Abstract
Free full text

Vision, challenges and opportunities for a Plant Cell Atlas
Associated Data
Abstract
With growing populations and pressing environmental problems, future economies will be increasingly plant-based. Now is the time to reimagine plant science as a critical component of fundamental science, agriculture, environmental stewardship, energy, technology and healthcare. This effort requires a conceptual and technological framework to identify and map all cell types, and to comprehensively annotate the localization and organization of molecules at cellular and tissue levels. This framework, called the Plant Cell Atlas (PCA), will be critical for understanding and engineering plant development, physiology and environmental responses. A workshop was convened to discuss the purpose and utility of such an initiative, resulting in a roadmap that acknowledges the current knowledge gaps and technical challenges, and underscores how the PCA initiative can help to overcome them.
Location-to-function knowledge can unlock new discoveries in plant science
The relationship between the sequence, structure and function of a protein has been explored from many angles, from biophysics to drug discovery (Brausemann et al., 2017; Rajendran et al., 2018; Shang et al., 2020). This long-standing paradigm has driven the development of innovative technologies in imaging, mass spectrometry, genomics and bioinformatics (Camp et al., 2019; Mallis et al., 2020; Nakane et al., 2020). Yet an evolving paradigm stating that the location of molecules is essential for their function at all scales, from molecular complexes to cells, organs, organisms and whole communities, also holds true (Figure 1).
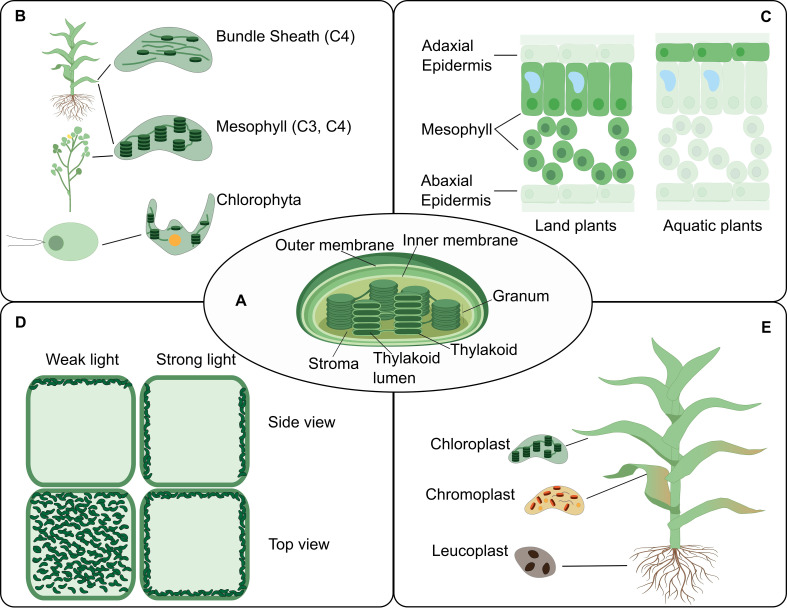
The illustration shows the levels of organization of a plant (left) and a few examples highlight how location determines function at each level (right). At the molecular level, functions of protein complexes can be determined by their localization in membrane microdomains (Jarsch et al., 2014) or by dynamic protein interactions (Obata, 2019). At the cellular level, a protein can be located differentially through transport mechanisms, including vesicle trafficking (Goring and Di Sansebastiano, 2018) and nuclear translocation (Marchive et al., 2013), regulating its function. At the tissue level, cell position can drive its fate into a specialized cell type (Shao and Dong, 2016). Metabolic pathways can operate specifically in specialized cell types across tissues (Marchive et al., 2013; Schlüter and Weber, 2020). At the next level, the existence of non-cell-autonomous transcription factors can transverse intercellular scales across plant organs (Han et al., 2014). Also, an organ-dependent post-translational proteome has been described as a mechanism of protein function regulation (Uhrig et al., 2019). At the organism level, plant interaction with biotic (Harrison et al., 2002) and abiotic factors (Michaud et al., 2017) can occur through a localized cue perception.
At the molecular level, compelling examples of the location-to-function paradigm include the multi-enzyme complexes that work as molecular machines (so-called ‘metabolons’) to channel intermediate metabolites and produce specific molecular products in a highly controlled manner (Obata, 2019).
At the cellular level, the partitioning of proteins and lipids into organelles – and even membrane microdomains within organelles – plays crucial roles in molecular trafficking and signal transduction, which have great impacts in many biological processes such as plant development and defence responses (Jarsch et al., 2014; Xing et al., 2019; Yu et al., 2020; Heinze et al., 2020; Shimizu et al., 2021). The differential location of a single molecule can determine one of several alternative functions (see, for example, Smirnoff and Arnaud, 2019). Protein function can also be dynamically regulated by a change in location, such as the activation of transcription factors by translocation into the nucleus (Jiang et al., 2020; Marchive et al., 2013; Ramon et al., 2019; Pastor-Cantizano et al., 2020). Furthermore, protein translocation to different cellular domains results in different functional outcomes (see for instance, Kim et al., 2020).
At the tissue level, C4 photosynthesis is a classic example of how differential molecular localization between cell types enhances plant performance: differential expression of enzymes in bundle sheath and mesophyll cells in C4 plants can concentrate CO2 in close proximity to Rubisco, which is not possible in C3 plants where the enzymes are present, but not differentially expressed between the cells (Schlüter and Weber, 2020).
Considering the opportunities in science and technology today, we contend that this ‘location-to-function’ paradigm will likely usher in conceptual leaps and transformative technologies, as was the case for the structure-to-function paradigm.
One particular area of potential success is agriculture, as the success of molecular approaches for crop improvement depends on a detailed understanding of the spatio-temporal regulation of genes and proteins and the production of metabolites (see Wang et al., 2008 for an example of how controlling the location of gene expression impacts rice grain size and yield). The timing of expression is also a key determinant in imparting functional specification and influencing traits. For instance, engineered early expression of WRINKLED1 (WRI1, an AP2 class transcription factor) during maturation increased Arabidopsis seed size and oil content (Kanai et al., 2016). More recently, an ambitious goal to globally sequester extra CO2 into roots was established, which predicates on understanding what controls the development of a specific tissue, periderm, and the cell-type-specific expression of suberin, a lipophilic polymer found in plant cell walls. These examples illustrate how a thorough understanding of the cell- or tissue-specific expression of plant genes may enable the development of more effective biotechnological tools to facilitate crop improvement, plant resilience and climate change mitigation. Currently, these spatially- or cell-type-resolved expression data are missing or incomplete for most crop plants and there is no centralized repository for their integration. These shortcomings limit the rapid development of improved crops based on location-to-function data.
Unlocking location-to-function: The first Plant Cell Atlas (PCA)
The PCA workshop
Establishing a comprehensive picture of where molecules are located and how their locations inform function in higher plants will require concerted efforts from diverse groups of scientists. To catalyze such a community, a series of workshops were organized by Carnegie Institution for Science, New York University and Stanford University scientists around the topic of a cell atlas for plants, a framework to generate and synthesize molecular and cellular data on the development, dynamic functions and specialization of plant cells. This effort would also strive to determine the state of the field, identify necessary components and people and discuss a path forward. Three workshops were held virtually during May and June of 2020. Over 400 scientists participated, of which 70% were early-career researchers, and 33% non-US based (Rice et al., 2020). Attendees of this first series of workshops became the basis of the PCA community. Following the success of the inaugural meeting, two technology-oriented workshops on single-cell sequencing and spatial proteomics and a career planning workshop were held virtually in 2021, respectively attracting 273, 253 and 42 participants. Additional workshops and symposia will be announced on the PCA website.
The PCA goals
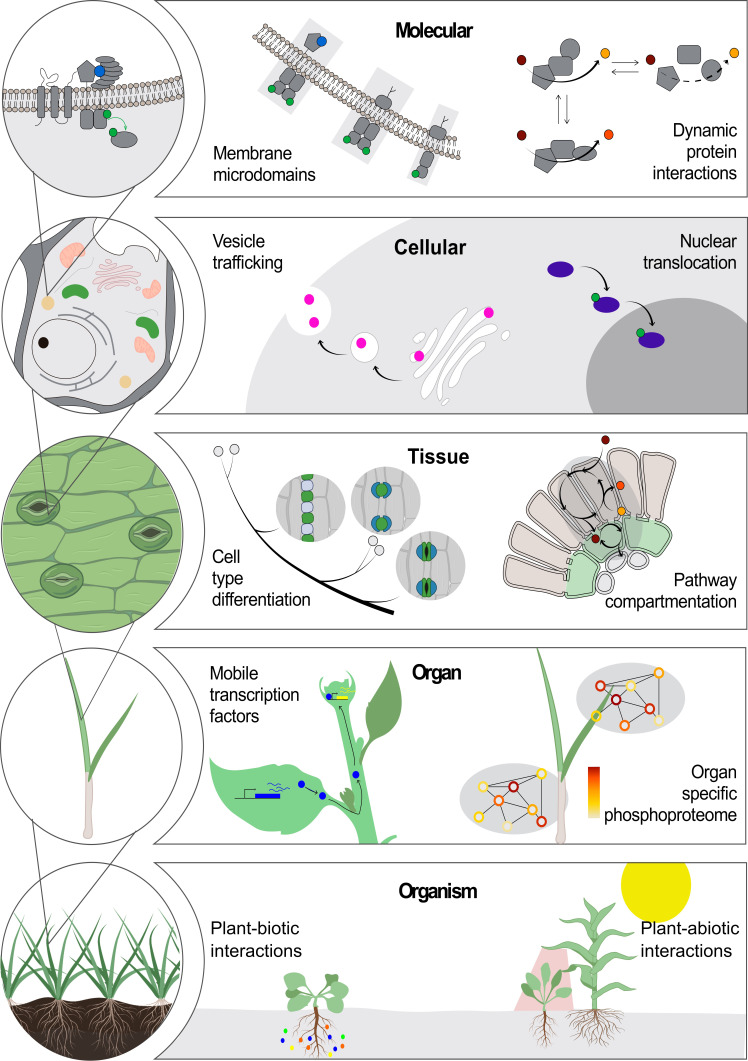
The goal of the PCA is to bridge gaps in knowledge, providing critical localization, dynamics and interaction information at the cellular and subcellular levels. The participants in the PCA Research Coordination Network envision a set of concrete milestones over the next decade to reach that vision, based on community goals set during these meetings in 2020 and 2021 (Figure 2).
PCA milestones for the next 10 years and beyond in data generation (yellow bars), data analysis (purple bars), software development (green bars), and building PCA community (orange bar).
Identify and quantify relative abundances of all definable cell types in every organ of several strategically selected reference species (e.g. a single-celled organism, a non-vascular plant, a eudicot, a monocot).
Profile the developmental trajectory of every specialized cell type in each organ in the reference species through single-cell sequencing.
Develop a ‘reference’ clearinghouse where new experiments based on single-cell profiling can be mapped onto comprehensive atlases representing the diversity of known transcriptional states among cell types.
Profile the responses of major organ systems to a common set of environmental perturbations at a single-cell resolution.
Determine the intraspecific variation of developmental trajectories and environmental responses at a single-cell resolution.
Establish data integration platforms and community-supported data standards to better enable data integration.
Develop a community-supported data benchmarking platform that will accomplish simulation and validation of data and evaluation metrics.
Determine protein subcellular localization, interactions and dynamics in the major cell types of leaves, roots and flowers for every gene in reference species.
Curate empirical datasets and develop data analysis and visualization tools through community-driven challenges (e.g., the DREAM single-cell transcriptomics challenge; Alonso et al., 2019; Pham et al., 2020).
Establish mechanisms for fostering new collaborations and funding for data generation, data integration as well as new technology and method development or adoption for plants.
Some of these goals can be accomplished quickly, as already existing technologies such as single-cell and single-nucleus RNA-seq make them realistic. Others require the development of technologies to collect information at the single-cell level. As technology and discovery reshape the vision of the PCA, new aspirations should emerge from the community. Nonetheless, these ambitious milestones represent key gaps in knowledge that will accelerate plant research (if addressed) and are feasible to achieve given the opportunities presented by recent technological advances. The PCA community can enable this vision in several ways.
The PCA vision
First, the participants in the PCA Research Coordination Network envision a one-stop, user-friendly website with illustrated and graphical details about cellular organization across different developmental stages. This information, modeled after findable, accessible, interoperable and reusable (FAIR) data principles, could be widely accessed and used to advance research and develop educational tools (Figure 3; Wilkinson et al., 2016). Although single-cell genomics data (mostly transcriptomic) have been generated and analyzed in individual studies for several years, how well the broader community can use these datasets in comparative analyses has been limited. This is largely due to the difficulty in integrating and comparing data generated by different methods, growth conditions and collection methods. The PCA will be critical in harnessing the power of single-cell data by establishing and promoting community-supported best practices in sample preparation, data generation, analysis and integration – including data benchmarking platforms, evaluation metrics and reference cell maps for major organ/cell types in model species. Important considerations for such infrastructure include how data will be gathered, curated and stored in order to integrate resources and contributions from different laboratories. It is necessary to develop and incentivize standards for both data collection (i.e. experimental protocols) and curation to allow integration of disparate datasets into a unified resource such as the PCA. This should include application programming interfaces (APIs) for programmatic access to ensure that PCA is accessible to all in a FAIR format. Similar standards have been produced for instance for plant phenotypic data (Papoutsoglou et al., 2020).
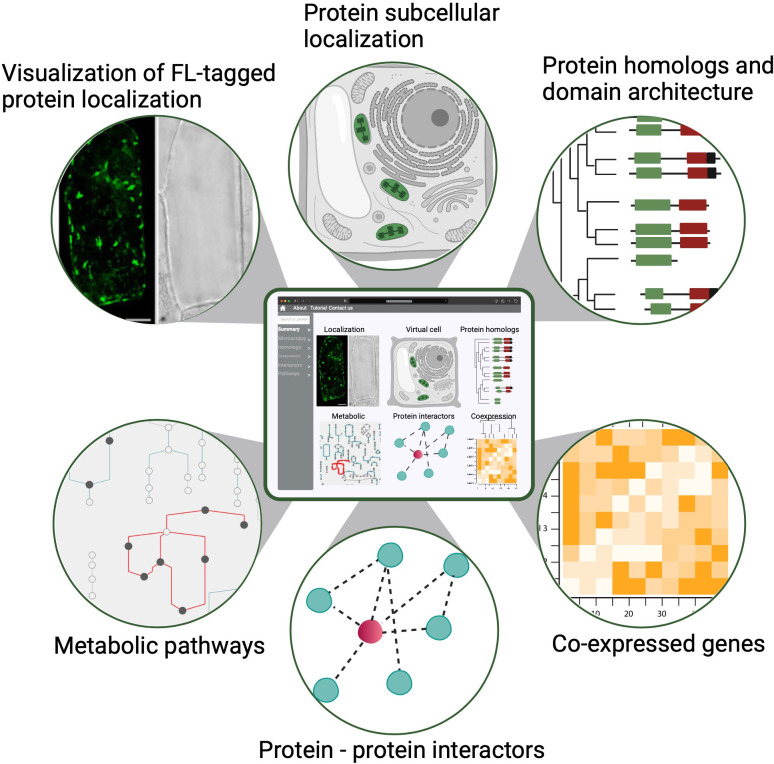
An example of a PCA user interface that integrates various data types from molecular, biochemical, cellular and evolutionary contexts to connect location to function. Shown are example data types that would be seamlessly connected to enable easy navigation and discovery (FL: fluorescence).
Second, we challenge and support the community to create a 4D representation of a developing plant from root to fruit (Henkhaus et al., 2020) with data collected from single-cell omics platforms (genomics, epigenomics, transcriptomics, proteomics and metabolomics) for each cell or cell type that are mapped onto tissue atlases of organ development. The data collected from the single-cell platforms form the basis of these atlases and serve as a reference for mapping further experimental data (e.g. from perturbation experiments). The PCA efforts include integration of not only single-cell omics data, but also spatiotemporal dynamics of single cells and their connection to cell types and developmental trajectories. It therefore facilitates a comprehensive understanding of how different complex tissues and organs are formed during plant growth and development.
Third, we wish to generate excitement and awareness about the potential for plant science research to address society’s most pressing challenges in agriculture, food security, bioenergy, resource management, as well as ecosystem stewardship and robustness in response to climate change. The PCA community needs to overcome the growing gap between ongoing research and societal perception with public communication and education. The PCA efforts should include outreach, training and education of the general public to understand the motivations, successes and limitations of this research. The initiative will help to build public awareness of how translational research that arises from model organisms can positively impact society and help feed the world. It will also demonstrate how understanding plants as foundational components of the ecosystem helps to predict and mitigate the consequences of climate change, and to be better stewards of the planet. Without these efforts, we fear that growing skepticism about science will impair the ability to aid society with the power of plant research, as apparent in the non-evidence-based regulation of gene editing in the European Union (Jorasch, 2020; Ruffell, 2018; Zimny et al., 2019).
