Abstract
Methods
Data for transplant candidates from 2010 to 2019 were retrieved from the Dutch Transplant Foundation database. Diagnosis categories and outcomes were compared between the periods before and after the introduction of the LAS. Time-dependent Cox regression and Fine-Gray analyses were performed to compare the chance for transplantation before and after introduction of the LAS.Results
The cohort comprised 1276 patients. After introduction of the LAS, the annual number of transplantations and waiting list mortality did not change. The proportion of patients on the waiting list and transplanted patients with pulmonary fibrosis increased (25%-37%, P < 0.001; 22%-39%, P < 0.001). The chance of transplantation increased significantly for patients with pulmonary fibrosis after introduction of the LAS (hazard ratio 1.9 [95% confidence interval 1.4-2.9]). Patients who died on the waiting list had an increased LAS compared to the time of placement on the waiting list, reflecting clinical deterioration. This was not the case in patients with chronic obstructive pulmonary disease (P < 0.001). Overall survival was similar after introduction of the LAS (5-y survival 68%, compared to 74% [P = 0.171]).Conclusions
After the introduction of the LAS in The Netherlands, an increased proportion of transplantations was performed for patients with pulmonary fibrosis. Overall survival after transplantation did not change.Free full text

Waiting List Dynamics and Lung Transplantation Outcomes After Introduction of the Lung Allocation Score in The Netherlands
Associated Data
Background.
The Netherlands was the third country to adopt the lung allocation score (LAS) for national allocation of donor lungs in April 2014. Evaluations of the introduction of the LAS in the United States and Germany showed mainly beneficial effects, including increased survival after transplantation.
Methods.
Data for transplant candidates from 2010 to 2019 were retrieved from the Dutch Transplant Foundation database. Diagnosis categories and outcomes were compared between the periods before and after the introduction of the LAS. Time-dependent Cox regression and Fine-Gray analyses were performed to compare the chance for transplantation before and after introduction of the LAS.
Results.
The cohort comprised 1276 patients. After introduction of the LAS, the annual number of transplantations and waiting list mortality did not change. The proportion of patients on the waiting list and transplanted patients with pulmonary fibrosis increased (25%–37%, P < 0.001; 22%–39%, P < 0.001). The chance of transplantation increased significantly for patients with pulmonary fibrosis after introduction of the LAS (hazard ratio 1.9 [95% confidence interval 1.4-2.9]). Patients who died on the waiting list had an increased LAS compared to the time of placement on the waiting list, reflecting clinical deterioration. This was not the case in patients with chronic obstructive pulmonary disease (P < 0.001). Overall survival was similar after introduction of the LAS (5-y survival 68%, compared to 74% [P = 0.171]).
Conclusions.
After the introduction of the LAS in The Netherlands, an increased proportion of transplantations was performed for patients with pulmonary fibrosis. Overall survival after transplantation did not change.
Lung transplantation can be a life-saving treatment for patients with end-stage lung disease. However, demand has always outstripped the supply of donor lungs, and the waiting list mortality is significant. Survival after lung transplantation is still limited compared to other solid-organ transplants, with a median survival of 6.7 y for patients transplanted between 2010 and 2017.1 To decrease waiting list mortality and to improve outcomes after lung transplantation, the lung allocation score (LAS) was developed.2 The LAS is used to create a ranking order of patients on the waiting list and is based on a “net benefit” concept. The score incorporates the estimated medical urgency for lung transplantation, as well as the probability of success after transplantation.3
The LAS was introduced in the United States in 2005 and in Germany in 2011. Favorable outcomes have been observed in both countries. The annual number of transplant procedures increased, even though the number of available donors did not. Waiting list mortality decreased, and 1-y survival after transplantation increased. The introduction of the LAS was associated with a relative increase of the number of transplant procedures for patients with pulmonary fibrosis compared to patients with obstructive lung disease.4,5
In The Netherlands, the LAS was introduced for national allocation in April 2014. The Dutch lung allocation system is also incorporated in the supranational Eurotransplant allocation system. Donor lungs are exchanged within Eurotransplant based on the LAS while maintaining a balanced exchange between countries. Candidates with a LAS ≥50 are put on top of the donor match list if there is a negative total balance between the candidate and donor country. Patients with a LAS <50 are sorted among the donor country matches in accordance with their LAS values if there is a negative total balance between the candidate and donor country.6
The Netherlands was the third country where the LAS was introduced, but the effects of this allocation system change have not been reported. Here, we report on the waiting list dynamics and outcomes after lung transplantation in The Netherlands before and after the introduction of the LAS.
