Abstract
Background and purpose
Recurrent seizures have been reported to induce neuronal loss in the hippocampus. It is unclear whether seizure control influences hippocampal volume. The aims of this study were to determine if there was a change in total or subfield hippocampal volume over time in children with focal drug-resistant epilepsy, and whether seizure control influenced total or subfield hippocampal volumes.Methods
Using FreeSurfer's automated segmentation of brain magnetic resonance imaging scans, we calculated the total and subfield (including CA1, CA3, CA4, subiculum, presubiculum, parasubiculum, molecular layer and dentate gyrus) hippocampal volumes of children with non-lesional focal epilepsy. Seizure frequency and hippocampal volumes were assessed at baseline and follow-up. Patients were classified into those who were seizure free or have improvement in seizures (group 1) and those with no improvement in seizures (group 2) at follow-up.Results
Thirty-seven patients were included, with mean age 10.31 ± 3.68 years at baseline. The interval between the two magnetic resonance imaging scans was 2.59 ± 1.25 years. There was no significant difference in the total and subfield hippocampal volumes for the whole cohort at follow-up compared to baseline (all P > 0.002). Seizure control of the two groups did not predict total or subfield hippocampal volume, after controlling for baseline volume, age, severity of seizure frequency at baseline and time interval between the magnetic resonance imaging scans (all P > 0.002).Conclusion
We have found that total and subfield hippocampal volumes did not change, and seizure control did not predict hippocampal volumes at follow-up in children with drug-resistant epilepsy.Free full text

Seizure control does not predict hippocampal subfield volume change in children with focal drug-resistant epilepsy
Abstract
Background and purpose
Recurrent seizures have been reported to induce neuronal loss in the hippocampus. It is unclear whether seizure control influences hippocampal volume. The aims of this study were to determine if there was a change in total or subfield hippocampal volume over time in children with focal drug-resistant epilepsy, and whether seizure control influenced total or subfield hippocampal volumes.
Methods
Using FreeSurfer’s automated segmentation of brain magnetic resonance imaging scans, we calculated the total and subfield (including CA1, CA3, CA4, subiculum, presubiculum, parasubiculum, molecular layer and dentate gyrus) hippocampal volumes of children with non-lesional focal epilepsy. Seizure frequency and hippocampal volumes were assessed at baseline and follow-up. Patients were classified into those who were seizure free or have improvement in seizures (group 1) and those with no improvement in seizures (group 2) at follow-up.
Results
Thirty-seven patients were included, with mean age 10.31 ±
± 3.68 years at
baseline. The interval between the two magnetic resonance imaging scans was
2.59
3.68 years at
baseline. The interval between the two magnetic resonance imaging scans was
2.59 ±
± 1.25 years. There was no significant difference in the total and
subfield hippocampal volumes for the whole cohort at follow-up compared to
baseline (all P
1.25 years. There was no significant difference in the total and
subfield hippocampal volumes for the whole cohort at follow-up compared to
baseline (all P >
> 0.002). Seizure control of the two
groups did not predict total or subfield hippocampal volume, after
controlling for baseline volume, age, severity of seizure frequency at
baseline and time interval between the magnetic resonance imaging scans (all
P
0.002). Seizure control of the two
groups did not predict total or subfield hippocampal volume, after
controlling for baseline volume, age, severity of seizure frequency at
baseline and time interval between the magnetic resonance imaging scans (all
P >
> 0.002).
0.002).
Conclusion
We have found that total and subfield hippocampal volumes did not change, and seizure control did not predict hippocampal volumes at follow-up in children with drug-resistant epilepsy.