Fourth, we see the PCA embracing new technologies and fostering multidisciplinary collaborations. The PCA will aim to build a community by introducing research tools to new users, and by facilitating interactions among scientists, engineers and breeders worldwide. These goals can be met by organizing in-person events and online workshops with presentations from domain experts, followed by breakout rooms for targeted discussions and networking. The multidisciplinary nature of the PCA has enormous potential for accelerating the fundamental understanding of plant cells and how they connect to the whole organism, and for translating fundamental discoveries to agriculturally important crops across the globe. The dream of the PCA initiative in this regard is to actively engage applied plant scientists and breeders by facilitating the formation of joint national and international funding schemes across scientific disciplines. This will allow diverse communities to benefit from each other's expertise and for ideas and technologies to be transferred from lab to land. Enhancement in research translatability will address globally relevant societal questions that are aligned with the UN Sustainable Development Goals (Biermann et al., 2017; Costanza et al., 2016; Griggs et al., 2013), including food sustainability (Goals # 1–3), resources to tackle climate change (Goal #13) and the overall survival of the biosphere (Goals # 14–15). We envision that starting with a comprehensive cell atlas of model organisms will help to better understand non-model species and eventually expand to an ecosystem-scale analysis of interactions between organisms.
Inspiration from similar projects
What the participants in the PCA Research Coordination Network envisage is ambitious and not yet available for any system. However, examples of components of our vision will serve as guides. Among the animal whole organism atlases (Lähnemann et al., 2020), the mouse brain and adult mouse cell atlases are models for comprehensive coverage and community accessibility (Rosenberg et al., 2018; Saunders et al., 2018; Zeisel et al., 2018; Tabula Muris Consortium, 2018; Han et al., 2018). The Human Cell Atlas’ data portal already includes data from 55 organs and 12 million cells, and it is becoming a model for making community-generated single-cell data available at a single portal, along with APIs and data analysis pipelines made easily accessible. In addition, the OpenWorm project provides an excellent example for modeling, visualizing and simulating various aspects of the biology and behavior of an organism (Szigeti et al., 2014). For plants, response to the environment would serve as an analogous level to behavior. EMBL-EBI’s Single Cell Expression Atlas recently started hosting plant single-cell transcriptome data, which is an exciting development (Papatheodorou et al., 2020). Finally, the Global Natural Product Social Molecular Networking initiative provides a model for a one-stop-shop web infrastructure to enable data generation, integration, analysis and publication from mass spectrometry (Wang et al., 2016).
The envisioned PCA community
The PCA will be developed, maintained and used by a global community with diverse scientific, technical, creative and educational backgrounds (Figure 4). Helped by rapidly evolving online tools that increase inclusivity and transparency, the initiative should seek ways to facilitate the engagement, communication and collective decision-making among all groups involved. This work will aim to be grounded in active listening, psychological safety, teamwork, mutual respect and integrity. An inclusive and bottom-up ethos will underpin the development of global biological databases built with scientific rigor and transparency.
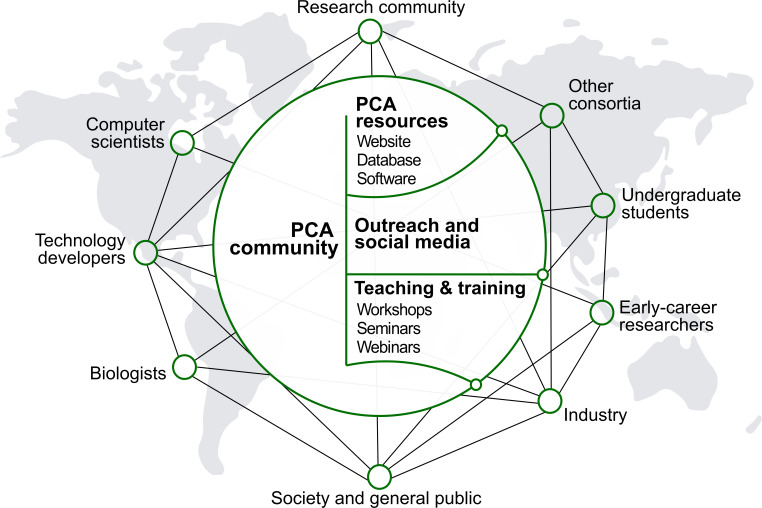
Major stakeholders of the PCA are described. The goal is to establish a broad network of collaboration between developers and users through the creation of an accessible platform, educational tools and outreach activities. The PCA community should strive to be inclusive, diverse and transparent.
The participants in the PCA Research Coordination Network anticipate that the success of the PCA will hinge on an open and frequent dialogue between developers and users, with facilitation and guidance from a steering group. Recruiting scientists from diverse disciplines as well as from different career stages will be crucial in the successful development of the PCA. In particular, the initiative should continue to provide an opportunity for early-career researchers and foster the development of future leaders in plant biology.
Developers
The mission of the PCA community will be achieved by groups who will contribute to the development and maintenance of the database, as well as those who generate or use the data and provide inputs for improvement. Developing the PCA database will be done with the expertise of scientists, software engineers, modelers, scientific illustrators and animators coming together to fine-tune the display of cellular organization and collecting information. Data and computational scientists will have to be involved in the curation and annotation of data. Engagement will extend to consortia pursuing similar activities in different biological systems (e.g., Human Cell Atlas). As the PCA will heavily depend on data visualization, user interface and experience design will also benefit from advisors in art and design fields. Additionally, collaboration with cartographers will enhance the understanding of spatial mapping for developing the PCA database. These influences from outside the field of plant research will complement and bolster the expertise of plant scientists in the PCA community.
Users
The participants in the PCA Research Coordination Network expect several types of users. One of the major user groups will be the data generators, which will involve plant scientists from many disciplines, including single-cell profiling, protein-protein interactions, cell and developmental biology, imaging or synthetic biology. To engage scientists from different career stages and areas of plant science, it will be important to develop mechanisms attributing and giving credit to data contributors. Another type of users will be researchers who wish to explore the PCA data to make discoveries. Engaging these users through a bottom-up process driven by their needs will be required for a vital and thriving PCA. Finally, the third group of users, both within and outside of the research community, are those who will access the PCA for educational purposes. Simple and intuitive interfaces combined with an attractive display of information and compelling visualizations will attract a large number and range of educators seeking information for teaching.
Integrating the PCA in the wider bioinformatics community
Robust computational infrastructure development will be essential to support the research, community-building and outreach activities of the PCA (Figure 5). We envision the PCA infrastructure (that is, the technologies, standards and computational platforms used by developers to build a framework to house and make available the PCA-relevant data) to have a seamless integration of multi-omics data with localization data at multiple scales of the plant. A governance structure, contribution guide and code of conduct for participants in infrastructure development should be established to define and support community values. These values include open-source development, FAIR principles and outcomes-based planning and implementation. Stakeholders should be included in the decision-making process. To implement these values, existing efforts with similar values and goals should be leveraged. For example, the DREAM challenges group could be leveraged for developing data analysis, visualization and access tools (Pham et al., 2020).
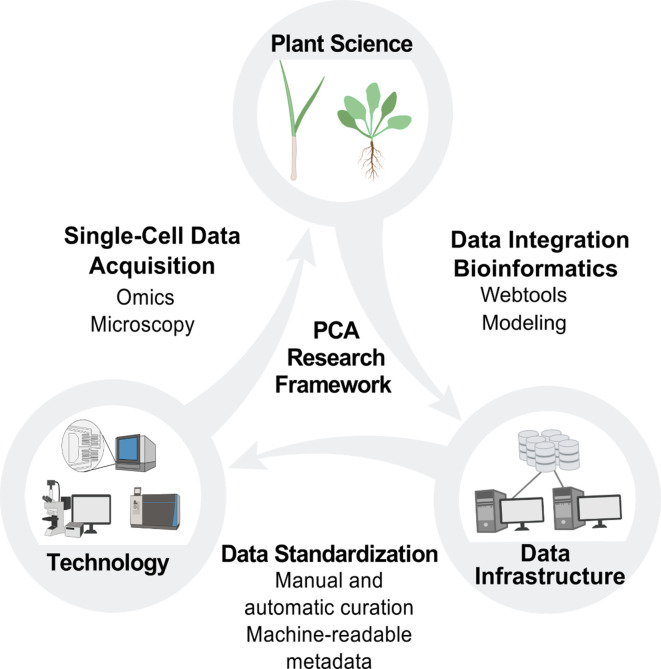
Building the PCA will require cooperation and coordination between plant science, technology and data infrastructure. Plant scientists will use new technologies to generate data at the single-cell level, which will be integrated by data scientists into the PCA infrastructure through manual and automated curation. This data integration will enable the development of new technologies and drive new hypotheses and investigations for plant scientists, who will then contribute additional data to the PCA resource.
While new tools and resources will be needed to build a PCA community platform, existing resources should be strong guiding principles when developing the PCA infrastructure. The PCA should make efforts to liaison with data repositories, knowledge bases and data analysis hubs such as ATTED, eFP Browser, ePlant, Ensembl Plants, Gramene, MaizeGDB, TAIR, The 1001 Arabidopsis Genomes phenotype database, NCBI’s GEO, EMBL-EBI’s Expression Atlas, AraPheno and others (Obayashi et al., 2007; Sullivan et al., 2019; Howe et al., 2020; Tello-Ruiz et al., 2021; Portwood, 2018; Berardini et al., 2015; Seren et al., 2016; Barrett et al., 2005; Papatheodorou et al., 2020; Waese et al., 2017). For example, OMERO is a global consortium effort aiming to standardize biological imaging data, which includes data depository as well as image processing software to translate image files into a universal format. Cross-species information may be mapped to the latest phylogenetic tree updated by the Tree of Life Web Project and Bioschemas. Engagement should also extend to consortia that are undertaking related work in other organisms, such as the Human Cell Atlas. To create a collaborative research environment, projects such as Synapse, Cyverse and KBase could be evaluated for strategic partnership. To develop a framework to represent the PCA at multiple scales, it will be important to implement conceptual frameworks such as the common coordinate framework (Rood et al., 2019), developmental trajectories (‘pseudo-time’) and spatial gradients (‘pseudo-space’; Stewart et al., 2019).
Driving the community forward
The PCA will receive diverse sets of data from different types of techniques and experiments, many of which are constantly pushing the boundaries of the state of the art. As such, the PCA needs to position itself and its community at the frontier of plant biology, looking out for emerging findings and technologies. This could be achieved through online workshops that enable pioneering researchers and developers to introduce emerging technologies to the community. Other training activities might include hands-on workshops on single-cell, spatially resolved experimental techniques, data annotation and analysis tutorials, as well as hackathons and virtual office hours to help novice code developers, data scientists and other researchers.
Gaps in knowledge: How the PCA could address unresolved questions in plant science
Many unresolved questions in plant biology require new technologies and approaches that the PCA could drive forward, as well as benefit from. Examples of such major knowledge gaps are described here (see also Figure 6).
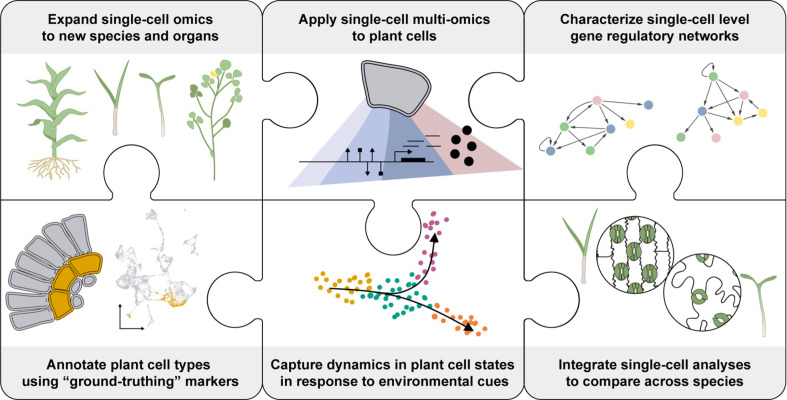
Several limitations inherent to plants (shown as pieces of a jigsaw puzzle) must be overcome to enhance the understanding of plant cells as biological systems. To characterize the unique molecular attributes of each cell type composing a plant, plant biologists will need to develop reliable methods to broadly access the different cell types across various plant species. Also, single-cell multi-omics technologies will be necessary to get a deeper understanding of plant cell processes across all layers of molecular regulation. This data must be gained in the context of functional annotation of the cells. This would require the identification of reliable marker genes for each type of plant cell-. Ultimately, the spatiotemporal distribution of the molecular attributes of each cell will help to understand their dynamic regulation during plant cell development and in response to environmental stresses. Integrating the information collected using bioinformatics tools will enable the characterization of regulatory networks at a single-cell resolution and comparative analyses across plant species at the cellular level. This data will fuel the establishment of new synthetic biology strategies to enhance plant biology.
Plant development
The PCA could unlock answers to questions that require understanding the molecular changes in cells during development. The potential to characterize embryonic development comprehensively at single-cell resolution and in the multicellular context of complex tissues could help decipher the ‘rules’ underlying the earliest stages of plant tissue formation (ten Hove et al., 2015). In post-embryonic development, plant meristems are remarkable multicellular machines, continually producing new cells that must be specified to fate and then guided through phases of maturation (Esau, 1953; Sinnott, 1960). A major challenge is to understand the mechanisms that coordinate these cell maturation and fate specification events within meristems. Furthermore, plants present a fascinating contrast to mammalian systems with respect to the plasticity they maintain (Borges, 2008). For example, perennial plants often show meristematic plasticity, where only a subset of meristems per season are activated, and with the meristematic activity frequently modulated in response to external and internal cues (Tylewicz et al., 2018). The power of single-cell omics to capture the dynamic molecular coordination of tissue maturation at fine scales promises to help advance these classic questions in plant development (McFaline-Figueroa et al., 2020).
Response to the environment
As for problems in development, how plants respond to environmental stimuli requires understanding the molecular changes taking place in individual cells and how these changes are communicated cell to cell, across tissues and among organisms. An example can be found in how the threat of invasion of foreign biota into cellular and intercellular spaces often induces localized hypersensitive responses of programmed cell death (Jones and Dangl, 2006; Ngou et al., 2021). The question remains as to how the signals induced at the attack sites are communicated to neighboring cells and propagated to distal regions. Do hidden pre-patterned states among seemingly homogenous cells help the plant anticipate attacks? Or is there heterogeneity in cellular responses that could reveal a more complex response to infection? How do these processes differ between susceptible and resistant plant varieties? Heterogeneous responses to infection around invasion sites, as well as differences in molecular signals that correlate with cellular immunity, could provide new insights into how successful defense responses are coordinated.
A parallel set of questions emerge from biotic interactions involved in symbioses, such as the ancient association of land plants with mycorrhizal fungi, or more recent symbioses like those formed by members of the nitrogen-fixing Fabids clade (Wang and Qiu, 2006). In the latter case, infection by the symbiont through the root hairs takes place asynchronously, so bulk sampling often leads to profiles that are a heterogeneous mixture of cell states (Libault et al., 2010). Can new technologies help to gain a discrete view of how individual cells respond to the symbiont over time? Can fresh insights be gained into plant-symbiont interactions by studying root cells and specialized symbiont structures (i.e., arbuscules, nodules) simultaneously? In addition, can the chain of cellular communication in the plant throughout the stages of microbial colonization be better understood? Lastly, by comparing these processes across plants with differential symbiotic specificities, is it possible to start understanding the hallmarks of a robust symbiotic relationship?
Cellular evolution
From rhizoids to pollen, plants have evolved many new specialized cell types, allowing adaptation to new ecological niches. In addition, specific cell types in the root harbor molecular mechanisms to perceive and respond to environmental stimuli, suggesting they may act as ‘gatekeepers’ for these responses (Henderson and Gilliham, 2015). What new perspectives on cellular evolution could we gain by producing comparable single-cell omics data sets on strategically selected species within the plant kingdom? Previous attempts to use expression patterns of homologous genes in distantly related species to assess functional orthology have provided insights (Adamski et al., 2020). However, bulk tissues collected in different species may have substantially different composition of cell types (Huang and Schiefelbein, 2015). Furthermore, the presence of novel cell types in some species may be obscured in whole organ samples (Kajala et al., 2021). By focusing on the fundamental unit of organs – cell types and cell states – comparative studies of species representing key plant lineages could identify minimal molecular toolkits driving conserved plant cellular functions. In addition, these single-cell approaches could highlight the differences in developmental pathways that lead to phenotypic diversity and adaptation to a myriad of environmental conditions.