MATERIALS AND METHODS
Data were retrieved from the Dutch Transplant Foundation database. All patients on the waiting list from January 1, 2010, to December 31, 2019, were included. The available information included diagnosis, age at placement on the waiting list, outcomes on the waiting list, LAS at placement on the waiting list, and LAS during follow-up, as well as outcomes after transplantation for transplanted patients. Follow-up for all patients ended at December 31, 2019. We excluded patients who were <18 y old at the time of listing for transplantation (n = 39), patients who were listed for a combined heart-lung transplantation (n = 5), patients who did not have a registered diagnosis (n = 5), patients who were listed for retransplantation (n = 27), and patients who were eventually transplanted outside of the Eurotransplant region (n=2). A flow chart is shown in Figure S1, SDC, http://links.lww.com/TXD/A363. Patients were removed from the waiting list because they were unfit for transplantation (n = 73), because they had recovered (n = 11), or because of other reasons (N = 44).
Diagnoses were subdivided into 5 categories: (1) chronic obstructive pulmonary disease (COPD) or emphysema; (2) pulmonary fibrosis; (3) cystic fibrosis; (4) pulmonary hypertension; and (5) other. A detailed list is provided in Table S1, SDC, http://links.lww.com/TXD/A363. These categories are not the same as initially used when developing the LAS,2 but comparable to those that were used by Egan and Edwards4 in a more recent analysis of the impact of the introduction of the LAS. We think that the classification that we used provides results that can be translated to clinical practice more easily.
Proportions were compared using Chi-squared and Fisher exact tests, where appropriate. Median values are reported with interquartile ranges (IQRs) and were compared using the Wilcoxon signed rank test or Mann–Whitney U test as appropriate. Mean values are reported with 95% confidence intervals (95% CIs) and were compared with students’ T-test or ANCOVA as appropriate.
We performed time-dependent analyses to determine the risk (ie, chance) of transplantation and waiting list mortality for all patients before and under the LAS system. This type of analysis is more reliable than comparing the chances of transplantation for all patients transplanted before and after the introduction the LAS, as a proportion of the patients was on the waiting list before the introduction of the LAS, and was then carried over into the LAS system. Two approaches are possible: a cause-specific Cox regression and the Fine-Gray subdistribution method.7 With the first approach, candidates are censored when they experience competing risks (death or removal from the waiting list), whereas with the second approach, they stay in the risk set but are no longer at risk for the event (ie, transplantation). There is no consensus on which method is preferable, so we performed both. The models included the period (ie, before or after introduction of the LAS), diagnosis category, and transplant center. Both analyses give proportional hazards, so reference categories are needed. For the diagnosis categories, COPD/emphysema was chosen as a reference category, as this comprised the largest group of patients.
For the comparison of the LAS value at time of waiting list placement and outcome, ANCOVA was used. The model included the time, as well as diagnosis category and outcome category. For survival analyses, Kaplan–Meier curves were made and factors were compared using a Log-rank test for univariate and Cox regression for multivariate analyses. Patients were censored at the last follow-up or at retransplantation. All analyses were performed using IBM SPSS Statistics for Windows, version 26 (IBM Corp, Armonk, NY), except for the Fine-Gray analysis, which was performed using SAS software, version 9.4 for Windows (SAS Institute Inc, Cary, NC), with the PROC PHREG procedure using the EVENTCODE option. A P value <0.05 was considered to represent statistical significance. This study is approved by the thoracic advisory committee of the Dutch Transplant Society and Dutch Transplant Foundation. Patients have consented to the use of data for research purposes.
RESULTS
The total cohort comprised 1276 patients. Five hundred fifty-eight patients (44%) were diagnosed with COPD/emphysema, 395 patients had pulmonary fibrosis (31%), 176 patients had cystic fibrosis (14%), 62 patients had pulmonary hypertension (5%), and 85 patients had other diagnoses (7%) (Table (Table1).1). The number of patients on the waiting list per diagnosis category is shown in Figure Figure1.1. Before the introduction of the LAS the median number of patients on the waiting list was 200 (IQR 195–217) versus 198 (IQR 188–206) after the introduction of the LAS (P < 0.001). The number of patients with COPD/emphysema, cystic fibrosis, and other diagnoses decreased after introduction of the LAS (P < 0.001), whereas the number of patients with pulmonary fibrosis remained stable (P = 0.12), and the number of patients with pulmonary hypertension increased (P < 0.001).
TABLE 1.
Baseline characteristics for the 1276 included patients
| Variable | |
|---|---|
| Diagnosis, n (%) | |
 • COPD/emphysema • COPD/emphysema | 558 (44) |
 • Pulmonary fibrosis • Pulmonary fibrosis | 395 (31) |
 • Cystic fibrosis • Cystic fibrosis | 176 (14) |
 • Pulmonary hypertension • Pulmonary hypertension | 62 (5) |
 • Other • Other | 85 (7) |
| Median age at placement on waiting list (IQR), y | 54 (46–59) |
| Period, n (%) | |
 • Placement on waiting list before LAS, outcome before LAS • Placement on waiting list before LAS, outcome before LAS | 452 (35) |
 • Placement on waiting list before LAS, outcome after LAS • Placement on waiting list before LAS, outcome after LAS | 180 (14) |
 • Placement on waiting list after LAS, outcome after LAS • Placement on waiting list after LAS, outcome after LAS | 644 (51) |
| Outcome, n (%) | |
 • Died on waiting list • Died on waiting list | 167 (13) |
 • Removed from waiting list • Removed from waiting list | 128 (10) |
 • Still on waiting list • Still on waiting list | 217 (17) |
 • Transplanted • Transplanted | 764 (60) |
  • Unilateral lung transplant • Unilateral lung transplant | 96 (13) |
  • Bilateral lung transplant • Bilateral lung transplant | 668 (87) |
COPD, chronic obstructive pulmonary disease; IQR, interquartile range; LAS, lung allocation score.