Introduction
Drug-resistant epilepsy (DRE) has been defined as the ‘failure of adequate trials of two tolerated, appropriately chosen and used antiseizure medications to achieve seizure freedom’. 1 Several studies have shown that children with mesial temporal lobe and extra-temporal lobe epilepsy could have hippocampal volume reduction compared to controls.2–8 Decreased volume of the hippocampal head, body and tail and total hippocampal volume were identified even when the hippocampus appeared normal by visual inspection on magnetic resonance imaging (MRI) in children with non-lesional focal epilepsy. 3 Hippocampal volume has not been shown to be associated with age at seizure onset or duration of epilepsy in children with focal epilepsy.4,9 However, in adults with temporal lobe epilepsy, volume reduction in the hippocampus has been shown to correlate with the duration of epilepsy.10–12 Most studies that have assessed hippocampal volumes in children with epilepsy have evaluated total or regional hippocampal volume; that is, hippocampal head, body and tail, and have been cross-sectional in design.4–6,8,11,13,14 Hence, it is uncertain if hippocampal volumes decline over time with ongoing seizures.
The aims of this study were to determine if there was a change in total or subfield hippocampal volume over time in children with focal DRE, and whether seizure control influenced total or subfield hippocampal volume.
Methods
Subjects
This study was approved by the research ethics board at the Hospital for Sick Children. Informed consent was obtained from parents and assent from children. Children with non-lesional focal epilepsy and had two sequential MRIs on 3T with dedicated high-resolution epilepsy protocol at least one year apart were included in the study. Exclusion criteria included children less than 6 years of age at baseline as they were unable to undergo the MRI without general anaesthesia. The baseline MRI was done as part of a research study, and the follow-up MRI was done as part of clinical MRI. Demographic and clinical variables were collected through chart reviews. Focal epilepsy was based on ictal and interictal video-electroencephalography (EEG), magnetoencephalography (MEG) and fluorodeoxyglucose-positron emission tomography (FDG-PET) scan.
MRI scanning
MRIs were performed on a Philips 3T scanner (Achieva, Philips Medical System,
Best, The Netherlands) using an eight channel phased array head coil in all
patients. The imaging in patients and controls included axial volumetric T1
(TR/TE 4.9/2.3 msec, slice thickness 1 mm, field of view (FOV) 22 cm, matrix 220
9 220), angled to the anterior commissure-posterior commissure plane. Additional
sequences done on the same scanner included axial and coronal fluid-attenuated
inversion recovery (FLAIR), T2-weighted and proton density.
mm, field of view (FOV) 22 cm, matrix 220
9 220), angled to the anterior commissure-posterior commissure plane. Additional
sequences done on the same scanner included axial and coronal fluid-attenuated
inversion recovery (FLAIR), T2-weighted and proton density.
FreeSurfer automated hippocampal subfield segmentation
Automated segmentation of the whole hippocampus and hippocampal subfield volumes
including cornu ammonis (CA)1, CA2/3, CA4, subiculum, presubiculum,
parasubiculum, molecular layer and dentate gyrus volumes was carried out using
FreeSurfer, v6.0 (http://surfer.nmr.mgh.harvard.edu). FreeSurfer uses
probabilistic information estimated from a large training set of expert
measurements to assign automatically a neuroanatomical label to each voxel in
the brain.
15
The technical details of the FreeSurfer whole brain segmentation
procedure have been described in detail in previous publications.16–19 In short, FreeSurfer was
used to detect automatically the grey matter and white matter of all brain
structures.20,21 After that, the subroutine of the hippocampal subfield detection
22
was implemented to generate subfield volumes including CA4, CA3 +
+ CA2
(delineated in combination by the algorithm), CA1, hippocampal tail, subiculum,
presubiculum, parasubiculum, molecular layer hippocampus (HP), granule cell and
molecular layer of the dentate gyrus (GC-ML-DG), hippocampal amygdala transition
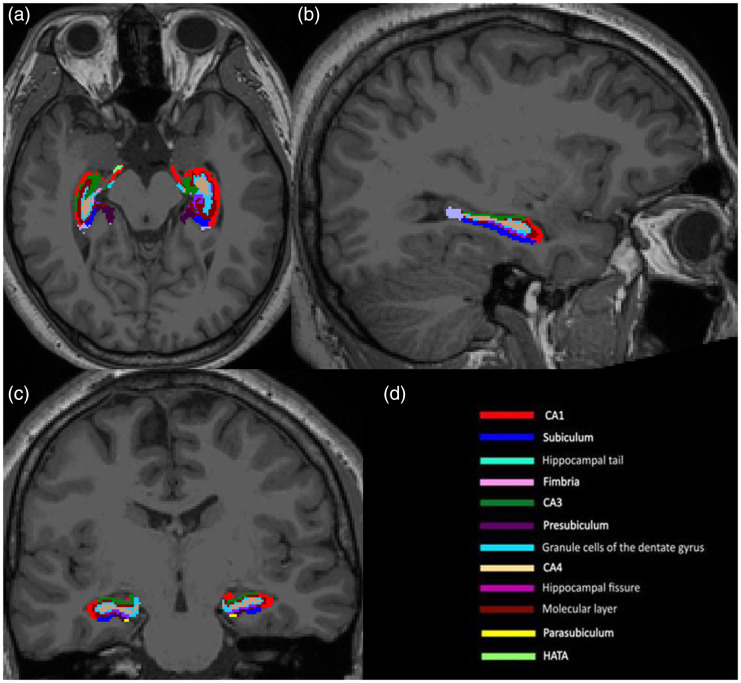
area (HATA), fimbria and total hippocampus (Figure 1). FreeSurfer segmentations of
the whole hippocampus and hippocampal subfields were visually inspected for
accuracy.