Technical challenges faced in the development of the PCA
Critical challenges must be overcome to achieve the data generation, analysis and software development milestones of the PCA (Figure 2). Many mirror those faced by the larger single-cell initiatives that include animal models (Lähnemann et al., 2020), but we focus here on those that specifically affect the plant research community (summarized in Box 1).
Isolating individual plant cells
A major challenge is dissociating plant cells: due to their rigid cell walls, some tissues and many species are recalcitrant to digestion (Himmel et al., 2007; Yoo et al., 2007). This feature limits the ability to sample mature tissues that often mediate environmental stresses. In addition, it can be difficult to isolate even young, meristematic cells in some key species that present cellular innovations. One emerging approach that has the potential to address this issue in plants is single-nucleus profiling, which avoids the need for cell wall digestion (Giacomello, 2021). Such nuclear isolation methods have already shown promise (Farmer et al., 2021; Marand et al., 2021). Recently developed proximity labeling approaches bypass the need for isolating cells or organelles by tagging proteins and RNAs near a target protein in living cells and organisms. These methods can profile components of protein complexes, dynamic protein-protein interactions, specific cell types and subcellular locations of proteins or RNAs (Zhang et al., 2019a; Mair et al., 2019; Huang et al., 2020; Kim et al., 2019; Wang et al., 2019).
Tracking cell provenance
Another challenge is mapping cells (or nuclei) back to their tissue location in the whole plant. Methods that can rapidly and conveniently generate ‘ground truth’ markers of cell types in situ will be especially important as more tissues and species with few existing markers are profiled. Adapting so-called spatial transcriptomic approaches to plants will be a critical tool. In particular, untargeted methods (that is, methods based on unbiased sequencing rather than hybridization of a limited set of predefined probes) have the potential for the resolution and throughput needed (Giacomello et al., 2017). In addition, nomenclature systems will be necessary to link the reference maps from different species that have varying organ anatomy and specialized cell types. The hierarchical approach proposed by the common coordinate framework for humans can serve as a starting point (Rood et al., 2019). However, unlike the coordinate systems used in animal organ atlases which focus on accounting for differences between individuals, plant coordinate systems should instead be geared toward discovering differences among representative species and populations. Any such system should be able to accommodate differences in cell type composition (Huang and Schiefelbein, 2015) and the development of novel cell types (Kajala et al., 2021).
Gene homology
Another set of issues surrounds gene homology in comparisons across populations and species. Plants have a well-known capacity for duplication at many scales, ranging from single gene to whole genome. An informative molecular profiling of cells will require high-quality genome annotations that consider the gene family expansion, gene loss and complex orthology relationships which frequently occur when extensive gene duplication follows speciation. Subfunctionalization and neofunctionalization can complicate assumptions about functional orthologs. On the other hand, difficulty identifying distant homologs that share low sequence similarity could obscure functional conservation. Advancement in sequence alignment methods, such as incorporating structural information, could help to discover these distant homologs (Morton et al., 2020). This will probably be an iterative process, where increased resolution of gene expression at the highly resolved cellular or subcellular level could allow inferences about functional orthology across species in large gene families.
Building the database and infrastructure of the PCA
Collection, standardization, curation, integration and visualization of data are key elements of a PCA platform, and the core PCA infrastructure should function as a data resource onto which services and tools are built.
Experimental and analytical standards
Establishing a vetted and community-approved set of experimental and analytical standards will be important. Such an approach was key to the success of the Encyclopedia of DNA Elements project, which ensured that data from multiple international laboratories were easily compared and integrated (ENCODE Project Consortium, 2011). Likewise, the 1001 Arabidopsis Genomes website provided a user-friendly interface to download all sequencing data as phases were completed – allowing the community to begin analyses and discoveries even before publication – and was complemented with an interactive tool to explore phenotypes (Seren et al., 2016) and their genomic associations (Togninalli et al., 2018). The standards were made easily available and updated over time as technologies advanced.
These platforms serve as data infrastructure blueprints as well as knowledge banks and inspiration. This would allow the PCA to be extended from an atlas of a single reference species and clone, to a set of atlases capturing natural variation (within and between species) in cell types and functions. For example, the interactive PCA platform could hyperlink the gene found in each tissue or cell type to the web portal of that gene in natural variation panels of the species of interest (such as the AraGWAS Catalog, Togninalli et al., 2020). Data structure scaffolds in the PCA could also be created so that the template can be replicated across genotypes of a species (e.g. different ecotypes) or different species, and allow users to select their desired organism or compare between the atlases.
Data visualization and exploration tools
In addition to data analysis and interpretation, the PCA infrastructure should include tools that provide effective ways to visualize real or virtual plant cells, and the dynamic processes that form and define them. This could enable hypothesis-generating in silico experiments that predict how plant cell types would respond to stimuli, or the properties of cells with a set of desired features. Creating these digital cells, tissues and other structures would require developing modeling, simulation, artificial intelligence, as well as data visualization and exploration tools similar to those being set up for other projects such as the OpenWorm (Sarma et al., 2018). Computational modeling and simulation-based predictions could then inform experimental work, the results of which could feed back into the PCA to improve models and predictions (Radivojević et al., 2020; Zhang et al., 2020).
Curating the PCA databases
To achieve the PCA’s vision of building a 4D representation of a developing plant, a wealth of existing data, resources and tools will need to be leveraged. For example, it is now routine to deposit next-generation sequencing, processed expression data, proteomics, metabolomics and protein structures in numerous repositories worldwide (Deutsch et al., 2017; Kaminuma et al., 2010; Leinonen et al., 2011; NCBI Resource Coordinators, 2018; wwPDB consortium, 2019). A combination of automated searches and manual curation of datasets from existing data repositories can be used to build the PCA data infrastructure. Reference genome databases such as TAIR, Phytozome, Gramene, EnsemblPlants and others that provide gene and pathway annotation resources (e.g. Gene Ontology) could enable an initial assessment of gene structure, location and function (Berardini et al., 2015; Goodstein et al., 2012; Howe et al., 2020; Tello-Ruiz et al., 2021; The Gene Ontology Consortium, 2019).
Building a platform that brings these data together with data exploration and visualization tools that allow a user to explore at multiple scales, from molecular to cellular to organismal, is a challenge. To this end, single-cell datasets should be made available in both raw and processed data formats and linked from the public-facing PCA website. This resource could additionally be populated using automated literature search tools, followed by manual curation by the PCA community. A selection of high-quality ‘reference’ single-cell datasets directly hosted by the PCA may also prove to be useful for benchmarking purposes. For all datasets, curation standards should be imposed to include minimal metadata falling into several categories: (1) experimental metadata should include a project name, details regarding how plants were grown, treated and sampled, as well as quality control metrics and definitions used to evaluate sample integrity; (2) cell metadata should include a cell identifier (where applicable), cell type annotations and other cell-level data that have come from standard or specific analysis of the dataset; and (3) gene metadata that include structural and functional annotations should be required.
In addition to providing links to data repositories, the PCA portal should include web-based tools to generate, access and query relevant data and metadata using a standardized ontology for metadata, similar to the Single Cell Expression Atlas (Papatheodorou et al., 2020) and the Human Cell Atlas implementation of metadata types. This will facilitate interoperability of datasets. Depending on the data type, scripts that implement standardized pipelines for single-cell data analysis could also be made available that reflect the current best practices, including those that implement methods for cross-platform and multimodal data integration as well as label transfer (e.g., Lotfollahi et al., 2020; Hie et al., 2019; Korsunsky et al., 2019; Kang et al., 2021; Stuart et al., 2019). Version-controlled source code that demonstrates analyses using the information in the PCA as a starting point should also be made available. These resources should promote greater use of the rapidly growing number of single-cell datasets.
Multiscale networks can provide an intuitive method to discretize and integrate different data types (Duran-Nebreda and Bassel, 2017). Other informatics frontiers that will be useful to explore include multi-dimensional image visualization and analysis tools such as Napari and Squidpy (Sofroniew et al., 2021; Palla et al., 2021), graph-based knowledgebases for integrating datasets (e.g., Reactome; Fabregat et al., 2018), natural language processing for automating curation (Braun and Lawrence-Dill, 2020), as well as machine learning and artificial intelligence methods for integrating heterogeneous data (Ma et al., 2020).
Challenges to the PCA infrastructure
Despite the widespread availability of data types described above, additional data that need to be integrated to achieve the PCA vision (metabolomics, imaging and phenotypic observations) are currently available in a variety of heterogeneous sources such as data sharing platforms (e.g. EMBL-EBI, Dryad, Figshare, and Zenodo), institutional data repositories, researcher websites or embedded within scientific publication records. A lack of centrality in data deposition makes these data less accessible.
In addition, methods for data standardization and curation will be critical tools to mitigate challenges with existing data, create best practices for collecting new data and ensure consistency to allow for data exchange and interoperability (Sansone et al., 2012). Experiments, datasets and other resources should be described in sufficient detail to be easily accessible and reproducible. Validating data with resource description frameworks is a means to ensure data compliance against the conceptual model it follows, data consistency and completeness. Data records and metadata should be machine-readable by using standardized vocabularies (i.e. cell type ontologies; Bard et al., 2005) to enable interoperability. Existing solutions and concepts of such ontologies are broadly applied in vertebrates and are available for plants, though they need to be widely adopted by the PCA community (Avraham et al., 2008; Bard et al., 2005 ; Diehl et al., 2016; Ilic et al., 2007; Xia and Yanai, 2019). Mapping ontologies to the 4D representation of the PCA will facilitate linking and visualizing the growing knowledge of plant cells.
Originality of the PCA infrastructure
The long-term vision for the PCA infrastructure is similar in scope to other moonshot ideas such as the Human Cell Atlas (Regev et al., 2017), the Transparent Plant (Henkhaus et al., 2020) and the Virtual Plant (Kost, 2001). However, the PCA vision is different in several important ways. The Human Cell Atlas is for a single organism with over 200 cell types, whereas the PCA community embraces cell atlas data from a diverse array of plants. A typical plant has about 100 cell types (https://www.ebi.ac.uk/ols/ontologies/po; Ilic et al., 2007). Understanding the diversity and origin of cell types across the plant lineage will be a central part of the initiative, as illustrated in Box 2 with the chloroplast as an example. The PCA is an essential step towards realizing the vision of a Transparent Plant, a simulated environment where virtual plants can be built from parts and behaviors (Henkhaus et al., 2020). In order to materialize such a simulation environment, where the parts are and how they work together must be understood. Finally, we see the Virtual Plant database (Katari et al., 2010) as an essential component of the PCA vision and infrastructure.
Potential sources of funding for the PCA
The PCA will require adequate funding to achieve its full potential – including translational impact for agriculture (Bartuska, 2017; Bol et al., 2018; Gök et al., 2016; Hu, 2020; Jaffe et al., 2015; Rosenbloom et al., 2015). As the PCA has an international reach, programs that facilitate collaboration across borders will be valuable in advancing the goals of the initiative, such as partnering programs by the Biotechnology and Biological Sciences Research Council (BBSRC) in the United Kingdom, European Commission’s Horizon Europe and the United States’ Fulbright scholars program. Similar programs exist that foster alliances between scientists from the US with those in Japan, Israel and Germany; and between India and other countries. As big data, nanotechnology, computation and artificial intelligence have now become an integral part of biological research, funds to support the interdisciplinary vision of PCA will be important to foster integration of these disciplines.
While governments are the major sources of funding for basic research, their level of funding has been steadily decreasing (Khan et al., 2020). Foundations are the major source of private basic research funding and are less constrained by time, politics and topic. They have a high potential for funding initiatives in emerging areas such as the PCA. However, it is challenging for foundations and individuals to find worthy initiatives. To fill this gap, the Science Philanthropy Alliance was established. One of their success stories is the Chan Zuckerberg Initiative providing substantial funds to develop technologies for the Human Cell Atlas initiative. Such a partnership for the PCA would take its vision to new heights.
Industry is another potential source of funding. Over 70% of applied research and development in the US is funded by industry (Khan et al., 2020). A thorough understanding of plant systems is essential for designing effective agro-biotech solutions leading to new crop varieties, and to innovative crop protection products that can leverage sustainable food production. A partnership with the PCA initiative would accelerate development of new solutions for customers of the agbiotech industry. Thus, funding and collaborations, ranging over multiple disciplines and countries, and multiway interactions amongst academia, industry and philanthropy will be essential to realize the vision of the PCA.
Conclusion and outlook
Future economies will be increasingly plant-based. Biomass is projected to be a major source of primary energy by 2050 (Reid et al., 2020), plant-based meat and dairy products are already transforming the food industry (Shepon et al., 2018), and plant-based vaccines against several diseases are increasingly being researched (reviewed by Shahid and Daniell, 2016). With the rapidly changing climate, economy and values in our society, now is the time to reimagine plant science as a critical component of the future not only for agriculture, but also the environment, energy, health care, manufacturing and technology.
Today, plant science constitutes a minority of life sciences in terms of funding and workforce. To meet the demands of the future economy, we need to strengthen plant science, attract talent from other fields and train the next generation of plant scientists (Henkhaus et al., 2020). In 2004, the evolutionary biologist John Avise wrote that ‘‘despite nearly three decades of experience with recombinant DNA techniques, the ultimate contribution to the broader human enterprise remains uncertain’’ (Avise, 2004). Two decades after this statement, in 2020, recombinant DNA technology ushered in the beginning of an end to a global pandemic that cost millions of lives, through a record-breaking runtime of development and deployment of mRNA and DNA-based viral vaccines. While still at its infancy, we hope that the PCA initiative has the potential to serve as a nucleator, bringing together scientists and engineers from a wide range of fields to solve fundamental problems in plant biology with innovative solutions from emerging technologies.
Acknowledgements
The PCA community-building activities are funded in part by the National Science Foundation grant numbers MCB-1916797 and MCB-2052590, Carnegie Institution for Science, and BASF. We thank Emily Fryer, Nick Melosh, Heather Meyer, Jason Thomas, Terri Tippets, Renate Weizbauer, Zhiyong Wang and Kangmei Zhao for helping with organizing the first workshop. We thank Emily Fryer and Julie Gosse at the Science Editors Network for developing the PCA website. We are grateful to the first PCA workshop steering committee members Jim Haseloff, David Jackson, Edward Marcotte, John Marioni, Marisa Otegui, Alberto Salleo, Waltraud Schulze, Edgar Spalding, Michael Sussman, Marja Timmermans and HS Philip Wong for their guidance and support. We thank Rachel Shahan, Shouling Xu, Kevin Cox and Erin Zess for their input in developing the manuscript. Some images in the figures were created with BioRender.com.