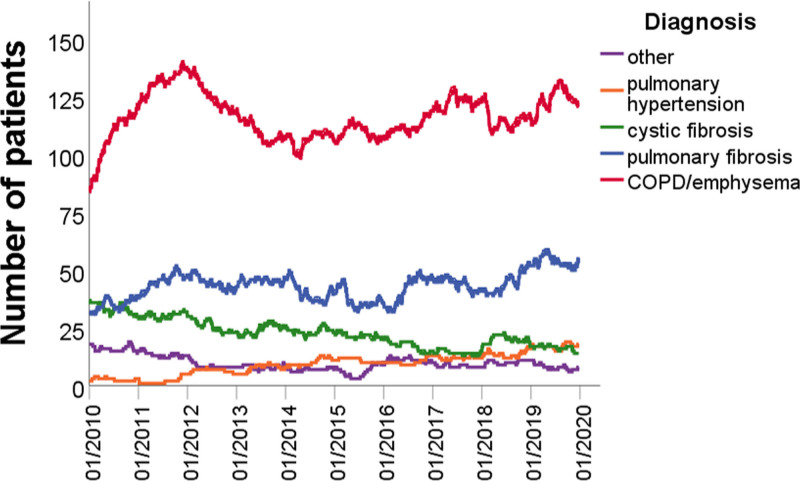
Number of patients on the waiting list per diagnosis category. The total number of patients on the waiting list was significantly higher before the introduction of the LAS (P < 0.001). The number of patients with COPD/emphysema significantly decreased after introduction of the LAS (P < 0.001), as did the number of patients with cystic fibrosis (P < 0.001) and other diagnoses (P < 0.001), whereas the number of patients with pulmonary fibrosis remained stable (P = 0.12) and the number of patients with pulmonary hypertension significantly increased (P < 0.001). COPD, chronic obstructive pulmonary disease; LAS, lung allocation score.
The number of patients placed on the waiting list every year per diagnosis category is shown in Figure Figure2.2. After introduction of the LAS, a higher percentage of transplant candidates had pulmonary fibrosis (P < 0.001), whereas the percentage of candidates with COPD/emphysema and cystic fibrosis decreased (P = 0.03 and P < 0.001). In absolute numbers, the median number of patients placed on the waiting list per year was 111 before the introduction of the LAS and 114 after the introduction of the LAS (P = 0.46). For patients with pulmonary fibrosis, this was 30 before the introduction of the LAS and 42 after the introduction of the LAS (P = 0.07). For COPD/emphysema, this was 47 and 44 (P = 0.46), and for cystic fibrosis 17 and 8, respectively (P = 0.11). The median number of patients removed from the waiting list each year was 9 before the introduction of the LAS and 12 after the introduction of the LAS (P = 1.00; Figure S2, SDC, http://links.lww.com/TXD/A363). Waiting list mortality per year is shown in Figure Figure3.3. Using a time-dependent Cox regression analysis, the overall chance of waiting list mortality was higher for patients with pulmonary fibrosis or pulmonary hypertension compared to COPD/emphysema (P < 0.001 and P = 0.002, respectively), but this did not significantly change after the introduction of the LAS (Table S2, SDC, http://links.lww.com/TXD/A363). The chance of either death on the waiting list or removal from the waiting list because a patient was unfit for transplantation was significantly higher for patients with pulmonary fibrosis (hazard ratio [HR] 2.48; P < 0.001) compared to patients with COPD/emphysema. The chance of waiting list mortality in combination with removal from the waiting list because patients were unfit for transplantation was significantly higher after the introduction of the LAS (HR 1.52; P = 0.048; Table S3, SDC, http://links.lww.com/TXD/A363).
