 |
| MSD p-tau181 | 178 | −0.107 (−0.196 to −0.019) | .02 | 0.274 | 0.254 |
| MSD p-tau217 | 178 | −0.104 (−0.190 to −0.017) | .02 | 0.273 | 0.254 |
| Simoa p-tau231 | 143 | −0.103 (−0.240 to 0.035) | .15 | 0.274 | 0.254 |
| Memory z score | |||||
| Simoa p-tau181 | 199 | −0.086 (−0.179 to 0.008) | .07 | 0.219 | 0.210 |
| MSD p-tau181 | 199 | −0.140 (−0.230 to −0.051) | .002 | 0.244 | 0.210 |
| MSD p-tau217 | 199 | −0.112 (−0.202 to −0.023) | .01 | 0.231 | 0.210 |
| Simoa p-tau231 | 163 | −0.149 (−0.291 to −0.006) | .04 | 0.258 | 0.210 |
| Attention z score | |||||
| Simoa p-tau181 | 191 | −0.064 (−0.154 to 0.027) | .17 | 0.337 | 0.334 |
| MSD p-tau181 | 191 | −0.068 (−0.156 to 0.019) | .13 | 0.339 | 0.334 |
| MSD p-tau217 | 191 | −0.081 (−0.167 to 0.006) | .07 | 0.343 | 0.334 |
| Simoa p-tau231 | 156 | 0.001 (−0.130 to 0.131) | .99 | 0.359 | 0.334 |
| Language z score | |||||
| Simoa p-tau181 | 196 | −0.069 (−0.168 to 0.030) | .18 | 0.173 | 0.170 |
| MSD p-tau181 | 196 | −0.056 (−0.153 to 0.041) | .26 | 0.171 | 0.170 |
| MSD p-tau217 | 196 | −0.061 (−0.156 to 0.035) | .21 | 0.172 | 0.170 |
| Simoa p-tau231 | 160 | −0.139 (−0.291 to 0.012) | .07 | 0.191 | 0.170 |
| Visual spatial z score | |||||
| Simoa p-tau181 | 183 | 0.009 (−0.088 to 0.105) | .86 | 0.132 | 0.137 |
| MSD p-tau181 | 183 | −0.046 (−0.140 to 0.049) | .34 | 0.136 | 0.137 |
| MSD p-tau217 | 183 | −0.056 (−0.148 to 0.036) | .23 | 0.139 | 0.137 |
| Simoa p-tau231 | 148 | −0.088 (−0.235 to 0.060) | .25 | 0.123 | 0.137 |
Abbreviations: FA, fractional anisotropy; Genu, genu of corpus callosum; HCB, hippocampal cingulum bundle; MSD, Meso Scale Discovery; PET, positron emission tomography; Simoa, single-molecule array; WMH, white matter hyperintensities.
MSD p-tau181 and p-tau217 were the only measures significantly associated with lower global cognition (Table 2). Although Simoa p-tau231 had a similar β estimate and R2, it was not statistically significant, likely because of the lower sample size and larger variation. For memory, MSD p-tau181 and Simoa p-tau231 were associated with lower performance. There were no other associations between any of the p-tau measures and other cognitive domains.
Sensitivity Analyses
In sensitivity analyses, we reran the previous models and restricted the analyses to the 164 participants with all 4 plasma p-tau biomarkers. The results were similar (eTables 4 to 8 in the Supplement).
Discussion
We conducted a head-to-head comparison of plasma p-tau181 and p-tau217 measured by MSD and p-tau181 and p-tau231 measured by Simoa among participants without dementia to compare p-tau species at the earliest stages of the AD continuum. All 4 p-tau measures correlated well with one another. However, the tightest correlations were found between p-tau species measured on the same platform. It is currently unclear if this is associated with analytical performance of the platform itself and/or the detection of different p-tau species. Both the Simoa p-tau181 and p-tau231 assays use a detection antibody to the far N-terminus of tau in addition to a phosphosite-specific capture antibody. In contrast, the MSD p-tau181 and p-tau217 assays use a detection antibody in the N-terminal to mid-domain of tau. Moreover, the MSD assays use peptides as calibrators, while the Simoa assays use the same in vitro phosphorylated tau protein. An important future direction is the harmonization of plasma p-tau measurements by developing reference measurement procedures, certified reference materials, and commutability studies among plasma p-tau measurements.38
Previous studies have demonstrated that plasma p-tau181, p-tau217, and p-tau231 show good discriminate accuracy for distinguishing those with amyloid and tau pathology, using either neuroimaging11,12,13,14,16,23,26 or neuropathological assessments,11,12,13,14,16,23,26 and those without in the more advanced stages of the AD clinical spectrum.11,12,13,14,16,23,26 A 2021 study of Simoa p-tau231 and p-tau18123 suggested similar diagnostic accuracy for the 2 p-tau measures, with increases in p-tau231 being earlier than p-tau181. In the present study, the 4 p-tau assays behaved similarly in relation to amyloid and tau PET outcomes. However, the fold change and diagnostic performance by PET status (amyloid positive vs amyloid negative or amyloid and tau positive vs amyloid positive) among CU participants was largest for MSD p-tau217 and lowest for Simoa p-tau231. Among participants with MCI, the fold change for amyloid positive vs amyloid negativity was highest for MSD p-tau217 and Simoa p-tau231. The discriminant accuracy of the plasma p-tau measures for amyloid PET, meta-ROI tau PET, and ERC tau PET did not significantly differ in most cases. The only significant association among CU participants was that MSD p-tau181 and MSD p-tau 217 were significantly more accurate predictors of abnormal ERC tau PET compared with Simoa p-tau181.