Biographies
Suryatapa Ghosh Jha is in the Department of Plant Biology, Carnegie Institution for Science, Stanford, United States
Alexander T Borowsky is in the Department of Botany and Plant Sciences, University of California, Riverside, Riverside, United States
Benjamin J Cole is in the Joint Genome Institute, Lawrence Berkeley National Laboratory, Walnut Creek, United States
Noah Fahlgren is in the Donald Danforth Plant Science Center, St. Louis, United States
Andrew Farmer is in the National Center for Genome Resources, Santa Fe, United States
Shao-shan Carol Huang is in the Center for Genomics and Systems Biology, New York University, New York, United States
Purva Karia is in the Department of Plant Biology, Carnegie Institution for Science, Stanford, United States and the Department of Cell and Systems Biology, University of Toronto, Toronto, Canada
Marc Libault is in the Department of Agronomy and Horticulture, University of Nebraska-Lincoln, Lincoln, United States
Nicholas J Provart is in the Department of Cell and Systems Biology and the Centre for the Analysis of Genome Evolution and Function, University of Toronto, Toronto, Canada
Selena L Rice is in the Department of Plant Biology, Carnegie Institution for Science, Stanford, United States
Maite Saura-Sanchez is in the Consejo Nacional de Investigaciones Científicas y Técnicas, Instituto de Investigaciones Fisiológicas y Ecológicas Vinculadas a la Agricultura, Facultad de Agronomía, Universidad de Buenos Aires, Buenos Aires, Argentina
Pinky Agarwal is in the National Institute of Plant Genome Research, New Delhi, India
Amir H Ahkami is in the Environmental Molecular Sciences Division, Pacific Northwest National Laboratory, Richland, United States
Christopher R Anderton is in the Environmental Molecular Sciences Division, Pacific Northwest National Laboratory, Richland, United States
Steven P Briggs is in the Department of Biological Sciences, University of California, San Diego, United States
Jennifer AN Brophy is in the Department of Biology, Stanford University, Stanford, United States
Peter Denolf is in the BASF Seeds & Traits, Ghent, Belgium
Luigi F Di Costanzo is in the Department of Agricultural Sciences, University of Naples Federico II, Napoli, Italy
Moises Exposito-Alonso is in the Department of Plant Biology, Carnegie Institution for Science, Stanford, United States
Stefania Giacomello is in the SciLifeLab, KTH Royal Institute of Technology, Solna, Sweden
Fabio Gomez-Cano is in the Department of Biochemistry and Molecular Biology, Michigan State University, East Lansing, United States
Kerstin Kaufmann is in the Department for Plant Cell and Molecular Biology, Institute for Biology, Humboldt-Universitaet zu Berlin, Berlin, Germany
Dae Kwan Ko is in the Great Lakes Bioenergy Research Center, Michigan State University, East Lansing, United States
Sagar Kumar is in the Department of Plant Breeding & Genetics, Mata Gujri College, Fatehgarh Sahib, Punjabi University, Patiala, India
Andrey V Malkovskiy is in the Department of Plant Biology, Carnegie Institution for Science, Stanford, United States
Naomi Nakayama is in the Department of Bioengineering, Imperial College London, London, United Kingdom
Toshihiro Obata is in the Department of Biochemistry, University of Nebraska-Lincoln, Lincoln, United States
Marisa S Otegui is in the Department of Botany, University of Wisconsin-Madison, Madison, United States
Gergo Palfalvi is in the Division of Evolutionary Biology, National Institute for Basic Biology, Okazaki, Japan
Elsa H Quezada-Rodríguez is in the Ciencias Agrogenómicas, Escuela Nacional de Estudios Superiores Unidad León, Universidad Nacional Autónoma de México, León, Mexico
Rajveer Singh is in the School of Agricultural Biotechnology, Punjab Agricultural University, Ludhiana, India
R Glen Uhrig is in the Department of Science, University of Alberta, Edmonton, Canada
Jamie Waese is in the Department of Cell and Systems Biology/Centre for the Analysis of Genome Evolution and Function, University of Toronto, Toronto, Canada
Klaas Van Wijk is in the School of Integrated Plant Science, Plant Biology Section, Cornell University, Ithaca, United States
R Clay Wright is in the Department of Biological Systems Engineering, Virginia Tech, Blacksburg, United States
David W Ehrhardt is in the Department of Plant Biology, Carnegie Institution for Science, Stanford, United States
Kenneth D Birnbaum is in the Center for Genomics and Systems Biology, New York University, New York, United States
Seung Y Rhee is in the Department of Plant Biology, Carnegie Institution for Science, Stanford, United States
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Peter Rodgers, eLife, United Kingdom,
Elsa Loissel, eLife, United Kingdom,
Plant Cell Atlas Consortium:
Jahed Ahmed, Louvain Institute of Biomolecular Science and Technology, UCLouvain, Louvain La Neuve, Belgium.
Oluwafemi Alaba, School of Biology and Ecology, University of Maine, Orono, United States.
Gazala Ameen, Washington State University, Pullman, United States.
Vaishali Arora, University of Delhi, Delhi, India.
Mario A Arteaga-Vazquez, Universidad Veracruzana, Xalapa, Mexico.
Alok Arun, Inter American University of Puerto Rico, Barranquitas, United States.
Julia Bailey-Serres, Center for Plant Cell Biology and Department of Botany and Plant Sciences, University of California, Riverside, Riverside, United States.
Laura E Bartley, Washington State University, Pullman, United States.
George W Bassel, University of Warwick, Coventry, United Kingdom.
Dominique C Bergmann, Stanford University and Howard Hughes Medical Institute, Stanford, United States.
Edoardo Bertolini, Donald Danforth Plant Science Center, St. Louis, United States.
Kaushal Kumar Bhati, Louvain Institute of Biomolecular Sciences, Catholic University of Louvain, Louvain La Neuve, Belgium.
Noel Blanco-Touriñán, Plant Vascular Development Group, ETH Zürich, Zurich, Switzerland.
Steven P Briggs, University of California San Diego, La Jolla, United States.
Javier Brumos, Instituto de Biologia Molecular y Celular de Plantas (IBMCP) Consejo Superior de Investigaciones Científicas (CSIC), Valencia, Spain.
Benjamin Buer, Bayer AG, Monheim am Rhein, Germany.
Adrien Burlaocot, HHMI, Department of Plant and Microbial Biology, University of California, Berkeley, Berkeley, United States.
Sergio Alan Cervantes-Pérez, LANGEBIO-CINVESTAV, Irapuato, Mexico.
Sixue Chen, Department of Biology, University of Florida, Gainesville, United States.
Bruno Contreras-Moreira, European Bioinformatics Institute - EMBL, Hinxton, United Kingdom.
Francisco J CORPAS, Estación Experimental del Zaidin (CSIC), Granada, Spain.
Alfredo Cruz-Ramirez, National Laboratory of Genomics for Biodiversity CINVESTAV, Irapuato, Mexico.
Cesar L Cuevas-Velazquez, Departamento de Bioquímica, Facultad de Química, Universidad Nacional Autónoma de México, Cd., Irapuato, Mexico.
Josh T Cuperus, University of Washington, Seattle, United States.
Lisa I David, North Carolina State University, Raleigh, United States.
Stefan de Folter, Unidad de Genómica Avanzada (UGA-LANGEBIO), Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional (CINVESTAV-IPN), Irapuato, Mexico.
Peter H Denolf, BASF Innovation Center Gent, Zwijnaarde, Belgium.
Pingtao Ding, Institute of Biology Leiden, Leiden University, Leiden, Netherlands.
William P Dwyer, Department of Plant Biology, Carnegie Institution for Science, Stanford, United States.
Matthew MS Evans, Department of Plant Biology, Carnegie Institution for Science, Stanford, United States.
Nancy George, European Bioinformatics Institute, Cambridge, United Kingdom.
Pubudu P Handakumbura, Environmental Molecular Sciences Division, Pacific Northwest National Laboratory, Richland, United States.
Maria J Harrison, Boyce Thompson Institute, Ithaca, United States.
Elizabeth S Haswell, Washington University in St. Louis, St. Louis, United States.
Venura Herath, University of Peradeniya, Peradeniya, Sri Lanka.
Yuling Jiao, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing, China.
Robert E Jinkerson, University of California, Riverside, Riverside, United States.
Uwe John, Alfrd-Wegener-Institute Helmholtz centre for Polar and Marine Research, Bremerhaven, Germany.
Sanjay Joshi, University of Kentucky, Lexington, United States.
Abhishek Joshi, Mohanlal Sukhadia University, Udaipur, India.
Lydia-Marie Joubert, Stanford University, Stanford, United States.
Ramesh Katam, Florida A&M University, Tallahassee, United States.
Harmanpreet Kaur, Punjab Agricultural University, Ludhiana, India.
Yana Kazachkova, Weizmann Institute of Science, Rehovot, Israel.
Sunil K Kenchanmane Raju, Michigan State University, East Lansing, United States.
Mather A Khan, Heinrich Heine University, Dusseldorf, Germany.
Rajdeep Khangura, Purdue University, West Lafayette, United States.
Ajay Kumar, ICAR-NIPB, New Delhi, India.
Arun Kumar, Biotechnology Division, CSIR-Institute of Himalayan Bioresource Technology, Palampur, India.
Pankaj Kumar, Dr Yashwant Singh Parmar University of Horticulture and Forestry Nauni Solan Himachal Pradesh, Solan, India.
Pradeep Kumar, Centre for Cellular & Molecular Biology, Hyderabad, India.
Dhruv Lavania, University of Alberta, Edmonton, Canada.
Tedrick Thomas Salim Lew, Institute of Materials Research and Engineering, Singapore, Singapore.
Mathew G Lewsey, La Trobe University, Bundoora, Australia.
Chien-Yuan Lin, Joint BioEnergy Institute, Lawrence Berkeley National Laboratory, Emeryville, United States.
Dianyi Liu, Donald Danforth Plant Science Center, Saint Louis, United States.
Le Liu, Department of Biology, University of Massachusetts Amherst, Amherst, United States.
Tie Liu, University of Florida, Gainesville, United States.
Ansul Lokdarshi, University of Tennessee, Knoxville, United States.
Ai My Luong, Louvain Institute of Biomolecular Science and Technology, Louvain-la-Neuve, Belgium.
Iain C Macaulay, Earlham Institute, Norwich, United Kingdom.
Sakil Mahmud, Plant Cell Biology, IZMB, University of Bonn, Bonn, Germany.
Ari Pekka Mähönen, Institute of Biotechnology, HiLIFE, University of Helsinki, Helsinki, Finland.
Kamal Kumar Malukani, Molecular Biology, Hyderabad, India.
Alexandre P Marand, University of Georgia, Athens, United States.
Carly A Martin, MIT, Cambridge, United States.
Claire D McWhite, Princeton University, Princeton, United States.
Devang Mehta, University of Alberta, Edmonton, Canada.
Miguel Miñambres Martín, Heinrich Heine University, Düsseldorf, Germany.
Jenny C Mortimer, University of Adelaide, Adelaide, Australia.
Lachezar A Nikolov, University of California, Los Angeles, Los, Angeles, United States.
Tatsuya Nobori, Salk Institute for Biological Studies, San, Diego, United States.
Trevor M Nolan, Duke University, Durham, United States.
Aaron J Ogden, Pacific Northwest National Laboratory, Richland, United States.
Marisa S Otegui, University of Wisconsin-Madison, Madison, United States.
Mark-Christoph Ott, Bayer AG, Monheim am Rhein, Germany.
José M Palma, Estación Experimental del Zaidín, CSIC, Granada, Spain.
Puneet Paul, University of Nebraska-Lincoln, Lincoln, United States.
Atique U Rehman, Department of Agronomy, Bahauddin Zakariya University Multan, Multan, Pakistan.
Maida Romera-Branchat, Institute of Plant Biology and Biotechnology, University of Münster, Muenster (Germany), Germany.
Luis C Romero, Instituto de Bioquimica Vegetal y Fotosintesis, CSIC-US, Seville, Spain.
Ronelle Roth, Department of Plant Sciences, University of Oxford, Oxford, United Kingdom.
Saroj K Sah, The Pennsylvania State University, University Park, United States.
Rachel Shahan, Duke University, Durham, United States.
Shyam Solanki, Washington State University, Pullman, United States.
Bao-Hua Song, University of North Carolina at Charlotte, Charlotte, United States.
Rosangela Sozzani, North Carolina State University, Raleigh, United States.
Gary Stacey, University of Missouri, Columbia, United States.
Anna N Stepanova, North Carolina State University, Raleigh, United States.
Nicolas L Taylor, The University of Western Australia, Perth, Australia.
Marcela K Tello-Ruiz, Cold Spring Harbor Laboratory, Cold Spring Harbor, United States.
Tuan M Tran, Nanyang Technological University, Singapore, Singapore.
Rajiv Kumar Tripathi, University of Manitoba, Winnipeg, Canada.
Batthula Vijaya Lakshmi Vadde Vadde, Cornell University, Ithaca, United States.
Tamas Varga, Environmental Molecular Sciences Division, Pacific Northwest National Laboratory, (PNNL), Richland, United States.
Marija Vidovic, Institute of Molecular Genetics and Genetic Engineering, University of Belgrade, Belgrade, Serbia.
Justin W Walley, Iowa State University, Ames, United States.
Zhiyong Wang, Carnegie Institution for Science, Dept. of Plant Biology, Stanford, United States.
Renate A Weizbauer, Carnegie Institution for Science, Dept. of Plant Biology, Stanford, United States.
James Whelan, La Trobe University, Melbourne, Australia.
Asela J Wijeratne, Arkansas State University, Jonesboro, United States.
Tingting Xiang, University of North Carolina at Charlotte, Charlotte, United States.
Shouling Xu, Carnegie Institution for Science, Stanford, United States.
Ramin Yadegari, University of Arizona, Tucson, United States.
Houlin Yu, Department of Biochemistry and Molecular Biology, University of Massachusetts Amherst, Amherst, United States.
Hai Ying Yuan, University of Saskatchewan, Saskatoon, Canada.
Fabio Zanini, UNSW, Sydney, Australia.
Feng Zhao, Laboratoire Reproduction et Développement des Plantes, Université de Lyon, Lyon, France.
Jie Zhu, University of California, Davis, Davis, United States.
Xiaohong Zhuang, Centre for Cell and Developmental Biology, State Key Laboratory of Agrobiotechnology, School of Life Sciences, The Chinese University of Hong Kong, Hong Kong SAR, Shatin, Hong Kong.
Funding Information
This paper was supported by the following grants:
National Science Foundation 1916797 to David W Ehrhardt, Kenneth D Birnbaum, Seung Yon Rhee.
National Science Foundation 2052590 to Seung Yon Rhee.
Additional information
Competing interests
No competing interests declared.
Author contributions
Writing - review and editing.
Supervision, Writing - original draft, Writing - review and editing.
Supervision, Visualization, Writing - original draft, Writing - review and editing.
Writing - original draft, Writing - review and editing.
Supervision, Writing - original draft, Writing - review and editing.
Supervision, Writing - original draft, Writing - review and editing.
Supervision, Writing - original draft, Writing - review and editing.
Visualization, Writing - original draft, Writing - review and editing.
Visualization, Writing - original draft, Writing - review and editing.
Supervision, Writing - original draft, Writing - review and editing.
Supervision, Writing - original draft, Project administration, Writing - review and editing.
Visualization, Writing - original draft, Writing - review and editing.
Writing - original draft, Writing - review and editing.
Writing - original draft, Writing - review and editing.
Writing - original draft, Writing - review and editing.
Writing - original draft, Writing - review and editing.
Writing - original draft, Writing - review and editing.
Writing - original draft, Writing - review and editing.
Writing - original draft, Writing - review and editing.
Writing - original draft, Writing - review and editing.
Writing - original draft, Writing - review and editing.
Writing - original draft, Writing - review and editing.
Writing - original draft, Writing - review and editing.
Writing - original draft, Writing - review and editing.
Visualization, Writing - original draft, Writing - review and editing.
Writing - original draft, Writing - review and editing.
Supervision, Writing - original draft, Writing - review and editing.
Writing - original draft, Writing - review and editing.
Writing - original draft, Writing - review and editing.
Writing - original draft, Writing - review and editing.
Writing - original draft, Writing - review and editing.
Writing - original draft, Writing - review and editing.
Visualization, Writing - review and editing.
Writing - review and editing.
Writing - original draft, Writing - review and editing.
Conceptualization, Funding acquisition, Writing - original draft, Writing - review and editing.
Conceptualization, Funding acquisition, Writing - original draft, Writing - review and editing.
Conceptualization, Supervision, Funding acquisition, Writing - original draft, Project administration, Writing - review and editing.
Data availability
No associated datasets.