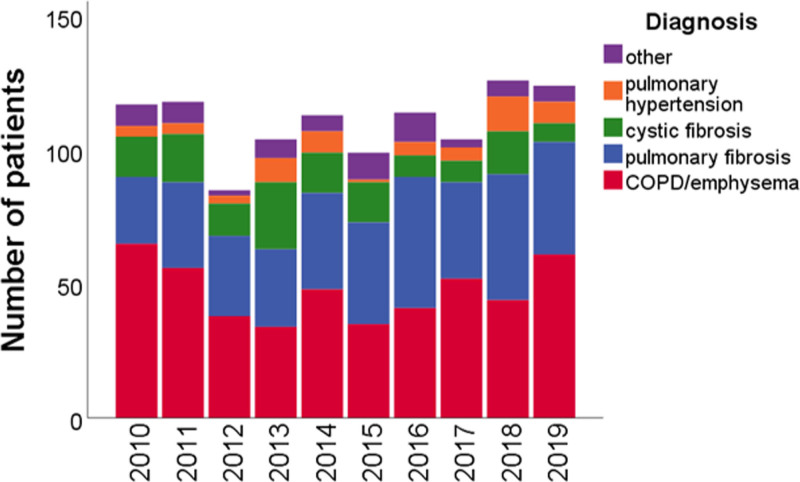
Patients placed on the waiting list per year, per diagnosis category. The annual number of patients placed on the waiting list did not significantly change after introduction of the LAS (P = 0.46; Wilcoxon signed ranks test; 2014 excluded). The percentages of patients placed on the waiting list who had COPD/emphysema or cystic fibrosis were significantly lower after introduction of the LAS (47% vs 41%, P = 0.03; 18% vs 12%; P < 0.001). The percentage of patients who had pulmonary fibrosis was significantly higher (25% vs 37%; P < 0.001). The percentages of patients who had pulmonary hypertension or other diagnoses were similar (4% vs 6%, P = 0.15; 7% vs 6%; P = 0.65). COPD, chronic obstructive pulmonary disease; LAS, lung allocation score.
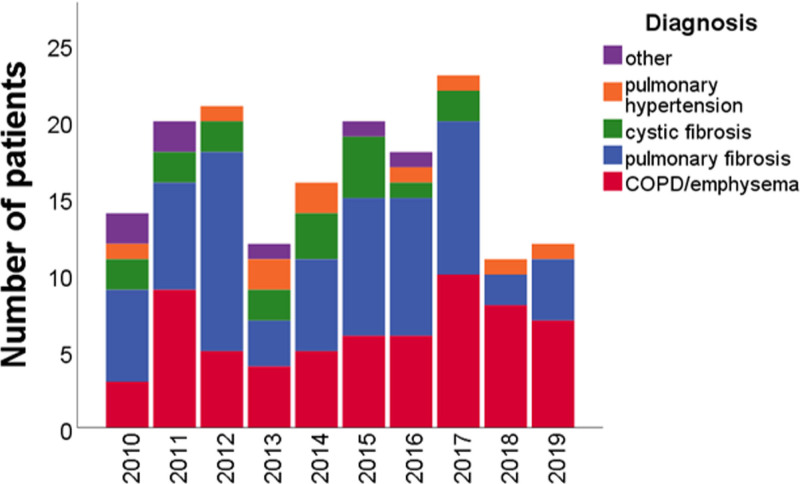
Patients who died on the waiting list per year, per diagnosis category. Annual waiting list mortality did not significantly change after introduction of the LAS (P = 0.58; Wilcoxon signed ranks test; 2014 excluded). The percentage of patients who died on the waiting list who had COPD/emphysema was not significantly different after the introduction of the LAS (P = 0.15), as were the percentages of patients who had pulmonary fibrosis (P = 0.43), cystic fibrosis (P = 0.80), pulmonary hypertension (P = 0.76), and other diagnoses (P = 0.25). COPD, chronic obstructive pulmonary disease; LAS, lung allocation score.
There was no significant change in the annual number of transplantations after the introduction of the LAS (Figure (Figure4).4). A higher percentage of transplantations was performed in patients with pulmonary fibrosis (P < 0.001), with a concomitant decrease in the percentage of patients with COPD/emphysema (P = 0.04) and cystic fibrosis (P < 0.001). In absolute numbers, the median number of transplantations was 69 per year before the introduction of the LAS, and 72 after the introduction of the LAS (P = 0.36). For patients with pulmonary fibrosis, this was 15 and 29 (P = 0.07), for patients with COPD/emphysema 32 and 30 (P = 0.20), and for patients with cystic fibrosis 17 and 10 (P = 0.07), respectively. Mean age at transplantation was significantly higher after introduction of the LAS (53.0 y [95% CI 51.9-54.0] compared to 49.2 y (47.8-50.6); P < 0.001]. Unilateral lung transplantations were significantly less common in the period after the introduction of the LAS (10% versus 17% of all lung transplantations; P = 0.003).