CA2
(delineated in combination by the algorithm), CA1, hippocampal tail, subiculum,
presubiculum, parasubiculum, molecular layer hippocampus (HP), granule cell and
molecular layer of the dentate gyrus (GC-ML-DG), hippocampal amygdala transition
area (HATA), fimbria and total hippocampus (Figure 1). FreeSurfer segmentations of
the whole hippocampus and hippocampal subfields were visually inspected for
accuracy.
Seizure control
We assessed seizure frequency at baseline and follow-up MRI, and compared seizure control at follow-up relative to baseline. Seizure control was adapted from the Engel classification.23,24 The patients were classified as free of disabling seizures (Engel 1), had rare disabling seizures (Engel II), worthwhile improvement in seizures (Engel III) and no worthwhile improvement of seizures (Engel IV) at follow-up compared to baseline. The patients were classified into two groups based on seizure control: group 1 consisted of those who were free of disabling seizures, had rare disabling seizures or worthwhile improvement in seizures, that is, Engel I–III; group 2 consisted of those without worthwhile improvement in seizures, that is, Engel IV. Seizure severity at baseline was assessed and binarised into (a) severe: more than once a month and (b) less severe: less than once a month.
Statistical analysis
Statistical analyses were performed with the statistical package for social sciences software (SPSS) version 18 (IBM SPSS, New York, USA). For baseline characteristics, continuous data were presented using mean and standard deviation (SD), and categorical data were presented using integers and percentages. Two-tailed t-tests were used to assess for group differences in age at baseline and follow-up MRI, as well as interval between the two MRI scans. We used a simple linear regression model to assess the total or subfield hippocampal volumes at follow-up related to the seizure control group adjusting for baseline total or subfield volumes, age and time interval between the MRI scans. Bonferroni correction was conducted to account for multiple comparison, and a P value of less than 0.002 (=0.05/24 (number of comparisons)) was considered as statistically significant. 25
Results
Subjects
A total of 140 children with epilepsy and no epileptogenic lesion older than 5
years of age were screened for baseline and follow-up MRI brain. Of these, 103
children did not have follow-up brain MRI. Thus, 37 children (12 girls and 25
boys) were included in this study. The mean age was 10.31 ±
± 3.68 years (range
3.79–17.34 years) at first MRI. Sixteen patients had left-sided epilepsy and 21
had right-sided epilepsy. Nineteen patients had frontal lobe epilepsy (nine
left-sided and 10 right-sided), seven had temporal lobe epilepsy (two left-sided
and five right-sided), six had parietal lobe epilepsy (four left-sided and two
right-sided) and five had multilobar epilepsy (one left-sided and four
right-sided). The mean age at seizure onset for all patients was 6.25
3.68 years (range
3.79–17.34 years) at first MRI. Sixteen patients had left-sided epilepsy and 21
had right-sided epilepsy. Nineteen patients had frontal lobe epilepsy (nine
left-sided and 10 right-sided), seven had temporal lobe epilepsy (two left-sided
and five right-sided), six had parietal lobe epilepsy (four left-sided and two
right-sided) and five had multilobar epilepsy (one left-sided and four
right-sided). The mean age at seizure onset for all patients was 6.25 ±
± 4.18
years and the mean duration of epilepsy at first MRI was 4.07
4.18
years and the mean duration of epilepsy at first MRI was 4.07 ±
± 3.47 years. The
median number of antiepileptic drugs at baseline for all patients was two (range
one to four).