References
- Adamski NM, Borrill P, Brinton J, Harrington SA, Marchal C, Bentley AR, Bovill WD, Cattivelli L, Cockram J, Contreras-Moreira B, Ford B, Ghosh S, Harwood W, Hassani-Pak K, Hayta S, Hickey LT, Kanyuka K, King J, Maccaferrri M, Naamati G, Pozniak CJ, Ramirez-Gonzalez RH, Sansaloni C, Trevaskis B, Wingen LU, Wulff BB, Uauy C. A roadmap for gene functional characterisation in crops with large genomes: lessons from polyploid wheat. eLife. 2020;9:e55646. 10.7554/eLife.55646. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Alonso AM, Carrea A, Diambra L. Prediction of cell position using single-cell transcriptomic data: an iterative procedure. F1000Research. 2019;8:1775. 10.12688/f1000research.20715.1. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Avise J. The hope, hype, and reality of genetic engineering: remarkable stories from agriculture, industry, medicine, and the environment. Choice. 2004;1:0283. 10.5860/choice.42-0283. [CrossRef] [Google Scholar]
- Avraham S, Tung C-W, Ilic K, Jaiswal P, Kellogg EA, McCouch S, Pujar A, Reiser L, Rhee SY, Sachs MM, Schaeffer M, Stein L, Stevens P, Vincent L, Zapata F, Ware D. The plant ontology database: a community resource for plant structure and developmental stages controlled vocabulary and annotations. Nucleic Acids Research. 2008;36:D449–D454. 10.1093/nar/gkm908. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Balasubramanian VK, Purvine SO, Liang Y, Kelly RT, Pasa-Tolic L, Chrisler WB, Blumwald E, Stewart CN, Zhu Y, Ahkami AH. Cell-type-specific proteomics analysis of a small number of plant cells by integrating laser capture microdissection with a nanodroplet sample processing platform. Current Protocols. 2021;1:e153. 10.1002/cpz1.153. [Abstract] [CrossRef] [Google Scholar]
- Bard J, Rhee SY, Ashburner M. An ontology for cell types. Genome Biology. 2005;6:R21. 10.1186/gb-2005-6-2-r21. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Barrett T, Suzek TO, Troup DB, Wilhite SE, Ngau WC, Ledoux P, Rudnev D, Lash AE, Fujibuchi W, Edgar R. NCBI GEO: mining millions of expression profiles-database and tools. Nucleic Acids Research. 2005;33:D562–D566. 10.1093/nar/gki022. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Barrett J, Girr P, Mackinder LCM. Pyrenoids: CO2-fixing phase separated liquid organelles. Biochimica Et Biophysica Acta (BBA) - Molecular Cell Research. 2021;1868:118949. 10.1016/j.bbamcr.2021.118949. [Abstract] [CrossRef] [Google Scholar]
- Bartuska A. USDA Testimony. U.S. Department of Agriculture; 2017. https://www.usda.gov/media/testimony [Google Scholar]
- Berardini TZ, Reiser L, Li D, Mezheritsky Y, Muller R, Strait E, Huala E. The Arabidopsis information resource: making and mining the "gold standard" annotated reference plant genome. Genesis. 2015;53:474–485. 10.1002/dvg.22877. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Biermann F, Kanie N, Kim RE. Global governance by goal-setting: the novel approach of the UN sustainable development goals. Current Opinion in Environmental Sustainability. 2017;26-27:26–31. 10.1016/j.cosust.2017.01.010. [CrossRef] [Google Scholar]
- Boehm CR, Bock R. Recent advances and current challenges in synthetic biology of the plastid genetic system and metabolism. Plant Physiology. 2019;179:794–802. 10.1104/pp.18.00767. [Abstract] [CrossRef] [Google Scholar]
- Bol T, de Vaan M, van de Rijt A. The Matthew effect in science funding. PNAS. 2018;115:4887–4890. 10.1073/pnas.1719557115. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Borges RM. Plasticity comparisons between plants and animals: concepts and mechanisms. Plant Signaling & Behavior. 2008;3:367–375. 10.4161/psb.3.6.5823. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Bouchnak I, van Wijk KJ. N-Degron pathways in plastids. Trends in Plant Science. 2019;24:917–926. 10.1016/j.tplants.2019.06.013. [Abstract] [CrossRef] [Google Scholar]
- Braun IR, Lawrence-Dill CJ. Automated methods enable direct computation on phenotypic descriptions for novel candidate gene prediction. Frontiers in Plant Science. 2020;10:1629. 10.3389/fpls.2019.01629. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Brausemann A, Gemmecker S, Koschmieder J, Ghisla S, Beyer P, Einsle O. Structure of phytoene desaturase provides insights into herbicide binding and reaction mechanisms involved in carotene desaturation. Structure. 2017;25:1222–1232. 10.1016/j.str.2017.06.002. [Abstract] [CrossRef] [Google Scholar]
- Camp JG, Platt R, Treutlein B. Mapping human cell phenotypes to genotypes with single-cell genomics. Science. 2019;365:1401–1405. 10.1126/science.aax6648. [Abstract] [CrossRef] [Google Scholar]
- Costanza R, Fioramonti L, Kubiszewski I. The UN sustainable development goals and the dynamics of well-being. Frontiers in Ecology and the Environment. 2016;14:59. 10.1002/fee.1231. [CrossRef] [Google Scholar]
- Decaestecker W, Buono RA, Pfeiffer ML, Vangheluwe N, Jourquin J, Karimi M, Van Isterdael G, Beeckman T, Nowack MK, Jacobs TB. CRISPR-TSKO: a technique for efficient mutagenesis in specific cell types, tissues, or organs in Arabidopsis. The Plant Cell. 2019;31:2868–2887. 10.1105/tpc.19.00454. [Abstract] [CrossRef] [Google Scholar]
- Deutsch EW, Csordas A, Sun Z, Jarnuczak A, Perez-Riverol Y, Ternent T, Campbell DS, Bernal-Llinares M, Okuda S, Kawano S, Moritz RL, Carver JJ, Wang M, Ishihama Y, Bandeira N, Hermjakob H, Vizcaíno JA. The ProteomeXchange consortium in 2017: supporting the cultural change in proteomics public data deposition. Nucleic Acids Research. 2017;45:D1100–D1106. 10.1093/nar/gkw936. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Diehl AD, Meehan TF, Bradford YM, Brush MH, Dahdul WM, Dougall DS, He Y, Osumi-Sutherland D, Ruttenberg A, Sarntivijai S, Van Slyke CE, Vasilevsky NA, Haendel MA, Blake JA, Mungall CJ. The cell ontology 2016: enhanced content, modularization, and ontology interoperability. Journal of Biomedical Semantics. 2016;7:44. 10.1186/s13326-016-0088-7. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Duran-Nebreda S, Bassel GW. Bridging scales in plant biology using network science. Trends in Plant Science. 2017;22:1001–1003. 10.1016/j.tplants.2017.09.017. [Abstract] [CrossRef] [Google Scholar]
- Edwards GE, Franceschi VR, Voznesenskaya EV. Single-cell C(4) photosynthesis versus the dual-cell (Kranz) paradigm. Annual Review of Plant Biology. 2004;55:173–196. 10.1146/annurev.arplant.55.031903.141725. [Abstract] [CrossRef] [Google Scholar]
- ENCODE Project Consortium A user's guide to the encyclopedia of DNA elements (ENCODE) PLOS Biology. 2011;9:e1001046. 10.1371/journal.pbio.1001046. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Erlinghaeuser M, Hagenau L, Wimmer D, Offermann S. Development, subcellular positioning and selective protein accumulation in the dimorphic chloroplasts of single-cell C4 species. Current Opinion in Plant Biology. 2016;31:76–82. 10.1016/j.pbi.2016.03.017. [Abstract] [CrossRef] [Google Scholar]
- Esau K. Plant anatomy. Soil Science. 1953;75:407. 10.1097/00010694-195305000-00014. [CrossRef] [Google Scholar]
- Fabregat A, Korninger F, Viteri G, Sidiropoulos K, Marin-Garcia P, Ping P, Wu G, Stein L, D'Eustachio P, Hermjakob H. Reactome graph database: efficient access to complex pathway data. PLOS Computational Biology. 2018;14:e1005968. 10.1371/journal.pcbi.1005968. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Farmer A, Thibivilliers S, Ryu KH, Schiefelbein J, Libault M. Single-nucleus RNA and ATAC sequencing reveals the impact of chromatin accessibility on gene expression in Arabidopsis roots at the single-cell level. Molecular Plant. 2021;14:372–383. 10.1016/j.molp.2021.01.001. [Abstract] [CrossRef] [Google Scholar]
- Giacomello S, Salmén F, Terebieniec BK, Vickovic S, Navarro JF, Alexeyenko A, Reimegård J, McKee LS, Mannapperuma C, Bulone V, Ståhl PL, Sundström JF, Street NR, Lundeberg J. Spatially resolved transcriptome profiling in model plant species. Nature Plants. 2017;3:17061. 10.1038/nplants.2017.61. [Abstract] [CrossRef] [Google Scholar]
- Giacomello S. A new era for plant science: spatial single-cell transcriptomics. Current Opinion in Plant Biology. 2021;60:102041. 10.1016/j.pbi.2021.102041. [Abstract] [CrossRef] [Google Scholar]
- Gök A, Rigby J, Shapira P. The impact of research funding on scientific outputs: evidence from six smaller european countries. Journal of the Association for Information Science and Technology. 2016;67:715–730. 10.1002/asi.23406. [CrossRef] [Google Scholar]
- Goodstein DM, Shu S, Howson R, Neupane R, Hayes RD, Fazo J, Mitros T, Dirks W, Hellsten U, Putnam N, Rokhsar DS. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Research. 2012;40:D1178–D1186. 10.1093/nar/gkr944. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Goring DR, Di Sansebastiano GP. Protein and membrane trafficking routes in plants: conventional or unconventional? Journal of Experimental Botany. 2018;69:1–5. 10.1093/jxb/erx435. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Griggs D, Stafford-Smith M, Gaffney O, Rockström J, Ohman MC, Shyamsundar P, Steffen W, Glaser G, Kanie N, Noble I. Policy: sustainable development goals for people and planet. Nature. 2013;495:305–307. 10.1038/495305a. [Abstract] [CrossRef] [Google Scholar]
- Han X, Kumar D, Chen H, Wu S, Kim JY. Transcription factor-mediated cell-to-cell signalling in plants. Journal of Experimental Botany. 2014;65:1737–1749. 10.1093/jxb/ert422. [Abstract] [CrossRef] [Google Scholar]
- Han X, Wang R, Zhou Y, Fei L, Sun H, Lai S, Saadatpour A, Zhou Z, Chen H, Ye F, Huang D, Xu Y, Huang W, Jiang M, Jiang X, Mao J, Chen Y, Lu C, Xie J, Fang Q, Wang Y, Yue R, Li T, Huang H, Orkin SH, Yuan G-C, Chen M, Guo G. Mapping the mouse cell atlas by Microwell-Seq. Cell. 2018;172:1091–1107. 10.1016/j.cell.2018.02.001. [Abstract] [CrossRef] [Google Scholar]
- Han S, Maberly SC, Gontero B, Xing Z, Li W, Jiang H, Huang W. Structural basis for C4 photosynthesis without Kranz anatomy in leaves of the submerged freshwater plant Ottelia alismoides. Annals of Botany. 2020;125:869–879. 10.1093/aob/mcaa005. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Harrison MJ, Dewbre GR, Liu J. A phosphate transporter from Medicago truncatula involved in the acquisition of phosphate released by arbuscular mycorrhizal fungi. The Plant Cell. 2002;14:2413–2429. 10.1105/tpc.004861. [Abstract] [CrossRef] [Google Scholar]
- Heinze L, Freimuth N, Rößling AK, Hahnke R, Riebschläger S, Fröhlich A, Sampathkumar A, McFarlane HE, Sauer M. EPSIN1 and MTV1 define functionally overlapping but molecularly distinct trans-Golgi network subdomains in Arabidopsis. PNAS. 2020;117:25880–25889. 10.1073/pnas.2004822117. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Henderson SW, Gilliham M. In: Molecular Mechanisms in Plant Adaptation. Laitinen R. A. E, editor. John Wiley & Sons, Inc; 2015. pp. 83–115. [CrossRef] [Google Scholar]
- Henkhaus N, Bartlett M, Gang D, Grumet R, Jordon-Thaden I, Lorence A, Lyons E, Miller S, Murray S, Nelson A, Specht C, Tyler B, Wentworth T, Ackerly D, Baltensperger D, Benfey P, Birchler J, Chellamma S, Crowder R, Donoghue M, Dundore-Arias JP, Fletcher J, Fraser V, Gillespie K, Guralnick L, Haswell E, Hunter M, Kaeppler S, Kepinski S, Li FW, Mackenzie S, McDade L, Min Y, Nemhauser J, Pearson B, Petracek P, Rogers K, Sakai A, Sickler D, Taylor C, Wayne L, Wendroth O, Zapata F, Stern D. Plant science decadal vision 2020-2030: reimagining the potential of plants for a healthy and sustainable future. Plant Direct. 2020;4:e00252. 10.1002/pld3.252. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Hie B, Bryson B, Berger B. Efficient integration of heterogeneous single-cell transcriptomes using scanorama. Nature Biotechnology. 2019;37:685–691. 10.1038/s41587-019-0113-3. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Himmel ME, Ding SY, Johnson DK, Adney WS, Nimlos MR, Brady JW, Foust TD. Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science. 2007;315:804–807. 10.1126/science.1137016. [Abstract] [CrossRef] [Google Scholar]
- Howe KL, Contreras-Moreira B, De Silva N, Maslen G, Akanni W, Allen J, Alvarez-Jarreta J, Barba M, Bolser DM, Cambell L, Carbajo M, Chakiachvili M, Christensen M, Cummins C, Cuzick A, Davis P, Fexova S, Gall A, George N, Gil L, Gupta P, Hammond-Kosack KE, Haskell E, Hunt SE, Jaiswal P, Janacek SH, Kersey PJ, Langridge N, Maheswari U, Maurel T, McDowall MD, Moore B, Muffato M, Naamati G, Naithani S, Olson A, Papatheodorou I, Patricio M, Paulini M, Pedro H, Perry E, Preece J, Rosello M, Russell M, Sitnik V, Staines DM, Stein J, Tello-Ruiz MK, Trevanion SJ, Urban M, Wei S, Ware D, Williams G, Yates AD, Flicek P. Ensembl genomes 2020-enabling non-vertebrate genomic research. Nucleic Acids Research. 2020;48:D689–D695. 10.1093/nar/gkz890. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Hu AGZ. Public funding and the ascent of Chinese science: evidence from the national natural science foundation of China. Research Policy. 2020;49:103983. 10.1016/j.respol.2020.103983. [CrossRef] [Google Scholar]
- Huang A, Tang Y, Shi X, Jia M, Zhu J, Yan X, Chen H, Gu Y. Proximity labeling proteomics reveals critical regulators for inner nuclear membrane protein degradation in plants. Nature Communications. 2020;11:3284. 10.1038/s41467-020-16744-1. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Huang L, Schiefelbein J. Conserved gene expression programs in developing roots from diverse plants. The Plant Cell. 2015;27:2119–2132. 10.1105/tpc.15.00328. [Abstract] [CrossRef] [Google Scholar]
- Ilic K, Kellogg EA, Jaiswal P, Zapata F, Stevens PF, Vincent LP, Avraham S, Reiser L, Pujar A, Sachs MM, Whitman NT, McCouch SR, Schaeffer ML, Ware DH, Stein LD, Rhee SY. The plant structure ontology, a unified vocabulary of anatomy and morphology of a flowering plant. Plant Physiology. 2007;143:587–599. 10.1104/pp.106.092825. [Abstract] [CrossRef] [Google Scholar]
- Irieda H, Takano Y. Epidermal chloroplasts are defense-related motile organelles equipped with plant immune components. Nature Communications. 2021;12:2739. 10.1038/s41467-021-22977-5. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Isoda R, Yoshinari A, Ishikawa Y, Sadoine M, Simon R, Frommer WB, Nakamura M. Sensors for the quantification, localization and analysis of the dynamics of plant hormones. The Plant Journal. 2021;105:542–557. 10.1111/tpj.15096. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Jaffe AB, Gush J, Larsen V, Laws A. The effect of public funding on research output. The New Zealand Marsden Fund. 2015;52:1–22. 10.1080/00779954.2017.1325921. [CrossRef] [Google Scholar]
- Jarsch IK, Konrad SS, Stratil TF, Urbanus SL, Szymanski W, Braun P, Braun KH, Ott T. Plasma membranes are subcompartmentalized into a plethora of coexisting and diverse microdomains in Arabidopsis and Nicotiana benthamiana. The Plant Cell. 2014;26:1698–1711. 10.1105/tpc.114.124446. [Abstract] [CrossRef] [Google Scholar]
- Jensen PE, Scharff LB. Engineering of plastids to optimize the production of high-value metabolites and proteins. Current Opinion in Biotechnology. 2019;59:8–15. 10.1016/j.copbio.2019.01.009. [Abstract] [CrossRef] [Google Scholar]
- Jiang X, Hoehenwarter W, Scheel D, Lee J. Phosphorylation of the CAMTA3 transcription factor triggers its destabilization and nuclear export. Plant Physiology. 2020;184:1056–1071. 10.1104/pp.20.00795. [Abstract] [CrossRef] [Google Scholar]
