Abstract
Background and objectives
To report the clinical, biological, and imaging features and clinical course of a French cohort of patients with glial fibrillary acidic protein (GFAP) autoantibodies.Methods
We retrospectively included all patients who tested positive for GFAP antibodies in the CSF by immunohistochemistry and confirmed by cell-based assay using cells expressing human GFAPα since 2017 from 2 French referral centers.Results
We identified 46 patients with GFAP antibodies. Median age at onset was 43 years, and 65% were men. Infectious prodromal symptoms were found in 82%. Other autoimmune diseases were found in 22% of patients, and coexisting neural autoantibodies in 11%. Tumors were present in 24%, and T-cell dysfunction in 23%. The most frequent presentation was subacute meningoencephalitis (85%), with cerebellar dysfunction in 57% of cases. Other clinical presentations included myelitis (30%) and visual (35%) and peripheral nervous system involvement (24%). MRI showed perivascular radial enhancement in 32%, periventricular T2 hyperintensity in 41%, brainstem involvement in 31%, leptomeningeal enhancement in 26%, and reversible splenial lesions in 4 cases. A total of 33 of 40 patients had a monophasic course, associated with a good outcome at last follow-up (Rankin Score ≤2: 89%), despite a severe clinical presentation. Adult and pediatric features are similar. Thirty-two patients were treated with immunotherapy. A total of 11/22 patients showed negative conversion of GFAP antibodies.Discussion
GFAP autoimmunity is mainly associated with acute/subacute meningoencephalomyelitis with prodromal symptoms, for which tumors and T-cell dysfunction are frequent triggers. The majority of patients followed a monophasic course with a good outcome.Free full text

Glial Fibrillary Acidic Protein Autoimmunity
Abstract
Background and Objectives
To report the clinical, biological, and imaging features and clinical course of a French cohort of patients with glial fibrillary acidic protein (GFAP) autoantibodies.
Methods
We retrospectively included all patients who tested positive for GFAP antibodies in the CSF by immunohistochemistry and confirmed by cell-based assay using cells expressing human GFAPα since 2017 from 2 French referral centers.
Results
We identified 46 patients with GFAP antibodies. Median age at onset was 43 years, and 65% were men. Infectious prodromal symptoms were found in 82%. Other autoimmune diseases were found in 22% of patients, and coexisting neural autoantibodies in 11%. Tumors were present in 24%, and T-cell dysfunction in 23%. The most frequent presentation was subacute meningoencephalitis (85%), with cerebellar dysfunction in 57% of cases. Other clinical presentations included myelitis (30%) and visual (35%) and peripheral nervous system involvement (24%). MRI showed perivascular radial enhancement in 32%, periventricular T2 hyperintensity in 41%, brainstem involvement in 31%, leptomeningeal enhancement in 26%, and reversible splenial lesions in 4 cases. A total of 33 of 40 patients had a monophasic course, associated with a good outcome at last follow-up (Rankin Score ≤2: 89%), despite a severe clinical presentation. Adult and pediatric features are similar. Thirty-two patients were treated with immunotherapy. A total of 11/22 patients showed negative conversion of GFAP antibodies.
Discussion
GFAP autoimmunity is mainly associated with acute/subacute meningoencephalomyelitis with prodromal symptoms, for which tumors and T-cell dysfunction are frequent triggers. The majority of patients followed a monophasic course with a good outcome.
Glial fibrillary acidic protein (GFAP) is the predominant intermediate filament protein in adult astrocytes. Antibodies (Ab) targeting GFAP have been identified as the biomarker of a recently reported disorder: autoimmune GFAP astrocytopathy.1 Detection of GFAP-Ab is recommended in the CSF by indirect immunofluorescence on rat brain tissues and cell-based assays (IFA and CBA) using GFAPα.2 Autoimmune GFAP astrocytopathy is a rare entity that has been described in adults and children mainly as a meningoencephalomyelitis with or without optic disc edema and is often accompanied by a hallmark brain linear perivascular radial enhancement on MRI.2,3 Coexisting tumor or autoimmune disorders and associated neural antibodies are not rare.2,3 Steroid responsiveness is usual, although relapsing cases and rare fatal issues have been described.2,3 Although some important studies at the national level have been published since the initial report,4-7 there are still several open questions about GFAP astrocytopathy: the pathogenesis is poorly understood; clinical heterogeneity has been reported and there are no uniform diagnostic criteria; prognosis factors to assess the risk of relapse and disability outcome are missing; and we are lacking treatment recommendations. The aim of our study was to report the first French cohort of patients with GFAP autoantibodies; to describe their clinical, biological, and imaging features; and to assess the clinical course and outcome.