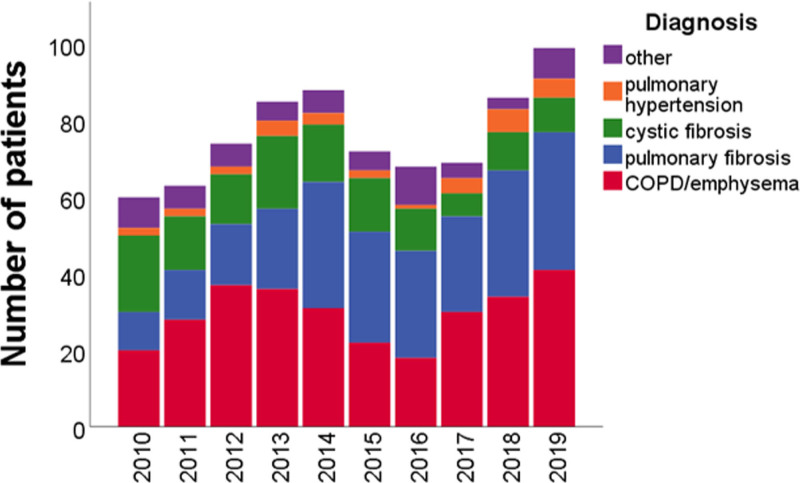
Transplanted patients per year, per diagnosis category. The annual number of transplantations did not significantly change after introduction of the LAS (P = 0.36; Wilcoxon signed ranks test; 2014 excluded). The percentage of transplanted patients who had COPD/emphysema or cystic fibrosis was significantly lower after introduction of the LAS (43% vs 36%, P = 0.04; 23% vs 13%, P = 0.001). The percentage of patients with pulmonary fibrosis was significantly higher (22% vs 39%; P < 0.001). The percentage of patients with pulmonary hypertension or other diagnoses was similar (4% vs 4%, P = 0.85; 9% vs 7%, P = 0.50). COPD, chronic obstructive pulmonary disease; LAS, lung allocation score.
In general, patients with pulmonary fibrosis, cystic fibrosis, and other diagnoses had a higher chance of transplantation compared to patients with COPD/emphysema (Table S4, SDC, http://links.lww.com/TXD/A363). The time-dependent HRs for transplantation are shown in Table Table2.2. The chance of transplantation for patients with pulmonary fibrosis was significantly higher after introduction of the LAS (HR 1.991 [95% CI 1.385-2.861); P < 0.001]. When using a Fine-Gray analysis instead of time-dependent Cox regression, the results were comparable (Table S5, SDC, http://links.lww.com/TXD/A363).
TABLE 2.
Hazard ratios for transplantation before and after introduction of the LAS, dependent on diagnosis category
| Variable | Hazard ratio (95% CI) | P |
|---|---|---|
| LAS vs pre-LAS | 1.101 (0.874-1.369) | 0.414 |
| Center 2 vs center 1 | 1.064 (0.862-1.283) | 0.519 |
| Center 3 vs center 1 | 1.614 (1.362-1.912) | <0.001 |
| LAS vs pre-LAS per diagnosis (compared to COPD) | ||
 Pulmonary fibrosis Pulmonary fibrosis | 1.991 (1.385-2.861) | <0.001 |
 Cystic fibrosis Cystic fibrosis | 1.259 (0.832-1.907) | 0.275 |
 Pulmonary hypertension Pulmonary hypertension | 0.819 (0.290-1.325) | 0.217 |
 Other Other | 1.605 (0.922-2.795) | 0.094 |
Hazard ratios were calculated using time-dependent Cox regression. The overall chance of transplantation was not different before and after the introduction of the LAS. After introduction of the LAS, the chance of transplantation significantly increased for patients with pulmonary fibrosis (P < 0.001). The interaction between center and period was not significant and was removed from the model. COPD/emphysema was used as the reference diagnosis category.
CI, confidence interval; COPD, chronic obstructive pulmonary disease; LAS, lung allocation score.
The mean LAS at the time of placement on the waiting list and at the time of transplantation or death on the waiting list are shown in Figure Figure5.5. In a linear model (Table S6, SDC, http://links.lww.com/TXD/A363), diagnosis category and outcome had a significant effect on the value of the LAS. For patients with COPD/emphysema who died on the waiting list, the LAS at the time of death was significantly lower compared to patients with pulmonary fibrosis or cystic fibrosis (P < 0.001). For patients with COPD/emphysema or pulmonary hypertension who were transplanted, the LAS at time of transplantation was significantly lower compared to patients with pulmonary fibrosis (P < 0.001).