- Jiang J, Dehesh K. Plastidial retrograde modulation of light and hormonal signaling: an odyssey. New Phytologist. 2021;230:931–937. 10.1111/nph.17192. [Abstract] [CrossRef] [Google Scholar]
- Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. 10.1038/nature05286. [Abstract] [CrossRef] [Google Scholar]
- Jorasch P. Will the EU stay out of step with science and the rest of the world on plant breeding innovation? Plant Cell Reports. 2020;39:163–167. 10.1007/s00299-019-02482-2. [Abstract] [CrossRef] [Google Scholar]
- Kajala K, Gouran M, Shaar-Moshe L, Mason GA, Rodriguez-Medina J, Kawa D, Pauluzzi G, Reynoso M, Canto-Pastor A, Manzano C, Lau V, Artur MAS, West DA, Gray SB, Borowsky AT, Moore BP, Yao AI, Morimoto KW, Bajic M, Formentin E, Nirmal NA, Rodriguez A, Pasha A, Deal RB, Kliebenstein DJ, Hvidsten TR, Provart NJ, Sinha NR, Runcie DE, Bailey-Serres J, Brady SM. Innovation, conservation, and repurposing of gene function in root cell type development. Cell. 2021;184:3333–3348. 10.1016/j.cell.2021.04.024. [Abstract] [CrossRef] [Google Scholar]
- Kaminuma E, Mashima J, Kodama Y, Gojobori T, Ogasawara O, Okubo K, Takagi T, Nakamura Y. DDBJ launches a new archive database with analytical tools for next-generation sequence data. Nucleic Acids Research. 2010;38:D33–D38. 10.1093/nar/gkp847. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Kanai M, Mano S, Kondo M, Hayashi M, Nishimura M. Extension of oil biosynthesis during the mid-phase of seed development enhances oil content in Arabidopsis seeds. Plant Biotechnology Journal. 2016;14:1241–1250. 10.1111/pbi.12489. [Abstract] [CrossRef] [Google Scholar]
- Kang JB, Nathan A, Zhang F, Millard N, Rumker L, Branch Moody D, Korsunsky I, Raychaudhuri S. Efficient and precise single-cell reference atlas mapping with symphony. bioRxiv. 2021 10.1101/2020.11.18.389189. [Europe PMC free article] [Abstract] [CrossRef]
- Katari MS, Nowicki SD, Aceituno FF, Nero D, Kelfer J, Thompson LP, Cabello JM, Davidson RS, Goldberg AP, Shasha DE, Coruzzi GM, Gutiérrez RA. VirtualPlant: a software platform to support systems biology research. Plant Physiology. 2010;152:500–515. 10.1104/pp.109.147025. [Abstract] [CrossRef] [Google Scholar]
- Khan B, Robbins C, Okrent A. The state of US. science and engineering. 2020. [September 18, 2020]. https://ncses.nsf.gov/pubs/nsb20201/u-s-r-d-performance-and-funding
- Kim T-W, Park CH, Hsu C-C, Zhu J-Y, Hsiao Y, Branon T, Xu S-L, Ting AY, Wang Z-Y. Application of TurboID-mediated proximity labeling for mapping a GSK3 kinase signaling network in Arabidopsis. bioRxiv. 2019 10.1101/636324. [CrossRef]
- Kim SC, Guo L, Wang X. Nuclear moonlighting of cytosolic glyceraldehyde-3-phosphate dehydrogenase regulates Arabidopsis response to heat stress. Nature Communications. 2020;11:3439. 10.1038/s41467-020-17311-4. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Kong SG, Wada M. Recent advances in understanding the molecular mechanism of chloroplast photorelocation movement. Biochimica Et Biophysica Acta (BBA) - Bioenergetics. 2014;1837:522–530. 10.1016/j.bbabio.2013.12.004. [Abstract] [CrossRef] [Google Scholar]
- Korsunsky I, Millard N, Fan J, Slowikowski K, Zhang F, Wei K, Baglaenko Y, Brenner M, Loh PR, Raychaudhuri S. Fast, sensitive and accurate integration of single-cell data with harmony. Nature Methods. 2019;16:1289–1296. 10.1038/s41592-019-0619-0. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Kost B. Towards a virtual Arabidopsis plant. Genome Biology. 2001;2:REPORTS4019. 10.1186/gb-2001-2-8-reports4019. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Kuhnert F, Stefanski A, Overbeck N, Drews L, Reichert AS, Stühler K, Weber APM. Rapid single-step affinity purification of HA-tagged plant mitochondria. Plant Physiology. 2020;182:692–706. 10.1104/pp.19.00732. [Abstract] [CrossRef] [Google Scholar]
- Lähnemann D, Köster J, Szczurek E, McCarthy DJ, Hicks SC, Robinson MD, Vallejos CA, Campbell KR, Beerenwinkel N, Mahfouz A, Pinello L, Skums P, Stamatakis A, Attolini CS, Aparicio S, Baaijens J, Balvert M, Barbanson B, Cappuccio A, Corleone G, Dutilh BE, Florescu M, Guryev V, Holmer R, Jahn K, Lobo TJ, Keizer EM, Khatri I, Kielbasa SM, Korbel JO, Kozlov AM, Kuo TH, Lelieveldt BPF, Mandoiu II, Marioni JC, Marschall T, Mölder F, Niknejad A, Raczkowski L, Reinders M, Ridder J, Saliba AE, Somarakis A, Stegle O, Theis FJ, Yang H, Zelikovsky A, McHardy AC, Raphael BJ, Shah SP, Schönhuth A. Eleven grand challenges in single-cell data science. Genome Biology. 2020;21:31. 10.1186/s13059-020-1926-6. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Larkum AWD, Davey PA, Kuo J, Ralph PJ, Raven JA. Carbon-concentrating mechanisms in seagrasses. Journal of Experimental Botany. 2017;68:3773–3784. 10.1093/jxb/erx206. [Abstract] [CrossRef] [Google Scholar]
- Leinonen R, Akhtar R, Birney E, Bower L, Cerdeno-Tárraga A, Cheng Y, Cleland I, Faruque N, Goodgame N, Gibson R, Hoad G, Jang M, Pakseresht N, Plaister S, Radhakrishnan R, Reddy K, Sobhany S, Ten Hoopen P, Vaughan R, Zalunin V, Cochrane G. The European Nucleotide Archive. Nucleic Acids Research. 2011;39:D28–D31. 10.1093/nar/gkq967. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Libault M, Brechenmacher L, Cheng J, Xu D, Stacey G. Root hair systems biology. Trends in Plant Science. 2010;15:641–650. 10.1016/j.tplants.2010.08.010. [Abstract] [CrossRef] [Google Scholar]
- Libault M, Pingault L, Zogli P, Schiefelbein J. Plant systems biology at the single-cell level. Trends in Plant Science. 2017;22:949–960. 10.1016/j.tplants.2017.08.006. [Abstract] [CrossRef] [Google Scholar]
- Llorente B, Torres-Montilla S, Morelli L, Florez-Sarasa I, Matus JT, Ezquerro M, D'Andrea L, Houhou F, Majer E, Picó B, Cebolla J, Troncoso A, Fernie AR, Daròs JA, Rodriguez-Concepcion M. Synthetic conversion of leaf chloroplasts into carotenoid-rich plastids reveals mechanistic basis of natural chromoplast development. PNAS. 2020;117:21796–21803. 10.1073/pnas.2004405117. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Lotfollahi M, Naghipourfar M, Luecken MD, Khajavi M, Büttner M, Avsec Z, Misharin AV, Theis FJ. Query to reference single-cell integration with transfer learning. bioRxiv. 2020 10.1101/2020.07.16.205997. [CrossRef]
- Ma A, McDermaid A, Xu J, Chang Y, Ma Q. Integrative methods and practical challenges for single-cell multi-omics. Trends in Biotechnology. 2020;38:1007–1022. 10.1016/j.tibtech.2020.02.013. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Mair A, Xu SL, Branon TC, Ting AY, Bergmann DC. Proximity labeling of protein complexes and cell-type-specific organellar proteomes in Arabidopsis enabled by TurboID. eLife. 2019;8:e47864. 10.7554/eLife.47864. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Mallis CS, Zheng X, Qiu X, McCabe JW, Shirzadeh M, Lyu J, Laganowsky A, Russell DH. Development of native MS capabilities on an extended mass range Q-TOF MS. International Journal of Mass Spectrometry. 2020;458:116451. 10.1016/j.ijms.2020.116451. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Marand AP, Chen Z, Gallavotti A, Schmitz RJ. A cis-regulatory atlas in maize at single-cell resolution. Cell. 2021;184:3041–3055. 10.1016/j.cell.2021.04.014. [Abstract] [CrossRef] [Google Scholar]
- Marchive C, Roudier F, Castaings L, Bréhaut V, Blondet E, Colot V, Meyer C, Krapp A. Nuclear retention of the transcription factor NLP7 orchestrates the early response to nitrate in plants. Nature Communications. 2013;4:1713. 10.1038/ncomms2650. [Abstract] [CrossRef] [Google Scholar]
- McFaline-Figueroa JL, Trapnell C, Cuperus JT. The promise of single-cell genomics in plants. Current Opinion in Plant Biology. 2020;54:114–121. 10.1016/j.pbi.2020.04.002. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Michaud O, Fiorucci AS, Xenarios I, Fankhauser C. Local auxin production underlies a spatially restricted neighbor-detection response in Arabidopsis. PNAS. 2017;114:7444–7449. 10.1073/pnas.1702276114. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Morton JT, Strauss CEM, Blackwell R, Berenberg D, Gligorijevic V, Bonneau R. Protein structural alignments from sequence. bioRxiv. 2020 10.1101/2020.11.03.365932. [Abstract] [CrossRef]
- Muñoz P, Munné-Bosch S. Oxylipins in plastidial retrograde signaling. Redox Biology. 2020;37:101717. 10.1016/j.redox.2020.101717. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Nakane T, Kotecha A, Sente A, McMullan G, Masiulis S, Brown P, Grigoras IT, Malinauskaite L, Malinauskas T, Miehling J, Uchański T, Yu L, Karia D, Pechnikova EV, de Jong E, Keizer J, Bischoff M, McCormack J, Tiemeijer P, Hardwick SW, Chirgadze DY, Murshudov G, Aricescu AR, Scheres SHW. Single-particle cryo-EM at atomic resolution. Nature. 2020;587:152–156. 10.1038/s41586-020-2829-0. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- NCBI Resource Coordinators Database resources of the national center for biotechnology information. Nucleic Acids Research. 2018;46:D8–D13. 10.1093/nar/gkx1095. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Ngou BPM, Ahn HK, Ding P, Jones JDG. Mutual potentiation of plant immunity by cell-surface and intracellular receptors. Nature. 2021;592:110–115. 10.1038/s41586-021-03315-7. [Abstract] [CrossRef] [Google Scholar]
- Niehaus M, Straube H, Künzler P, Rugen N, Hegermann J, Giavalisco P, Eubel H, Witte CP, Herde M. Rapid affinity purification of tagged plant mitochondria (Mito-AP) for metabolome and proteome analyses. Plant Physiology. 2020;182:1194–1210. 10.1104/pp.19.00736. [Abstract] [CrossRef] [Google Scholar]
- Obata T. Metabolons in plant primary and secondary metabolism. Phytochemistry Reviews. 2019;18:1483–1507. 10.1007/s11101-019-09619-x. [CrossRef] [Google Scholar]
- Obayashi T, Kinoshita K, Nakai K, Shibaoka M, Hayashi S, Saeki M, Shibata D, Saito K, Ohta H. ATTED-II: a database of co-expressed genes and cis elements for identifying co-regulated gene groups in Arabidopsis. Nucleic Acids Research. 2007;35:D863–D869. 10.1093/nar/gkl783. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Okumoto S, Versaw W. Genetically encoded sensors for monitoring the transport and concentration of nitrogen-containing and phosphorus-containing molecules in plants. Current Opinion in Plant Biology. 2017;39:129–135. 10.1016/j.pbi.2017.07.004. [Abstract] [CrossRef] [Google Scholar]
- Palla G, Spitzer H, Klein M, Fischer D, Schaar AC, Kuemmerle LB, Rybakov S, Ibarra IL, Holmberg O, Virshup I, Lotfollahi M, Richter S, Theis FJ. Squidpy: a scalable framework for spatial single cell analysis. bioRxiv. 2021 10.1101/2021.02.19.431994. [Europe PMC free article] [Abstract] [CrossRef]
- Papatheodorou I, Moreno P, Manning J, Fuentes AM, George N, Fexova S, Fonseca NA, Füllgrabe A, Green M, Huang N, Huerta L, Iqbal H, Jianu M, Mohammed S, Zhao L, Jarnuczak AF, Jupp S, Marioni J, Meyer K, Petryszak R, Prada Medina CA, Talavera-López C, Teichmann S, Vizcaino JA, Brazma A. Expression atlas update: from tissues to single cells. Nucleic Acids Research. 2020;48:D77–D83. 10.1093/nar/gkz947. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Papoutsoglou EA, Faria D, Arend D, Arnaud E, Athanasiadis IN, Chaves I, Coppens F, Cornut G, Costa BV, Ćwiek-Kupczyńska H, Droesbeke B, Finkers R, Gruden K, Junker A, King GJ, Krajewski P, Lange M, Laporte MA, Michotey C, Oppermann M, Ostler R, Poorter H, Ramı Rez-Gonzalez R, Ramšak Ž, Reif JC, Rocca-Serra P, Sansone SA, Scholz U, Tardieu F, Uauy C, Usadel B, Visser RGF, Weise S, Kersey PJ, Miguel CM, Adam-Blondon AF, Pommier C. Enabling reusability of plant phenomic datasets with MIAPPE 1.1. New Phytologist. 2020;227:260–273. 10.1111/nph.16544. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Pastor-Cantizano N, Ko DK, Angelos E, Pu Y, Brandizzi F. Functional diversification of ER stress responses in Arabidopsis. Trends in Biochemical Sciences. 2020;45:123–136. 10.1016/j.tibs.2019.10.008. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Pesaresi P, Kim C. Current understanding of GUN1: a key mediator involved in biogenic retrograde signaling. Plant Cell Reports. 2019;38:819–823. 10.1007/s00299-019-02383-4. [Abstract] [CrossRef] [Google Scholar]
- Pham VVH, Li X, Truong B, Nguyen T, Liu L, Li J, Le TD. The winning methods for predicting cellular position in the DREAM single cell transcriptomics challenge. bioRxiv. 2020 10.1101/2020.05.09.086397. [Abstract] [CrossRef]
- Pinard D, Mizrachi E. Unsung and understudied: plastids involved in secondary growth. Current Opinion in Plant Biology. 2018;42:30–36. 10.1016/j.pbi.2018.01.011. [Abstract] [CrossRef] [Google Scholar]
- Portwood JL. The maize multi-genome genetics and genomics database. Nucleic Acids Research. 2018;1:D1146–D1154. 10.1093/nar/gky1046. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Radivojević T, Costello Z, Workman K, Garcia Martin H. A machine learning automated recommendation tool for synthetic biology. Nature Communications. 2020;11:4879. 10.1038/s41467-020-18008-4. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Rajendran V, Kalita P, Shukla H, Kumar A, Tripathi T. Aminoacyl-tRNA synthetases: structure, function, and drug discovery. International Journal of Biological Macromolecules. 2018;111:400–414. 10.1016/j.ijbiomac.2017.12.157. [Abstract] [CrossRef] [Google Scholar]
- Ramon M, Dang TVT, Broeckx T, Hulsmans S, Crepin N, Sheen J, Rolland F. Default activation and nuclear translocation of the plant cellular energy sensor SnRK1 regulate metabolic stress responses and development. The Plant Cell. 2019;31:1614–1632. 10.1105/tpc.18.00500. [Abstract] [CrossRef] [Google Scholar]
- Regev A, Teichmann SA, Lander ES, Amit I, Benoist C, Birney E, Bodenmiller B, Campbell P, Carninci P, Clatworthy M, Clevers H, Deplancke B, Dunham I, Eberwine J, Eils R, Enard W, Farmer A, Fugger L, Göttgens B, Hacohen N, Haniffa M, Hemberg M, Kim S, Klenerman P, Kriegstein A, Lein E, Linnarsson S, Lundberg E, Lundeberg J, Majumder P, Marioni JC, Merad M, Mhlanga M, Nawijn M, Netea M, Nolan G, Pe'er D, Phillipakis A, Ponting CP, Quake S, Reik W, Rozenblatt-Rosen O, Sanes J, Satija R, Schumacher TN, Shalek A, Shapiro E, Sharma P, Shin JW, Stegle O, Stratton M, Stubbington MJT, Theis FJ, Uhlen M, van Oudenaarden A, Wagner A, Watt F, Weissman J, Wold B, Xavier R, Yosef N, Human Cell Atlas Meeting Participants The human cell atlas. eLife. 2017;6:e27041. 10.7554/eLife.27041. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Reid WV, Ali MK, Field CB. The future of bioenergy. Global Change Biology. 2020;26:274–286. 10.1111/gcb.14883. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Rice S, Fryer E, Ghosh Jha S, Malkovskiy A, Meyer H, Thomas J, Weizbauer R, Zhao K, Birnbaum K, Ehrhardt D, Wang Z, Rhee SY, Plant Cell Atlas Consortium First plant cell atlas workshop report. Plant Direct. 2020;4:e00271. 10.1002/pld3.271. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Rolland N, Bouchnak I, Moyet L, Salvi D, Kuntz M. The main functions of plastids. Methods in Molecular Biology. 2018;1829:73–85. 10.1007/978-1-4939-8654-5_5. [Abstract] [CrossRef] [Google Scholar]