We also compared the plasma p-tau species in relation to neuroimaging measures of temporal lobe thickness, WMH, FA-Genu, and FA-HCB. Again, there were some differences across p-tau species and platforms. MSD p-tau181 and p-tau217 and Simoa p-tau181, but not Simoa p-tau231, were associated with greater WMH volume and lower FA-Genu. WMH have been associated with AD pathology,36 so the association with the p-tau measures may be explained by that mechanism. However, FA-Genu is a biomarker of cerebrovascular disease because loss of microstructural integrity in this region has previously been shown with worsening of system vascular health and cerebrovascular injury even after accounting for AD pathology.34 Thus, the mechanism underlying the association between vascular-related imaging measures and p-tau181 and p-tau217, but not p-tau231, is an important area of future research.
In this study, there were 2 assays measuring p-tau181, which differed in the antibody combination used as well as the platform. Although platform differences cannot be ruled out, the more likely reason for differences is the anti-tau antibody used in each assay. In the Simoa p-tau181 assay, an N-terminal antibody (tau12 targeting amino acids 6 to 18) was used for detection, and in the MSD p-tau181 assay, a mid-domain antibody (4G10E2 antibody targeting amino acids 111 to 130) was used. There are several splice forms of tau, as well as proteolytic fragments, that could be influential in disease-related changes in plasma or CSF levels and significantly affect clinical accuracy.4,5,6,7,10 In previous studies using the MSD p-tau181 assay in CSF,39,40 the fold changes between amyloid-negative CU patients and amyloid-positive patients with AD were almost 4-fold higher, whereas other p-tau181 assays have historically shown only 2-fold higher results.2 In this study, the difference in anti-tau antibody epitopes for the p-tau181 assays may contribute to the differences observed in the correlation analysis with p-tau217. This same assay design difference is the most likely reason for the higher fold change, correlation with amyloid and tau PET, and numerically higher discriminative accuracy when comparing the 2 p-tau181 assays. Direct comparison of p-tau assays developed on the same platform using either partner antibody may help address this.
Higher levels of all plasma p-tau measures were associated with worse performance across cognitive domains, with associations most pronounced for global cognitive z score and memory z score. Simoa p-tau181 was associated with less decline compared with MSD p-tau181 and p-tau217 and Simoa p-tau231. The most likely explanation for this observation is the anti-tau epitope difference, as described above.
Strengths and Limitations
A major strength of the study is the well-characterized individuals with MRI, amyloid PET, and tau PET data. However, limitations warrant consideration. First, our comparison was focused on the earliest changes in p-tau species, so patients with AD dementia were not included. Additional comparison of the p-tau species at later stages of the clinical and pathological spectrum is also needed. Second, we used amyloid and tau PET as our criterion standards for which to compare the plasma p-tau measures. These PET measures have measurement noise and are not perfectly indicative of underlying pathology. Future research should focus on the comparison of these plasma p-tau measures with autopsy-confirmed neuropathological changes. Third, the sample size of patients with data on p-tau231 was smaller than the other species. However, in additional analyses, restricting the sample to those with all 4 p-tau measures did not change the results. Forth, a minor weakness is that we did not adjust for multiple comparisons in the AUROC or neuroimaging analyses. Fifth, participants were primarily White, and the results may not be generalizable to more diverse populations.
Conclusions
In this head-to-head comparison of plasma p-tau181 and p-tau217 measured by MSD and p-tau181 and p-tau231 measured by Simoa, we observed some differences across plasma p-tau species and platforms for a tau PET ERC region and MRI measures of cerebrovascular and AD pathology among participants without dementia. Longitudinal studies of the plasma p-tau species and platforms across the AD clinical continuum are needed to better understand these differences. An important future direction will be to harmonize the plasma p-tau measurements.