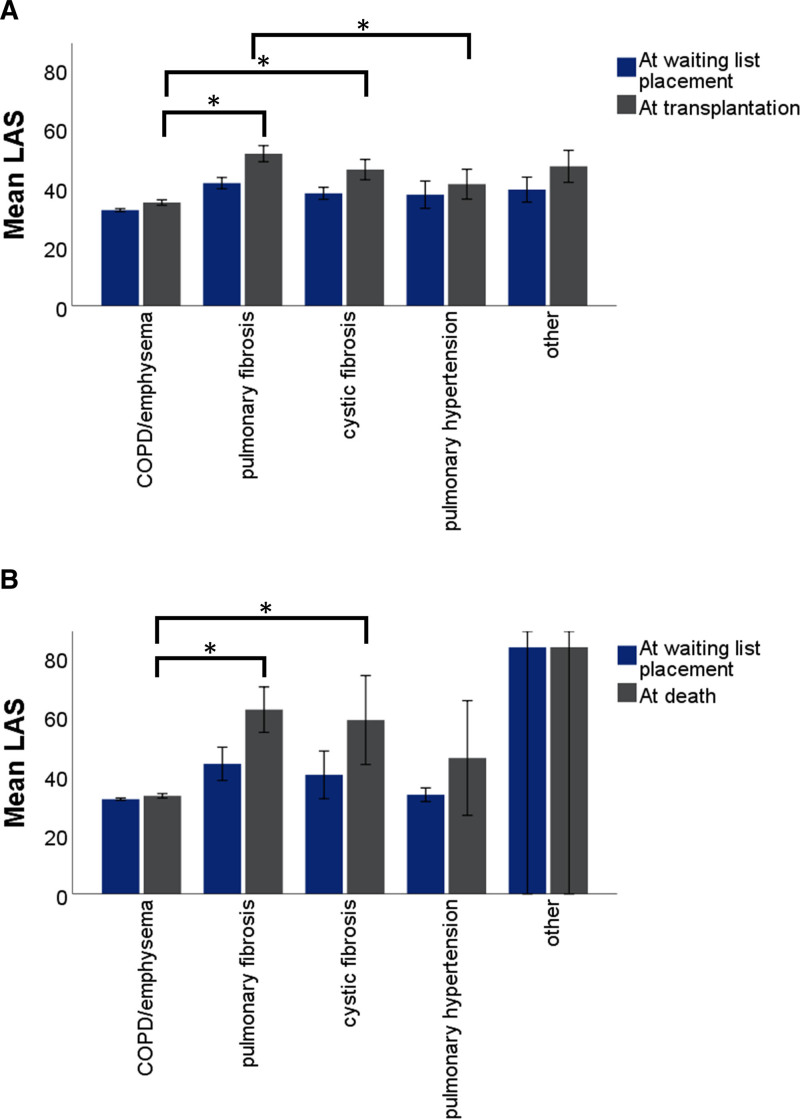
Comparison of LAS at time of placement on the waiting list to LAS at time of outcome. A, LAS at transplantation was significantly lower in patients with COPD/emphysema compared to other patients (P < 0.001). B, LAS at death was significantly lower in patients with COPD/emphysema compared to other patients (P < 0.001). Bars indicate 95% confidence intervals. *Statistically significant differences between diagnosis categories. Also see Table S5, SDC, http://links.lww.com/TXD/A363. COPD, chronic obstructive pulmonary disease; LAS, lung allocation score.
Survival after transplantation is shown in Figure Figure6.6. Overall survival was similar after the introduction of the LAS, with a 1-y survival of 84% compared to 87% before the introduction of the LAS. The 3-y survival was 75%, compared to 77% before introduction of the LAS, and 5-y survival was 68% versus 74% (P = 0.171). In a multivariate model (Table (Table3),3), younger age at transplantation was the only factor associated with longer survival (P = 0.006). Diagnosis categories were not significantly associated with survival.
TABLE 3.
Multivariate Cox regression analysis for factors influencing survival after lung transplantation
| Variable | Hazard ratio for death (95% CI) | P |
|---|---|---|
| Age at transplantation, y | 1.025 (1.007-1.042) | 0.005 |
| Diagnosis category | 0.615 | |
 Pulmonary fibrosis Pulmonary fibrosis | 0.851 (0.378-1.913) | 0.696 |
 Cystic fibrosis Cystic fibrosis | 0.736 (0.348-1.556) | 0.442 |
 Pulmonary hypertension Pulmonary hypertension | 1.072 (0.473-2.428) | 0.868 |
 Other Other | 0.846 (0.396-1.807) | 0.666 |
| Period after introduction of LAS | 0.868 (0.638-1.181) | 0.368 |
| Bilateral vs unilateral lung transplantation | 0.921 (0.619-1.372) | 0.686 |
Younger age at transplantation was significantly associated with increased survival after transplantation. A diagnosis of chronic obstructive pulmonary disease/emphysema and the period before introduction of the LAS was used as a reference category.
CI, confidence interval; LAS, lung allocation score.