- Rood JE, Stuart T, Ghazanfar S, Biancalani T, Fisher E, Butler A, Hupalowska A, Gaffney L, Mauck W, Eraslan G, Marioni JC, Regev A, Satija R. Toward a common coordinate framework for the human body. Cell. 2019;179:1455–1467. 10.1016/j.cell.2019.11.019. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Rosenberg AB, Roco CM, Muscat RA, Kuchina A, Sample P, Yao Z, Graybuck LT, Peeler DJ, Mukherjee S, Chen W, Pun SH, Sellers DL, Tasic B, Seelig G. Single-cell profiling of the developing mouse brain and spinal cord with split-pool barcoding. Science. 2018;360:176–182. 10.1126/science.aam8999. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Rosenbloom JL, Ginther DK, Juhl T, Heppert JA. The effects of research and development funding on scientific productivity: academic chemistry, 1990-2009. PLOS ONE. 2015;10:e0138176. 10.1371/journal.pone.0138176. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Ruffell D. The EU court of justice extends the GMO directive to gene-edited organisms. FEBS Letters. 2018;592:3653–3657. 10.1002/1873-3468.13293. [Abstract] [CrossRef] [Google Scholar]
- Sansone S-A, Rocca-Serra P, Field D, Maguire E, Taylor C, Hofmann O, Fang H, Neumann S, Tong W, Amaral-Zettler L, Begley K, Booth T, Bougueleret L, Burns G, Chapman B, Clark T, Coleman L-A, Copeland J, Das S, de Daruvar A, de Matos P, Dix I, Edmunds S, Evelo CT, Forster MJ, Gaudet P, Gilbert J, Goble C, Griffin JL, Jacob D, Kleinjans J, Harland L, Haug K, Hermjakob H, Sui SJH, Laederach A, Liang S, Marshall S, McGrath A, Merrill E, Reilly D, Roux M, Shamu CE, Shang CA, Steinbeck C, Trefethen A, Williams-Jones B, Wolstencroft K, Xenarios I, Hide W. Toward interoperable bioscience data. Nature Genetics. 2012;44:121–126. 10.1038/ng.1054. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Sarma GP, Lee CW, Portegys T, Ghayoomie V, Jacobs T, Alicea B, Cantarelli M, Currie M, Gerkin RC, Gingell S, Gleeson P, Gordon R, Hasani RM, Idili G, Khayrulin S, Lung D, Palyanov A, Watts M, Larson SD. OpenWorm: overview and recent advances in integrative biological simulation of Caenorhabditis elegans. Philosophical Transactions of the Royal Society B: Biological Sciences. 2018;373:20170382. 10.1098/rstb.2017.0382. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Saunders A, Macosko EZ, Wysoker A, Goldman M, Krienen FM, de Rivera H, Bien E, Baum M, Bortolin L, Wang S, Goeva A, Nemesh J, Kamitaki N, Brumbaugh S, Kulp D, McCarroll SA. Molecular diversity and specializations among the cells of the adult mouse brain. Cell. 2018;174:1015–1030. 10.1016/j.cell.2018.07.028. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Schlüter U, Weber APM. Regulation and evolution of C4 photosynthesis. Annual Review of Plant Biology. 2020;71:183–215. 10.1146/annurev-arplant-042916-040915. [Abstract] [CrossRef] [Google Scholar]
- Seren Ü, Grimm D, Fitz J, Weigel D, Nordborg M, Borgwardt K, Korte A. AraPheno: a public database for Arabidopsis thaliana phenotypes. Nucleic Acids Research. 2016;1:D1054–D1059. 10.1093/nar/gkw986. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Shahid N, Daniell H. Plant-based oral vaccines against zoonotic and non-zoonotic diseases. Plant Biotechnology Journal. 2016;14:2079–2099. 10.1111/pbi.12604. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, Geng Q, Auerbach A, Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. 10.1038/s41586-020-2179-y. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Shao W, Dong J. Polarity in plant asymmetric cell division: division orientation and cell fate differentiation. Developmental Biology. 2016;419:121–131. 10.1016/j.ydbio.2016.07.020. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Shepon A, Eshel G, Noor E, Milo R. The opportunity cost of animal based diets exceeds all food losses. PNAS. 2018;115:3804–3809. 10.1073/pnas.1713820115. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Shimizu Y, Takagi J, Ito E, Ito Y, Ebine K, Komatsu Y, Goto Y, Sato M, Toyooka K, Ueda T, Kurokawa K, Uemura T, Nakano A. Cargo sorting zones in the trans-Golgi network visualized by super-resolution confocal live imaging microscopy in plants. Nature Communications. 2021;12:1901. 10.1038/s41467-021-22267-0. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Sinnott EW. Plant morphogenesis. Nature. 1960;189:697–699. 10.1038/189697a0. [CrossRef] [Google Scholar]
- Smirnoff N, Arnaud D. Hydrogen peroxide metabolism and functions in plants. New Phytologist. 2019;221:1197–1214. 10.1111/nph.15488. [Abstract] [CrossRef] [Google Scholar]
- Sofroniew N, Lambert T, Evans K, Nunez-Iglesias J, Winston P, Bokota G, Yamauchi K, Solak AC, Peña-Castellanos G, ziyangczi BM, Buckley G, Pop DD. Napari. 0.4.8rc6Zenodo. 2021 10.5281/zenodo.3555620. [CrossRef]
- Solymosi K, Lethin J, Aronsson H. Diversity and plasticity of plastids in land plants. Methods in Molecular Biology. 2018;1829:55–72. 10.1007/978-1-4939-8654-5_4. [Abstract] [CrossRef] [Google Scholar]
- Stewart BJ, Ferdinand JR, Young MD, Mitchell TJ, Loudon KW, Riding AM, Richoz N, Frazer GL, Staniforth JUL, Vieira Braga FA, Botting RA, Popescu DM, Vento-Tormo R, Stephenson E, Cagan A, Farndon SJ, Polanski K, Efremova M, Green K, Del Castillo Velasco-Herrera M, Guzzo C, Collord G, Mamanova L, Aho T, Armitage JN, Riddick ACP, Mushtaq I, Farrell S, Rampling D, Nicholson J, Filby A, Burge J, Lisgo S, Lindsay S, Bajenoff M, Warren AY, Stewart GD, Sebire N, Coleman N, Haniffa M, Teichmann SA, Behjati S, Clatworthy MR. Spatiotemporal immune zonation of the human kidney. Science. 2019;365:1461–1466. 10.1126/science.aat5031. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, Mauck WM, Hao Y, Stoeckius M, Smibert P, Satija R. Comprehensive integration of single-cell data. Cell. 2019;177:1888–1902. 10.1016/j.cell.2019.05.031. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Sullivan A, Purohit PK, Freese NH, Pasha A, Esteban E, Waese J, Wu A, Chen M, Chin CY, Song R, Watharkar SR, Chan AP, Krishnakumar V, Vaughn MW, Town C, Loraine AE, Provart NJ. An 'eFP-Seq Browser' for visualizing and exploring RNA sequencing data. The Plant Journal : For Cell and Molecular Biology. 2019;100:641–654. 10.1111/tpj.14468. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Szigeti B, Gleeson P, Vella M, Khayrulin S, Palyanov A, Hokanson J, Currie M, Cantarelli M, Idili G, Larson S. OpenWorm: an open-science approach to modeling Caenorhabditis elegans. Frontiers in Computational Neuroscience. 2014;8:137. 10.3389/fncom.2014.00137. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Tabula Muris Consortium Single-cell transcriptomics of 20 mouse organs creates a Tabula muris. Nature. 2018;562:367–372. 10.1038/s41586-018-0590-4. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Tello-Ruiz MK, Naithani S, Gupta P, Olson A, Wei S, Preece J, Jiao Y, Wang B, Chougule K, Garg P, Elser J, Kumari S, Kumar V, Contreras-Moreira B, Naamati G, George N, Cook J, Bolser D, D'Eustachio P, Stein LD, Gupta A, Xu W, Regala J, Papatheodorou I, Kersey PJ, Flicek P, Taylor C, Jaiswal P, Ware D. Gramene 2021: harnessing the power of comparative genomics and pathways for plant research. Nucleic Acids Research. 2021;49:D1452–D1463. 10.1093/nar/gkaa979. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- ten Hove CA, Lu KJ, Weijers D. Building a plant: cell fate specification in the early Arabidopsis embryo. Development. 2015;142:420–430. 10.1242/dev.111500. [Abstract] [CrossRef] [Google Scholar]
- The Gene Ontology Consortium The gene ontology resource: 20 years and still GOing strong. Nucleic Acids Research. 2019;47:D330–D338. 10.1093/nar/gky1055. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Togninalli M, Seren Ü, Meng D, Fitz J, Nordborg M, Weigel D, Borgwardt K, Korte A, Grimm DG. The AraGWAS catalog: a curated and standardized Arabidopsis thaliana GWAS catalog. Nucleic Acids Research. 2018;46:D1150–D1156. 10.1093/nar/gkx954. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Togninalli M, Seren Ü, Freudenthal JA, Monroe JG, Meng D, Nordborg M, Weigel D, Borgwardt K, Korte A, Grimm DG. AraPheno and the AraGWAS catalog 2020: a major database update including RNA-Seq and knockout mutation data for Arabidopsis thaliana. Nucleic Acids Research. 2020;48:D1063–D1068. 10.1093/nar/gkz925. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Tylewicz S, Petterle A, Marttila S, Miskolczi P, Azeez A, Singh RK, Immanen J, Mähler N, Hvidsten TR, Eklund DM, Bowman JL, Helariutta Y, Bhalerao RP. Photoperiodic control of seasonal growth is mediated by ABA acting on cell-cell communication. Science. 2018;360:212–215. 10.1126/science.aan8576. [Abstract] [CrossRef] [Google Scholar]
- Uhrig RG, Schläpfer P, Roschitzki B, Hirsch-Hoffmann M, Gruissem W. Diurnal changes in concerted plant protein phosphorylation and acetylation in Arabidopsis organs and seedlings. The Plant Journal. 2019;99:176–194. 10.1111/tpj.14315. [Abstract] [CrossRef] [Google Scholar]
- Voon CP, Guan X, Sun Y, Sahu A, Chan MN, Gardeström P, Wagner S, Fuchs P, Nietzel T, Versaw WK, Schwarzländer M, Lim BL. ATP compartmentation in plastids and cytosol of Arabidopsis thaliana revealed by fluorescent protein sensing. PNAS. 2018;115:E10778–E10787. 10.1073/pnas.1711497115. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Waese J, Fan J, Pasha A, Yu H, Fucile G, Shi R, Cumming M, Kelley LA, Sternberg MJ, Krishnakumar V, Ferlanti E, Miller J, Town C, Stuerzlinger W, Provart NJ. ePlant: visualizing and exploring multiple levels of data for hypothesis generation in plant biology. The Plant Cell. 2017;29:1806–1821. 10.1105/tpc.17.00073. [Abstract] [CrossRef] [Google Scholar]
- Wang E, Wang J, Zhu X, Hao W, Wang L, Li Q, Zhang L, He W, Lu B, Lin H, Ma H, Zhang G, He Z. Control of rice grain-filling and yield by a gene with a potential signature of domestication. Nature Genetics. 2008;40:1370–1374. 10.1038/ng.220. [Abstract] [CrossRef] [Google Scholar]
- Wang M, Carver JJ, Phelan VV, Sanchez LM, Garg N, Peng Y, Nguyen DD, Watrous J, Kapono CA, Luzzatto-Knaan T, Porto C, Bouslimani A, Melnik AV, Meehan MJ, Liu WT, Crüsemann M, Boudreau PD, Esquenazi E, Sandoval-Calderón M, Kersten RD, Pace LA, Quinn RA, Duncan KR, Hsu CC, Floros DJ, Gavilan RG, Kleigrewe K, Northen T, Dutton RJ, Parrot D, Carlson EE, Aigle B, Michelsen CF, Jelsbak L, Sohlenkamp C, Pevzner P, Edlund A, McLean J, Piel J, Murphy BT, Gerwick L, Liaw CC, Yang YL, Humpf HU, Maansson M, Keyzers RA, Sims AC, Johnson AR, Sidebottom AM, Sedio BE, Klitgaard A, Larson CB, Cab P, Torres-Mendoza D, Gonzalez DJ, Silva DB, Marques LM, Demarque DP, Pociute E, O'Neill EC, Briand E, Helfrich EJN, Granatosky EA, Glukhov E, Ryffel F, Houson H, Mohimani H, Kharbush JJ, Zeng Y, Vorholt JA, Kurita KL, Charusanti P, McPhail KL, Nielsen KF, Vuong L, Elfeki M, Traxler MF, Engene N, Koyama N, Vining OB, Baric R, Silva RR, Mascuch SJ, Tomasi S, Jenkins S, Macherla V, Hoffman T, Agarwal V, Williams PG, Dai J, Neupane R, Gurr J, Rodríguez AMC, Lamsa A, Zhang C, Dorrestein K, Duggan BM, Almaliti J, Allard PM, Phapale P, Nothias LF, Alexandrov T, Litaudon M, Wolfender JL, Kyle JE, Metz TO, Peryea T, Nguyen DT, VanLeer D, Shinn P, Jadhav A, Müller R, Waters KM, Shi W, Liu X, Zhang L, Knight R, Jensen PR, Palsson BO, Pogliano K, Linington RG, Gutiérrez M, Lopes NP, Gerwick WH, Moore BS, Dorrestein PC, Bandeira N. Sharing and community curation of mass spectrometry data with global natural products social molecular networking. Nature Biotechnology. 2016;34:828–837. 10.1038/nbt.3597. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Wang P, Tang W, Li Z, Zou Z, Zhou Y, Li R, Xiong T, Wang J, Zou P. Mapping spatial transcriptome with light-activated proximity-dependent RNA labeling. Nature Chemical Biology. 2019;15:1110–1119. 10.1038/s41589-019-0368-5. [Abstract] [CrossRef] [Google Scholar]
- Wang X, Ye L, Lyu M, Ursache R, Löytynoja A, Mähönen AP. An inducible genome editing system for plants. Nature Plants. 2020;6:766–772. 10.1038/s41477-020-0695-2. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Wang B, Qiu YL. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza. 2006;16:299–363. 10.1007/s00572-005-0033-6. [Abstract] [CrossRef] [Google Scholar]
- Wilkinson MD, Dumontier M, Aalbersberg IJ, Appleton G, Axton M, Baak A, Blomberg N, Boiten JW, da Silva Santos LB, Bourne PE, Bouwman J, Brookes AJ, Clark T, Crosas M, Dillo I, Dumon O, Edmunds S, Evelo CT, Finkers R, Gonzalez-Beltran A, Gray AJ, Groth P, Goble C, Grethe JS, Heringa J, 't Hoen PA, Hooft R, Kuhn T, Kok R, Kok J, Lusher SJ, Martone ME, Mons A, Packer AL, Persson B, Rocca-Serra P, Roos M, van Schaik R, Sansone SA, Schultes E, Sengstag T, Slater T, Strawn G, Swertz MA, Thompson M, van der Lei J, van Mulligen E, Velterop J, Waagmeester A, Wittenburg P, Wolstencroft K, Zhao J, Mons B. The FAIR guiding principles for scientific data management and stewardship. Scientific Data. 2016;3:160018. 10.1038/sdata.2016.18. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- wwPDB consortium Protein Data Bank: the single global archive for 3D macromolecular structure data. Nucleic Acids Research. 2019;47:D520–D528. 10.1093/nar/gky949. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Xia B, Yanai I. A periodic table of cell types. Development. 2019;146:dev.169854. 10.1242/dev.169854. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Xing J, Li X, Wang X, Lv X, Wang L, Zhang L, Zhu Y, Shen Q, Baluška F, Šamaj J, Lin J. Secretion of phospholipase Dδ functions as a regulatory mechanism in plant innate immunity. The Plant Cell. 2019;31:3015–3032. 10.1105/tpc.19.00534. [Abstract] [CrossRef] [Google Scholar]
- Yoo SD, Cho YH, Sheen J. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nature Protocols. 2007;2:1565–1572. 10.1038/nprot.2007.199. [Abstract] [CrossRef] [Google Scholar]
- Yu M, Cui Y, Zhang X, Li R, Lin J. Organization and dynamics of functional plant membrane microdomains. Cellular and Molecular Life Sciences. 2020;77:275–287. 10.1007/s00018-019-03270-7. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zeisel A, Hochgerner H, Lönnerberg P, Johnsson A, Memic F, van der Zwan J, Häring M, Braun E, Borm LE, La Manno G, Codeluppi S, Furlan A, Lee K, Skene N, Harris KD, Hjerling-Leffler J, Arenas E, Ernfors P, Marklund U, Linnarsson S. Molecular architecture of the mouse nervous system. Cell. 2018;174:999–1014. 10.1016/j.cell.2018.06.021. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zhang Y, Song G, Lal NK, Nagalakshmi U, Li Y, Zheng W, Huang PJ, Branon TC, Ting AY, Walley JW, Dinesh-Kumar SP. TurboID-based proximity labeling reveals that UBR7 is a regulator of N NLR immune receptor-mediated immunity. Nature Communications. 2019a;10:3252. 10.1038/s41467-019-11202-z. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zhang TQ, Xu ZG, Shang GD, Wang JW. A single-cell RNA sequencing profiles the developmental landscape of Arabidopsis root. Molecular Plant. 2019b;12:648–660. 10.1016/j.molp.2019.04.004. [Abstract] [CrossRef] [Google Scholar]
- Zhang J, Petersen SD, Radivojevic T, Ramirez A, Pérez-Manríquez A, Abeliuk E, Sánchez BJ, Costello Z, Chen Y, Fero MJ, Martin HG, Nielsen J, Keasling JD, Jensen MK. Combining mechanistic and machine learning models for predictive engineering and optimization of tryptophan metabolism. Nature Communications. 2020;11:4880. 10.1038/s41467-020-17910-1. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zimny T, Sowa S, Tyczewska A, Twardowski T. Certain new plant breeding techniques and their marketability in the context of EU GMO legislation - recent developments. New Biotechnology. 2019;51:49–56. 10.1016/j.nbt.2019.02.003. [Abstract] [CrossRef] [Google Scholar]
Decision letter
Thank you for submitting your article "A Roadmap for the Plant Cell Atlas" to eLife for consideration as a Feature Article. Three peer reviewers have reviewed your article, and two editors from the eLife Features Team have overseen the evaluation.