3.47 years. The
median number of antiepileptic drugs at baseline for all patients was two (range
one to four).
The mean interval between the two MRI scans was 2.59 ±
± 1.25 years. Five patients
were free of disabling seizures, five had rare disabling seizures, three had
worthwhile improvement in seizures and 24 had no worthwhile improvement in
seizures. Eleven children (29.7%) had a less severe seizure burden at baseline.
Of these, two patients were in the group 1 category. Twenty-six patients (70.3%)
had a severe seizure burden at baseline. Of these, 11 patients were in the group
1 category.
1.25 years. Five patients
were free of disabling seizures, five had rare disabling seizures, three had
worthwhile improvement in seizures and 24 had no worthwhile improvement in
seizures. Eleven children (29.7%) had a less severe seizure burden at baseline.
Of these, two patients were in the group 1 category. Twenty-six patients (70.3%)
had a severe seizure burden at baseline. Of these, 11 patients were in the group
1 category.
Mean age at first and second MRI and interval between the scans were not
significantly different between groups 1 and 2 (all
P >
> 0.002). Table 1 summarises patient
characteristics according to their group.
0.002). Table 1 summarises patient
characteristics according to their group.
Table 1.
Characteristics of 37 patients.
Group 1 (n = = 13, 35%) 13, 35%) | Group 2 (n = = 24, 65%) 24, 65%) | P value | |
|---|---|---|---|
| Engel class I | 5 | N/A | |
| Engel class II | 5 | N/A | |
| Engel class III | 3 | N/A | |
| Engel class IV | N/A | 24 | |
| Female:male (absolute and ratio) | 3:10 (25%:40%) | 9:15 (75%:60%) | |
Mean age  ± ±  SD at first MRI (years) SD at first MRI (years) | 11.33 ± ± 4.65 4.65 | 9.77 ± ± 2.88 2.88 | 0.22 |
Mean age  ± ±  SD at second MRI (years) SD at second MRI (years) | 14.17 ± ± 5.15 5.15 | 12.22 ± ± 2.88 2.88 | 0.15 |
Mean interval  ± ±  SD between the scans (years) SD between the scans (years) | 2.84 ± ± 1.38 1.38 | 2.45 ± ± 1.56 1.56 | 0.46 |
| Right-sided:left-sided EEG findings | 9:4 (43%:25%) | 12:12 (57%:75%) | |
Mean duration of epilepsy  ± ±  SD at first MRI (years) SD at first MRI (years) | 2.74 ± ± 2.62 2.62 | 4.79 ± ± 3.65 3.65 | 0.08 |
| Median number and range of antiepileptic medications | 2 [1-4] | 2 [1-3] | |
| Seizure severity at baseline MRI, severe:less severe | 11:2 (42%:18%) | 15:9 (58%:82%) |
EEG: electroencephalography; MRI: magnetic resonance imaging; SD: standard deviation.
Total and subfield hippocampal volumes: whole cohort
The baseline and follow-up total and subfield hippocampal volumes for the whole
cohort are shown in Table
2. There was no significant difference in left versus right
hippocampal and hippocampal subfield volumes for the whole cohort (all
P >
> 0.002). No significant differences were found in the
total hippocampal and hippocampal subfield volumes for the whole cohort at
follow-up compared to baseline (all P
0.002). No significant differences were found in the
total hippocampal and hippocampal subfield volumes for the whole cohort at
follow-up compared to baseline (all P >
> 0.002).
0.002).
Table 2.
Whole cohort comparison of volumes in mean mm³  ±
± SD at baseline and at
follow-up MRI.