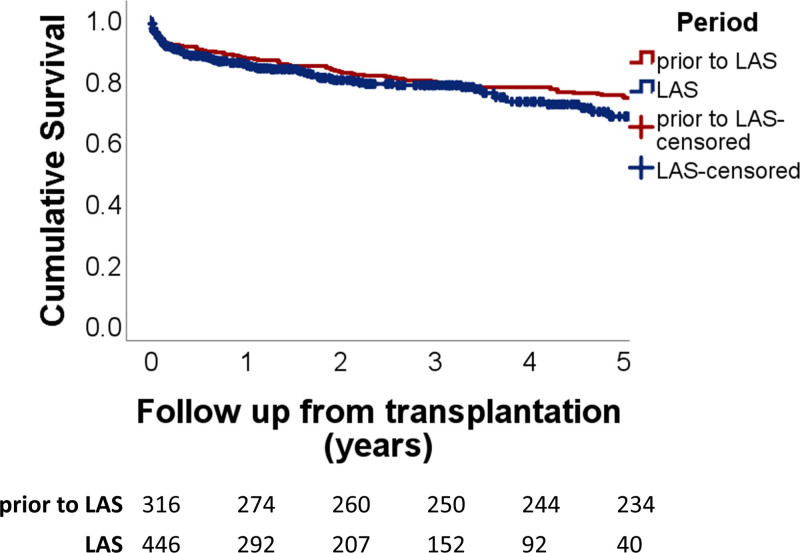
Survival after lung transplantation compared between the period before introduction of the LAS and after introduction of the LAS. Survival was similar after introduction of the LAS, with a 1-y survival of 84% compared to 87% before introduction of the LAS, 3-y survival 77% vs 79%, and 5-y survival 68% vs 74%; P = 0.171, Log-rank test. LAS, lung allocation score.
DISCUSSION
The Netherlands was the third country in the world to adopt the LAS for national allocation of donor lungs in April 2014. Here, we have studied waiting list dynamics and outcomes after transplantation after introduction of the LAS. The total number of patients on the waiting list was significantly lower after the introduction of the LAS, and this was driven by a decrease in patients with COPD/emphysema, cystic fibrosis, and other diagnoses. Patients with pulmonary fibrosis represented a significantly higher percentage of patients on the waiting list and transplantations after the introduction of the LAS.
For patients with pulmonary fibrosis, the introduction of the LAS has had a positive effect on their chances of transplantation. This was not seen for patients with COPD/emphysema, cystic fibrosis, and pulmonary hypertension, and the overall number of transplantations each year did not increase after the introduction of the LAS. The HR for transplantation in patients with pulmonary fibrosis after the introduction of the LAS compared to before the introduction of the LAS was 2.0 (95% CI 1.4-2.9), indicating an almost 2-fold increase in the chances of getting a lung transplantation in the current system compared to patients with COPD/emphysema. There was no significant change in waiting list mortality after the introduction of the LAS compared to before the introduction of the LAS.
These findings echo previously voiced concerns about the effect of the LAS on chances of transplantation for patients with COPD/emphysema, cystic fibrosis, and pulmonary hypertension compared to patients with pulmonary fibrosis.8,9 The LAS includes several parameters that are more representative for disease severity in pulmonary fibrosis than in other disease. For example, the LAS is not well tailored for the sickest patients with pulmonary hypertension.9 In the present study, we observed that the LAS in patients with COPD who died on the waiting list was significantly lower compared to patients with pulmonary fibrosis or cystic fibrosis and that they had a smaller increase of the LAS between the time of listing and death. This seems to reflect that clinical deterioration in patients with COPD/emphysema is not represented in the LAS as well as in patients with other diagnoses. Of note, our findings are in contrast with those from a study on outcomes in pulmonary hypertension patients in the United States.10 There the chances of transplantation increased, whereas the chances of dying on the waiting list decreased after the introduction of the LAS.
In the United States, the LAS has been updated,4 whereas the Eurotransplant region uses the old model, supplemented with specific business rules that better reflect the slightly different clinical characteristics of transplant recipients. The Eurotransplant business rules specify the possibility of an “exceptional LAS status” for patients with pulmonary hypertension,11,12 but there are well-defined inclusion criteria for this. An analysis of the waiting list in the Eurotransplant region in 2017 showed that it would not be significantly altered by using the updated model from the United States.11 As shown by Lehr et al,13 adding other clinical variables to the LAS, which are not included in either model now, might improve the chances of transplantation for patients with COPD/emphysema and cystic fibrosis.