Notes
Supplement.
eMethods.
eFigure 1. Scatterplots and spearman correlations between plasma p-tau measures.
eFigure 2. Boxplots of plasma p-tau measures by clinical diagnosis and elevated amyloid PET.
eFigure 3. Boxplots of plasma p-tau measures by clinical diagnosis and amyloid and meta-ROI tau PET.
eFigure 4. Boxplots of plasma p-tau measures by diagnosis and amyloid and entorhinal cortex tau PET.
eFigure 5. Comparison of the accuracy of the 4 plasma p-tau measures for a tau PET metaregion of interest.
eTable 1. Predictive accuracy of continuous plasma p-tau biomarkers in predicting elevated amyloid PET.
eTable 2. Predictive accuracy of continuous plasma p-tau biomarkers in predicting elevated tau PET meta-ROI.
eTable 3. Predictive accuracy of continuous plasma p-tau biomarkers in predicting elevated tau PET ERC.
eTable 4. Univariable associations between the plasma p-tau isoforms and neuroimaging and cognitive z scores.
eTable 5. Predictive accuracy of continuous plasma biomarkers in predicting elevated amyloid PET among the 164 participants with all plasma p-tau species.
eTable 6. Predictive accuracy of continuous plasma biomarkers in predicting elevated meta-ROI tau PET among the 164 participants with all plasma p-tau species.
eTable 7. Predictive accuracy of continuous plasma biomarkers in predicting elevated ERC tau PET among the 164 participants with all plasma p-tau species.
eTable 8. Multivariable associations between the plasma p-tau species and neuroimaging and cognitive z scores among the 164 participants with all plasma p-tau species.
References
Full text links
Read article at publisher's site: https://doi.org/10.1001/jamaneurol.2021.2293
Read article for free, from open access legal sources, via Unpaywall:
https://jamanetwork.com/journals/jamaneurology/articlepdf/2782135/jamaneurology_mielke_2021_oi_210038_1630683432.28069.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/110489389
Article citations
The Hidden Dangers of Sedentary Living: Insights into Molecular, Cellular, and Systemic Mechanisms.
Int J Mol Sci, 25(19):10757, 06 Oct 2024
Cited by: 0 articles | PMID: 39409085 | PMCID: PMC11476792
Review Free full text in Europe PMC
Blood-based biomarkers in the oldest old: towards Alzheimer's disease detection in primary care.
Lancet Reg Health Eur, 45:101077, 19 Sep 2024
Cited by: 0 articles | PMID: 39329097 | PMCID: PMC11426150
Association between blood-based protein biomarkers and brain MRI in the Alzheimer's disease continuum: a systematic review.
J Neurol, 271(11):7120-7140, 12 Sep 2024
Cited by: 0 articles | PMID: 39264441 | PMCID: PMC11560990
Review Free full text in Europe PMC
Navigating the Landscape of Plasma Biomarkers in Alzheimer's Disease: Focus on Past, Present, and Future Clinical Applications.
Neurol Ther, 13(6):1541-1557, 07 Sep 2024
Cited by: 0 articles | PMID: 39244522 | PMCID: PMC11541985
Review Free full text in Europe PMC
Alzheimer's disease biomarkers and their current use in clinical research and practice.
Mol Psychiatry, 04 Sep 2024
Cited by: 0 articles | PMID: 39232196
Review
Go to all (100) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Plasma phosphorylated tau 217 and phosphorylated tau 181 as biomarkers in Alzheimer's disease and frontotemporal lobar degeneration: a retrospective diagnostic performance study.
Lancet Neurol, 20(9):739-752, 01 Sep 2021
Cited by: 194 articles | PMID: 34418401 | PMCID: PMC8711249
Association of Phosphorylated Tau Biomarkers With Amyloid Positron Emission Tomography vs Tau Positron Emission Tomography.
JAMA Neurol, 80(2):188-199, 01 Feb 2023
Cited by: 67 articles | PMID: 36508198 | PMCID: PMC9856704
Associations of Plasma Phospho-Tau217 Levels With Tau Positron Emission Tomography in Early Alzheimer Disease.
JAMA Neurol, 78(2):149-156, 01 Feb 2021
Cited by: 154 articles | PMID: 33165506 | PMCID: PMC7653537
Tau proteins in blood as biomarkers of Alzheimer's disease and other proteinopathies.
J Neural Transm (Vienna), 129(2):239-259, 17 Feb 2022
Cited by: 8 articles | PMID: 35175385
Review
Funding
Funders who supported this work.
Medical Research Council (1)
Grant ID: UKDRI-1003
NIA NIH HHS (5)
Grant ID: R01 AG041851
Grant ID: P30 AG062677
Grant ID: R01 AG011378
Grant ID: R37 AG011378
Grant ID: U01 AG006786
NINDS NIH HHS (1)
Grant ID: R01 NS097495
 1
,
2
1
,
2