SD at baseline and at
follow-up MRI.
| 1st MRI | 2nd MRI | |
|---|---|---|
| Right | ||
 Hippocampal tail Hippocampal tail | 476.50 ± ± 64.69 64.69 | 474.96 ± ± 63.96 63.96 |
 Subiculum Subiculum | 418.28 ± ± 46.59 46.59 | 421.68 ± ± 49.17 49.17 |
 CA1 CA1 | 634.16 ± ± 82.03 82.03 | 632.03 ± ± 87.31 87.31 |
 Presubiculum Presubiculum | 300.80 ± ± 40.98 40.98 | 300.38 ± ± 44.01 44.01 |
 Parasubiculum Parasubiculum | 65.98 ± ± 11.91 11.91 | 66.38 ± ± 15.88 15.88 |
 Molecular layer HP Molecular layer HP | 559.15 ± ± 62.72 62.72 | 558.39 ± ± 63.15 63.15 |
 GC-ML-DG GC-ML-DG | 294.22 ± ± 32.17 32.17 | 292.58 ± ± 28.21 28.21 |
 CA3 CA3 | 208.00 ± ± 30.92 30.92 | 206.04 ± ± 30.67 30.67 |
 CA4 CA4 | 253.06 ± ± 26.50 26.50 | 249.93 ± ± 23.76 23.76 |
 Fimbria Fimbria | 88.79 ± ± 19.64 19.64 | 92.68 ± ± 21.73 21.73 |
 HATA HATA | 58.42 ± ± 8.59 8.59 | 59.72 ± ± 8.70 8.70 |
 Whole hippocampus Whole hippocampus | 3357.36 ± ± 341.72 341.72 | 3354.76 ± ± 351.79 351.79 |
| Left | ||
 Hippocampal tail Hippocampal tail | 482.63 ± ± 70.73 70.73 | 479.14 ± ± 68.38 68.38 |
 Subiculum Subiculum | 424.40 ± ± 47.58 47.58 | 427.21 ± ± 49.98 49.98 |
 CA1 CA1 | 613.58 ± ± 88.52 88.52 | 616.00 ± ± 85.64 85.64 |
 Presubiculum Presubiculum | 314.20 ± ± 34.39 34.39 | 316.15 ± ± 36.91 36.91 |
 Parasubiculum Parasubiculum | 68.78 ± ± 11.54 11.54 | 69.47 ± ± 13.73 13.73 |
 Molecular layer HP Molecular layer HP | 548.18 ± ± 66.68 66.68 | 551.88 ± ± 65.67 65.67 |
 GC-ML-DG GC-ML-DG | 280.51 ± ± 33.96 33.96 | 282.56 ± ± 33.00 33.00 |
 CA3 CA3 | 190.15 ± ± 35.92 35.92 | 189.38 ± ± 35.08 35.08 |
 CA4 CA4 | 239.16 ± ± 27.96 27.96 | 241.06 ± ± 28.51 28.51 |
 Fimbria Fimbria | 92.76 ± ± 20.44 20.44 | 94.37 ± ± 18.17 18.17 |
 HATA HATA | 55.91 ± ± 8.82 8.82 | 56.79 ± ± 8.49 8.49 |
 Whole hippocampus Whole hippocampus | 3310.25 ± ± 370.63 370.63 | 3324.02 ± ± 357.92 357.92 |
 Whole brain Whole brain  volume of volume of  1st and 2nd MRI 1st and 2nd MRI | 1,184,294.38 ±102,951.96 ±102,951.96 | 1,174,004.70 ±102,542.59 ±102,542.59 |
FreeSurfer results are shown for hippocampus and hippocampal subfield
segmentations in mm³ (mean  ±
± SD).
SD).
CA: cornu ammonis; HP: hippocampus; GC-ML-DG: granule cell and molecular layer of the dentate gyrus; HATA: hippocampal amygdala transition area; MRI: magnetic resonance imaging; SD: standard deviation.