In general, the introduction of the LAS in The Netherlands seems to have had a positive effect on the number of patients with pulmonary fibrosis who were transplanted but seems to have had less beneficial effects compared to the United States and Germany with regard to waiting list mortality. This could have several explanations. First, the analyses of the introduction of the LAS in the United States and Germany did not fully consider the effect of patients that were already on the waiting list under the old system and were transferred to the new system, which might have biased their results.4,5 Second, cooperation on a national level was already common before the introduction of the LAS, and the old allocation system in The Netherlands also included a high-urgency status for patients who were expected to need a transplant soon. A patient was given a high-urgency status only after agreement by all 3 centers. In the years 2010–2013, between 46% and 52% of transplants were performed in patients with a high-urgency status.14 Contrarily, implementation of the LAS in the United States was accompanied by increased coordination between transplant centers.4
Overall survival after transplantation in The Netherlands has remained similar after the introduction of the LAS. This contrasts with findings from the United States and Germany, where 1-y survival significantly increased.4,5 However, these studies did not assess survival beyond 1 y, and there have been concerns that long-term survival has actually worsened under the LAS system.15 The absence of a survival benefit in our study could be due to several factors. We found that patients transplanted after the introduction of the LAS were significantly older and that older age at transplantation was associated with significantly shorter survival. Furthermore, the LAS could have led to an increase in recipients who are critically ill before transplantation and subsequently to decreased survival after transplantation.16 Another factor might be the changed distribution of diagnosis categories, even though we did not find diagnosis category to be a predictor of survival. In addition, a recent study showed that the use of extended criteria organ donors has increased in the Eurotransplant region over the years and that this was associated with shorter survival after transplantation.17 Given these findings, the absence of a decrease in overall posttransplantation survival could actually represent a beneficial effect of the introduction of the LAS. It is important to note that an improved overall survival was never the goal of the LAS.2 The LAS does reflect the likely 1-y survival benefit of transplantation for a specific patient but only in relation to expected waiting list mortality.
This study has several limitations. First, no perfect comparison of 2 different organ allocation systems is possible, and our comparisons might have been influenced by time-dependent differences in the standard-of-care for transplant candidates and recipients, such as increased use of extracorporeal membrane oxygenation and ex vivo lung perfusion, increased double instead of single lung transplants, and the availability of new medication for the treatment of cystic fibrosis and idiopathic pulmonary fibrosis.18-20 Given that randomized controlled trials are unfeasible, we do think that our approach is the next best. Second, some comparisons, such as waiting list mortality, were limited by a small sample size. As The Netherlands is a smaller country than the United States and Germany, the number of patients with rare lung diseases such as pulmonary hypertension is lower. Third, the scarcity of clinical characteristics for the transplant recipients prohibited a thorough evaluation of the absence of a survival benefit after the introduction of the LAS.
In conclusion, after the introduction of the LAS in The Netherlands, the annual number of transplantations and waiting list mortality did not significantly change. There was a shift in diagnosis categories, and more patients with pulmonary fibrosis have been placed on the waiting list and transplanted. The chances of transplantation increased for patients with pulmonary fibrosis and decreased for patients with COPD/emphysema, cystic fibrosis, and pulmonary hypertension. This raises concerns about the capacity of the LAS to capture clinical deterioration in these patients. It should be investigated if specific changes to the LAS better reflect clinical deterioration in waiting list patients with all diagnosis types. Posttransplantation survival has remained similar after the introduction of the LAS, despite increased recipient age at transplantation.
ACKNOWLEDGMENTS
We thank Dr D. Goedhart for his advice on the statistical analyses.
Footnotes
Published online 7 September, 2021.
The authors declare no funding or conflicts of interest.
D.A.v.K. conceived of the study, A.C.H. acquired the data, T.W.H. and P.Z. performed data analyses, all authors contributed to the interpretation of the data, T.W.H. and D.A.v.K. wrote the initial draft of the manuscript, and all authors revised the manuscript for important intellectual content.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
Articles from Transplantation Direct are provided here courtesy of Wolters Kluwer Health
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/113648156
Article citations
A modular simulation framework for organ allocation.
J Heart Lung Transplant, 43(8):1326-1335, 04 May 2024
Cited by: 0 articles | PMID: 38705499 | PMCID: PMC11261589
Creating synthetic populations in transplantation: A Bayesian approach enabling simulation without registry re-sampling.
PLoS One, 19(3):e0296839, 21 Mar 2024
Cited by: 1 article | PMID: 38512928 | PMCID: PMC10956776
Sarcoidosis lung transplantation waitlist mortality, a national registry database study.
ERJ Open Res, 9(4):738-2022, 17 Jul 2023
Cited by: 3 articles | PMID: 37465560 | PMCID: PMC10350678
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Lung Transplantation in Germany Since the Introduction of the Lung Allocation Score.
Dtsch Arztebl Int, 114(11):179-185, 01 Mar 2017
Cited by: 35 articles | PMID: 28382903 | PMCID: PMC5387849
Munich lung transplant group: waiting list during the first 9 months of the lung allocation score era.
Thorac Cardiovasc Surg, 62(5):422-426, 19 Dec 2013
Cited by: 1 article | PMID: 24356999
Effect of Including Important Clinical Variables on Accuracy of the Lung Allocation Score for Cystic Fibrosis and Chronic Obstructive Pulmonary Disease.
Am J Respir Crit Care Med, 200(8):1013-1021, 01 Oct 2019
Cited by: 16 articles | PMID: 31199166
Lung allocation.
J Thorac Dis, 9(8):2670-2674, 01 Aug 2017
Cited by: 45 articles | PMID: 28932574 | PMCID: PMC5594149
Review Free full text in Europe PMC

 1,3
1,3