The reviewers and editors have discussed the reviews and we have drafted this decision letter to help you prepare a revised submission.
Summary:
On the heels of a 2020 workshop, this paper discusses the Plant Cell Atlas (PCA) initiative, which aims to create a resource to identify all plant cell types, and to annotate the localization and organization of molecules at cellular and tissue levels. The authors discuss the gaps in knowledge and technologies and the challenges of integrating different data types to enable the PCA infrastructure. This vision paper provides a compelling argument as to why the PCA is important for food security, environmental sustenance, and human health. However, a number of points need to be addressed to make the article suitable for publication.
Overall, the reviewers raised concerns about two aspects: ensuring that the paper is markedly different from the one published in 2019; and clarifying whether the manuscript is a clarion call for investment in plant science or a highly technical discussion of data collection at a finer and finer level. More specifically, you will find below a list of essential revisions for you to consider.
Essential revisions:
1. Overall: Please include a detailed, practical roadmap of how the PCA can come to be in the near future, which would include concrete steps of how it could be designed and rolled out. Without including this content, the reviewers fear the paper would be too similar to previous work and would lack the impact it could otherwise achieve on the community. This feedback constitutes one of the most important concerns from the reviewers.
2. Overall: Please address how the PCA consortium plans to tackle how to unify the growing number of single-cell data, and make them more accessible/comparable to the community. For instance, please consider including standardized techniques, pipelines and SOPs to maximize productive sharing and cross-comparison/analysis of these data. The reviewer is especially concerned about the fact that ever accumulating scRNA-seq (and other single-cell) data are under-utilized because it is very difficult, if not impossible, to compare different technological platforms. Indeed, different pipelines for dimension reduction/data presentation make it difficult to cross-analyze data generated from different laboratories in a meaningful way, and different genetic backgrounds, growth conditions, age, stress treatment, etc, make a comparison of Arabidopsis data even more challenging. This discussion could also include reflection on how to make single-cell data comparable to metazoan data.
3. Overall: Please consider strengthening your discussion of the roadblocks particular to plants (as opposed to other systems such as metazoans or humans) when leveraging experimental and computational technologies to create the atlas, and in particular how the data will actually be integrated and used.
4. Overall: Please also discuss more clearly how piecing together these different sources of data will result in a new, usable structure. The reviewer felt that discussing these issues was 'lost' amongst the text and could be made more prominent.
5. Overall: As natural variation is important in determining phenotypic diversity, please clarify how the PCA initiative is going to handle such complexity in terms of extending the functionality of cell atlas beyond one genotype.
6. Title: Please change the title of the article to reflect that it will not detail a prototype of PCA infrastructure, and to reflect that it is more a collection of focus areas or opportunities. Please ensure that this title is markedly different from the previous published work "Towards building a plant cell atlas (2019)".
7. Page 4, start of the page: Please discuss how PCA will improve on what is currently being done, as the benefits of understanding cell/tissue-specific expression have been known – and used – for decades.
8. Page 4, in "vision for a plant cell atlas": If possible, please describe other examples or prototypes in model organisms such as human, mouse or Drosophila, which would support that it is feasible for the PCA community to accomplish its vision in the ways you suggest (e.g. example creating "a one-stop, user-friendly website", "a 4D representation of a developing plant, from root to shoot with data collected from single-cell omics platforms for each cell".)
9. Page 7: Please consider trying to aggregate the gaps for investment, which at present are scattered through the review and sometimes not fully discussed. For instance, "Gaps in technology" is a comprehensive review of new techniques, but only seems to identify "more efficient capture technologies" as a specific gap.
10. End of page 12: Please consider further discussing and expanding on how the PCA compares to the roadmap of other projects and ideas, such as the Human Cell Atlas, and the actual concepts in the Decadal Vision ("The Transparent Plant"), which, while referenced, are not considered even though they outline similar issues.
11. End of page 12: Continuing this line of thoughts, please discuss how the PCA Consortium will/does coordinate with existing platforms to deliver the vision. For instance, the plant biology community already widely uses numerous data-curated plant atlas platforms such as BAR (http://bar.utoronto.ca/), ATTED-II (www.atted.jp/). Other examples include the human cell atlas project (https://www.humancellatlas.org/). It would be enlightening if the authors could discuss whether the PCA will stay separate and become yet another platform, or if it will be possible to actively coordinate and integrate the PCA platform with these existing platforms. What platform would be the most accessible to a variety of users (from students to senior researchers, from bioinformaticians to agricultural specialists)?
12. Page 12 and 13: Please add content to explain in more detail what will happen to bring the 4D project to life. For instance, please discuss the informatics frontiers and how we get to “hypothesis-generating in silico experiments" that could potentially meet some of the "people and culture objectives”.
13. Page 13: Please consider fleshing out the "people and culture" aspect beyond the two existing paragraphs.
Author response
Overall, the reviewers raised concerns about two aspects: ensuring that the paper is markedly different from the one published in 2019; and clarifying whether the manuscript is a clarion call for investment in plant science or a highly technical discussion of data collection at a finer and finer level. More specifically, you will find below a list of essential revisions for you to consider.
We thank the reviewers and editors for calling us out on this important point. We wish the paper to be “a clarion call for investment in plant science” and, in the revised version, we highlight both the opportunities and challenges in making this happen from technical, human resource, and investment perspectives. We have substantially revised the manuscript to provide evidence and argument supporting this primary message and detailed the mechanics of how we envision building out this important resource for plant science. We added 3 new figures and 2 boxes to address the reviewers’ and editors’ comments. We provide a detailed point-by-point response below that fully addresses the comments. Overall, we feel that the comments from the editors and reviewers guided us well in devising a much stronger and more impactful perspective article for which we are grateful.
Essential revisions:
1. Overall: Please include a detailed, practical roadmap of how the PCA can come to be in the near future, which would include concrete steps of how it could be designed and rolled out. Without including this content, the reviewers fear the paper would be too similar to previous work and would lack the impact it could otherwise achieve on the community. This feedback constitutes one of the most important concerns from the reviewers.
We substantially revised the manuscript, emphasizing how the PCA infrastructure can be built as a community-supported, integrated resource for assimilating molecular and cellular data to make accessible the dynamic organization of various plant cell types. First, we laid out a pragmatic set of achievable targets for the next 10+ years in the “Vision for a Plant Cell Atlas” section, and summarized the milestones in Figure 2 (new).
Second, we added a graphical illustration of a “one-stop shop” PCA resource in Figure 3 (new) that exemplifies the type of data integration and visualization we are envisioning.
Third, throughout the manuscript, we added details on how the PCA could be built. These include data integration approaches (“Vision for the Plant Cell Atlas” section); community building approaches (“The PCA Community” section); types of infrastructure needed for making single cell data accessible according to the FAIR principles (“Data and Infrastructure” section); and specific challenges that need to be overcome (“Challenges Faced in the Development of the PCA” section). All the new text are in blue.
Fourth, we summarized the major challenges that should be targeted for investment to enable the PCA and transform plant science in Box 1.
Finally, we added an example of plant biology, the chloroplast structure, function and dynamics, for which PCA efforts could transform its understanding and impact in plant science in Box 2 and associated Figure 7 (new).
2. Overall: Please address how the PCA consortium plans to tackle how to unify the growing number of single-cell data, and make them more accessible/comparable to the community. For instance, please consider including standardized techniques, pipelines and SOPs to maximize productive sharing and cross-comparison/analysis of these data. The reviewer is especially concerned about the fact that ever accumulating scRNA-seq (and other single-cell) data are under-utilized because it is very difficult, if not impossible, to compare different technological platforms. Indeed, different pipelines for dimension reduction/data presentation make it difficult to cross-analyze data generated from different laboratories in a meaningful way, and different genetic backgrounds, growth conditions, age, stress treatment, etc, make a comparison of Arabidopsis data even more challenging. This discussion could also include reflection on how to make single-cell data comparable to metazoan data.
To address how the PCA will facilitate access and use of the single cell data sets, we have laid out in more detail our vision of single-cell dataset accessibility in the ‘Data and Infrastructure’ section by adding a new paragraph.
3. Overall: Please consider strengthening your discussion of the roadblocks particular to plants (as opposed to other systems such as metazoans or humans) when leveraging experimental and computational technologies to create the atlas, and in particular how the data will actually be integrated and used.
We have included a description of challenges in a new section called “Challenges Faced in the Development of the PCA” that replaces the old section on “Gaps in Technology” discusses the challenges as well as ways to overcome those in the future. We also added a summary of key challenges in Box 1 that are particular to the goals of the PCA such as natural variation and interspecies comparison, polyploidy and multigene families, spatial constraints imposed by cell walls, differences between tissues in terms of accessibility, and the limitation of resources in the plant science community.
4. Overall: Please also discuss more clearly how piecing together these different sources of data will result in a new, usable structure. The reviewer felt that discussing these issues was 'lost' amongst the text and could be made more prominent.
We have addressed this by clarifying the vision statement. We have clarified what the benefits are for bringing all the pieces together and the revised manuscript now emphasizes integration, usability, and visualization in the “Vision for Plant Cell Atlas” section. We have also included a new figure (Figure 3) showing an integrated user interface that exemplifies the type of front-end that is made possible by the back end of integrated data we are envisioning.
5. Overall: As natural variation is important in determining phenotypic diversity, please clarify how the PCA initiative is going to handle such complexity in terms of extending the functionality of cell atlas beyond one genotype.
We agree with the reviewer that it is important to plan for natural variation to be incorporated in the PCA. We have incorporated references to genomic natural variation databases such as the 1001 Arabidopsis Genomes in the “Data and Infrastructure” section. We envision several degrees of integration of natural variation in the PCA, from hyper-linking gene IDs between PCA and natural variation catalogs to the preparation of data structure scaffolds that will enable in the future re-creation of PCA for multiple genotypes and species and interactively compare and study differences and similarities.
6. Title: Please change the title of the article to reflect that it will not detail a prototype of PCA infrastructure, and to reflect that it is more a collection of focus areas or opportunities. Please ensure that this title is markedly different from the previous published work "Towards building a plant cell atlas (2019)".
We changed the title to: “The Plant Cell Atlas”
[Editor’s note: The title has now been changed following discussions with the editor.]
7. Page 4, start of the page: Please discuss how PCA will improve on what is currently being done, as the benefits of understanding cell/tissue-specific expression have been known – and used – for decades.
We have addressed how PCA will improve the accessibility of tissue and single cell data and eliminate the constraint in using transcriptomic data in comparative analysis studies. The PCA community as well as the portal will be useful as it will promote community supported best practices in data generation and use and will also integrate metrics and reference maps for organ/cell types. This is elaborated in the “Vision for a Plant Cell Atlas” section.
8. Page 4, in "vision for a plant cell atlas": If possible, please describe other examples or prototypes in model organisms such as human, mouse or Drosophila, which would support that it is feasible for the PCA community to accomplish its vision in the ways you suggest (e.g. example creating "a one-stop, user-friendly website", "a 4D representation of a developing plant, from root to shoot with data collected from single-cell omics platforms for each cell".)
We added a paragraph to the “Vision for a Plant Cell Atlas” section to address this point.
9. Page 7: Please consider trying to aggregate the gaps for investment, which at present are scattered through the review and sometimes not fully discussed. For instance, "Gaps in technology" is a comprehensive review of new techniques, but only seems to identify "more efficient capture technologies" as a specific gap.
We have addressed this comment by including a revised section titled “Challenges Faced in the Development of PCA” to consolidate the limitations and describes the areas that should be targeted for investment in the future. This section is summarized in Box 2.
10. End of page 12: Please consider further discussing and expanding on how the PCA compares to the roadmap of other projects and ideas, such as the Human Cell Atlas, and the actual concepts in the Decadal Vision ("The Transparent Plant"), which, while referenced, are not considered even though they outline similar issues.
We added a paragraph in the “Data and Infrastructure” section to address this point.
11. End of page 12: Continuing this line of thoughts, please discuss how the PCA Consortium will/does coordinate with existing platforms to deliver the vision. For instance, the plant biology community already widely uses numerous data-curated plant atlas platforms such as BAR (http://bar.utoronto.ca/), ATTED-II (www.atted.jp/). Other examples include the human cell atlas project (https://www.humancellatlas.org/). It would be enlightening if the authors could discuss whether the PCA will stay separate and become yet another platform, or if it will be possible to actively coordinate and integrate the PCA platform with these existing platforms. What platform would be the most accessible to a variety of users (from students to senior researchers, from bioinformaticians to agricultural specialists)?
To address this comment, we overhauled the section entitled “The PCA Community” and added a new subsection called “PCA in the bioinformatics community” where we lay out how we see PCA positioning in itself in the context of the other existing resources.
12. Page 12 and 13: Please add content to explain in more detail what will happen to bring the 4D project to life. For instance, please discuss the informatics frontiers and how we get to hypothesis-generating in silico experiments" (p. 12) that could potentially meet some of the "people and culture objectives (p. 13).
We added examples of informatics areas we envision being important for realizing the PCA vision to the Data and Infrastructure section. First, we expanded the need for modeling, simulation, and visualization tools for developing a virtual plant cell resource, which would be conceptually similar to other digital organism projects like the OpenWorm. Second, we introduce several topic areas with references that will likely be important informatics frontiers for the PCA, including multi-dimensional image visualization and analysis, graph-based knowledge bases, natural language processing, and machine learning and artificial intelligence.
13. Page 13: Please consider fleshing out the "people and culture" aspect beyond the two existing paragraphs.
We have overhauled this section that is now called “The PCA community” and has four sections, “Developers”, “Users”, “PCA in the bioinformatics community”, and “Training”. This section has been expanded and moved up in the manuscript.
Articles from eLife are provided here courtesy of eLife Sciences Publications, Ltd
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/113042608
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.7554/elife.66877
Article citations
MAMS: matrix and analysis metadata standards to facilitate harmonization and reproducibility of single-cell data.
Genome Biol, 25(1):205, 01 Aug 2024
Cited by: 2 articles | PMID: 39090672 | PMCID: PMC11292877
Pleiotropy, a feature or a bug? Toward co-ordinating plant growth, development, and environmental responses through engineering plant hormone signaling.
Curr Opin Biotechnol, 88:103151, 31 May 2024
Cited by: 0 articles | PMID: 38823314
Review
AraLeTA: An Arabidopsis leaf expression atlas across diurnal and developmental scales.
Plant Physiol, 195(3):1941-1953, 01 Jun 2024
Cited by: 1 article | PMID: 38428997
Comprehensive integration of single-cell transcriptomic data illuminates the regulatory network architecture of plant cell fate specification.
Plant Divers, 46(3):372-385, 03 Apr 2024
Cited by: 2 articles | PMID: 38798726 | PMCID: PMC11119547
Spatiotemporal metabolic responses to water deficit stress in distinct leaf cell-types of poplar.
Front Plant Sci, 15:1346853, 01 Mar 2024
Cited by: 0 articles | PMID: 38495374 | PMCID: PMC10940329
Go to all (21) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
First plant cell atlas workshop report.
Plant Direct, 4(10):e00271, 15 Oct 2020
Cited by: 3 articles | PMID: 33083684 | PMCID: PMC7557347
Plant Systems Biology at the Single-Cell Level.
Trends Plant Sci, 22(11):949-960, 29 Sep 2017
Cited by: 47 articles | PMID: 28970001
Review
Towards Building a Plant Cell Atlas.
Trends Plant Sci, 24(4):303-310, 16 Feb 2019
Cited by: 45 articles | PMID: 30777643 | PMCID: PMC7449582
Review Free full text in Europe PMC
The Chlamydomonas heat stress response.
Plant J, 82(3):466-480, 27 Mar 2015
Cited by: 45 articles | PMID: 25754362
Review
Funding
Funders who supported this work.
NIGMS NIH HHS (2)
Grant ID: R35 GM138143
Grant ID: R01 GM123259
National Science Foundation (2)
Grant ID: 1916797
Grant ID: 2052590