Individually, 23 patients have an increase of more than 10% in subfield hippocampal volumes, and seven patients have an increase of more than 10% in total hippocampal volumes. Twenty-five patients have a decrease of more than 10% in subfield hippocampal volumes, and two patients have a decrease of more than 10% in total hippocampal volumes. One patient had right frontal epilepsy and showed a 12.1% decrease in the size of the right hippocampus, including a 51.43% decrease of the parasubiculum, 24.76% decrease of the presubiculum and a 16.16% decrease of the fimbria. The other patient had left parietal epilepsy and showed a 11.51% decrease in the size of the left hippocampus, including a 25.2% decrease of the hippocampal tail, a 19.27% decrease of the CA3 and a 15.65% decrease of the CA1.
Seizure control versus total and subfield hippocampal volumes
The right and left total and subfield hippocampal volumes of group 1 and group 2
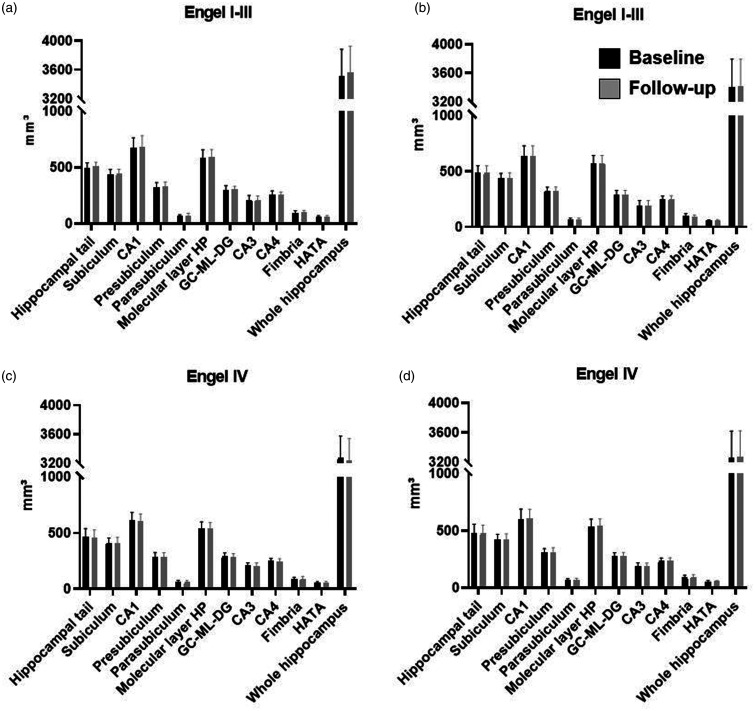
at baseline and follow-up are shown in Figure 2. There were no significant
associations between total or subfield hippocampal volumes at follow-up and
seizure control group, after adjusting for baseline volume, age, seizure
severity at baseline and time interval between the MRI scans (all
P >
> 0.002). Regression coefficients and
P values are summarised in Supplementary Table 1. From
group 1, nine patients demonstrated a hippocampal subfield volume decrease
greater than 10% including the fimbria (five patients), hippocampal tail (two
patients), parasubiculum (two patients), HATA (one patient), CA1 (one patient),
presubiculum (one patient) and CA3 (one patient). From group 2, 15 patients
demonstrated a hippocampus or a hippocampal subfield volume decrease greater
than 10% including parasubiculum (eight patients), fimbria (five patients), CA3
(four patients), HATA (three patients), presubiculum (three patients),
hippocampal tail (three patients), subiculum (three patients), CA1 (two
patients), CA4 (two patients), whole hippocampus (two patients), GC-ML-DG (one
patient) and molecular layer HP (one patient) (see Supplementary Table 2).
0.002). Regression coefficients and
P values are summarised in Supplementary Table 1. From
group 1, nine patients demonstrated a hippocampal subfield volume decrease
greater than 10% including the fimbria (five patients), hippocampal tail (two
patients), parasubiculum (two patients), HATA (one patient), CA1 (one patient),
presubiculum (one patient) and CA3 (one patient). From group 2, 15 patients
demonstrated a hippocampus or a hippocampal subfield volume decrease greater
than 10% including parasubiculum (eight patients), fimbria (five patients), CA3
(four patients), HATA (three patients), presubiculum (three patients),
hippocampal tail (three patients), subiculum (three patients), CA1 (two
patients), CA4 (two patients), whole hippocampus (two patients), GC-ML-DG (one
patient) and molecular layer HP (one patient) (see Supplementary Table 2).
Discussion
We did not find a difference in total or subfield hippocampal volume at follow-up compared to baseline for the whole cohort. Further, we found that there were no associations between total or subfield hippocampal volumes at follow-up and with seizure control (group 1 vs. group 2), after adjusting for baseline volumes, age and time interval between the MRI scans.
Polli et al. 26 evaluated the hippocampal volumes in rats treated with kainic acid or pilocarpine to induce status epilepticus, and their hippocampal volumes were measured on MRI at 3, 6 and 9 months. Both groups had a time-specific reduction in MRI hippocampal volume compared to controls, but the hippocampal volume reduction did not progress over time. The authors concluded that hippocampal atrophy once detected remains stable, despite continuous seizures. 26 Similar findings were reported in an adult study with 179 patients. 27 Thirty-three of the 179 patients were diagnosed with cryptogenic temporal lobe epilepsy. No significant correlation was observed between the frequency of convulsive seizures, partial seizures, epilepsy duration, antiepileptic drug use and change in ipsilateral hippocampal volume. 27 Our findings were in line with the literature, in that we did not find a significant volume loss of total or subfield hippocampal volumes at follow-up compared to baseline, nor volume loss based on seizure control over time in children with focal DRE.
Previous studies have evaluated hippocampal total or subregion volumes such as hippocampal head, body and tail volume, and demonstrated reduced hippocampal volumes in children with focal epilepsy.3–8,28,29 Reduced total and regional hippocampal volumes were identified in those with non-lesional epilepsy and have normal appearing hippocampus, both ipsilateral and contralateral to seizure focus. 3 Those studies were cross-sectional in design. To our knowledge, there has been no longitudinal study that has evaluated total or subfield hippocampal volumes in children with focal epilepsy. It is possible that hippocampal volume reduction may be time specific, occurring around the time of seizure onset, but does not progress with time, similar to that observed in an animal model. 26 Alternatively, there may be microstructural changes in the hippocampal subfields that may not be detected using volumetric measures. A prior study has also shown that there was no significant volume loss longitudinally in total, cerebral or hemispheric grey or white matter in children with focal epilepsy, 30 suggesting that volume loss does not occur despite ongoing seizures.
A prior systematic review and meta-analysis has shown that neuronal loss was observed in hippocampal subfields CA1 to CA4 in patients with hippocampal sclerosis. 31 In vivo MRI of hippocampal body subfield identified abnormality in subfield volume in patients with hippocampal sclerosis, which corresponded with International League Against Epilepsy (ILAE) hippocampal sclerosis subtypes based on histology. 32 Voets et al. 33 evaluated patients with MRI-negative temporal lobe epilepsy and found hippocampal subfield atrophy in nine out of 12 (75%) patients, commonly affecting CA3. Studies that have assessed hippocampal subfields have done so in adults with temporal lobe epilepsy, and were cross-sectional studies comparing hippocampal subfield volumes on MRI to histological assessment. Our study has evaluated hippocampal subfield volumes longitudinally in children. Although we did not find a difference in total or subfield volumes at follow-up relative to baseline for the whole cohort, 25 patients demonstrated a reduction in total and/or subfield volume by more than 10%. It is possible that there are individuals who may be more susceptible to injury related to ongoing seizures than others, and average group differences may not be sufficiently sensitive to identify subtle changes in hippocampal volumes.
Our study has limitations. We used FreeSurfer’s automated segmentation of the hippocampal subfields. Manual segmentation of the hippocampus was reported to be more sensitive in detecting hippocampal atrophy than automated segmentation,13,17,34 but it is time consuming and prone to operator error. Automated segmentation of the hippocampal subfields using FreeSurfer has the potential to provide rapid and accurate assessment of the hippocampal volume, and has been shown to be sensitive at detecting hippocampal atrophy.13,17,35,36 Whelan et al. showed that FreeSurfer version 6 is a reliable tool for segmenting hippocampal subfields, with intraclass correlation of 0.90 or greater. 37 Herten et al. recommended utilising both T1 and T2-weighted images for FreeSurfer’s automated segmentation of the hippocampus to increase accuracy. 38 However, volumetric T2-weighted images were not available for this study. We have included children with a variety of epilepsy types. It is uncertain whether those with temporal lobe epilepsy would be more likely to demonstrate hippocampal volume loss over time relative to extra-temporal epilepsy. Bernasconi et al. showed that in adults with temporal lobe epilepsy, hippocampal, amygdala and entorhinal atrophy was associated with the duration of epilepsy. 39 In addition, it is possible that with an increase in the time interval between baseline and follow-up, total and subfield hippocampal volumes could demonstrate significant volumetric changes. Other limitations are the moderate number of patients at various ages, the single-centre nature of this study and the relative lack of controls with longitudinal MRI. Recruiting and retaining healthy children as controls in longitudinal research is challenging. In a previous cross-sectional study, we have compared the hippocampal volumes in children with non-lesional focal epilepsy to healthy controls and found smaller hippocampal subregion volumes in patients relative to controls. 3
In conclusion, our study did not show a difference in total or subfield hippocampal volumes at follow-up compared to baseline, although 25 patients showed a 10% reduction in total or subfield hippocampal volumes at follow-up compared to baseline. We also found that seizure control did not predict total or subfield hippocampal volumes at follow-up. Future study is required to validate our findings.
Supplemental Material
Supplemental material, sj-pdf-1-neu-10.1177_19714009211049078 for Seizure control does not predict hippocampal subfield volume change in children with focal drug-resistant epilepsy by Matthias W. Wagner, Jovanka Skocic and Elysa Widjaja in The Neuroradiology Journal
Footnotes
Conflict of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: This study has the approval of institutional research ethics board.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by Sickkids Foundation/CIHR Institute of Human Development, Child and Youth Health, and EpLink – the Epilepsy Research Program of the Ontario Brain Institute. The Ontario Brain Institute is an independent non-profit corporation, funded partially by the Ontario government. The opinions, results and conclusions are those of the authors and no endorsement by the Ontario Brain Institute is intended or should be inferred.
ORCID iD: Matthias W. Wagner https://orcid.org/0000-0001-6501-839X
Supplemental material: Supplemental material for this article is available online.
References
Articles from The Neuroradiology Journal are provided here courtesy of SAGE Publications
Full text links
Read article at publisher's site: https://doi.org/10.1177/19714009211049078
Read article for free, from open access legal sources, via Unpaywall:
https://journals.sagepub.com/doi/pdf/10.1177/19714009211049078
Citations & impact
Impact metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1177/19714009211049078
Article citations
MRI Volumetric Analysis of the Hypothalamus and Limbic System across the Pediatric Age Span.
Children (Basel), 10(3):477, 27 Feb 2023
Cited by: 0 articles | PMID: 36980035 | PMCID: PMC10047273
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Automated subfield volumetric analysis of amygdala, hippocampus, and thalamic nuclei in mesial temporal lobe epilepsy.
World Neurosurg X, 19:100212, 13 May 2023
Cited by: 0 articles | PMID: 37304157 | PMCID: PMC10250154
In vivo hippocampal cornu ammonis 1-3 glutamatergic abnormalities are associated with temporal lobe epilepsy surgery outcomes.
Epilepsia, 62(7):1559-1568, 31 May 2021
Cited by: 3 articles | PMID: 34060082
Hippocampal subfield segmentation in temporal lobe epilepsy: Relation to outcomes.
Acta Neurol Scand, 137(6):598-608, 23 Mar 2018
Cited by: 10 articles | PMID: 29572865 | PMCID: PMC5969077
Quantification of subfield pathology in hippocampal sclerosis: a systematic review and meta-analysis.
Epilepsy Res, 108(8):1279-1285, 23 Jul 2014
Cited by: 31 articles | PMID: 25107686
Review
Funding
Funders who supported this work.