Methods
Study Participants
We included all patients testing positive in the CSF for GFAPα immunoglobulin G (IgG) between May 2017 and April 2020 from the Reference Center for Rare Brain and Spinal Cord Inflammatory Diseases (MIRCEM) in Lyon, France, and at the French National Reference Center for Paraneoplastic Neurologic Syndromes in Lyon, France.
Data of interest were retrospectively collected from the treating physicians using a structured questionnaire. It included demographic data, past history of tumor, coexisting autoimmunity, and T-cell dysregulation conditions. We collected clinical data: prodromal symptoms were defined as concomitant (or preceding by less than 1 month) constitutional symptoms (fever, fatigue, malaise, weight loss, nausea or vomiting); acute and subacute onsets were gathered and defined by 3 months or less between symptom onset and peak of symptoms whereas progressive onset was defined by more than 3 months between symptom onset and peak of symptoms; neurologic features and intensive care unit admission were evaluated. Modified Rankin scale (mRS) score was employed at diagnosis and at last reported date for a minimal follow-up period of 6 months.
Serologic data collected comprised anti-GFAP-IgG testing in serum and coexisting neural autoantibodies. The presence of IgG onconeural autoantibodies was evaluated by IFA and CBA in serum and CSF. The presence of aquaporin-4 (AQP4) IgG and myelin oligodendrocyte glycoprotein (MOG) IgG was evaluated by CBA in serum. Natremia and C-reactive protein (CRP) values were assessed. Routine CSF analysis included total white cell counts (neutrophils or lymphocytes), protein level, glucose level, and number of oligoclonal bands in CSF at diagnosis. Brain and spinal cord MRIs were reviewed by one neurologist (A.G.-D.) and one neuroradiologist (R.A.). Other paraclinical data included EMG and ophthalmic examinations (visual acuity, ocular fundus, visual field, retinal nerve fiber layer thickness at optical coherence tomography) when performed. First-line and long-term immunotherapy were collected. To assess treatment outcome, significant response was defined as a decrease of at least 2 points on mRS or full recovery. Longitudinal evaluation of anti-GFAP status in the CSF and MRI follow-up were reported, when available. Finally, we compared clinical, paraclinical, and outcome data of our cohort to the main published cohorts of GFAP astrocytopathy.
Assays
Indirect Immunofluorescence Assays
Both centers used indirect IFAs for screening (eMethods and eFigure 1, links.lww.com/WNL/B666).
Cell-Based Assays
Both centers used CBA for confirmation, with 100% agreement among positive results (eFigure 1, links.lww.com/WNL/B666). Some samples were shared and validated in the Mayo Clinic Neuroimmunology Laboratory. The CBA method from MIRCEM used human embryonic kidney 293 (HEK293) cells transfected with pEGFP-GFAP variant 1 plasmid using lipofectamine LTX. A total of 48 hours after transfection, cells were fixed with 1% paraformaldehyde (PFA) for 15 minutes and permeabilized with 0.2% Triton X-100 for 10 minutes. Cells were incubated overnight at 4°C (serum diluted at 1:100, CSF diluted at 1:2). Allophycocyanin-goat IgG-Fcγ fragment-specific was used as a secondary antibody (1:100, 45 minutes) and signal intensity evaluation was performed with fluorescence-activated cell sorting. The CBA of the French National Reference Center for Paraneoplastic Neurologic Syndromes employed HEK293 cells grown on glass coverslips in Dulbecco modified Eagle medium with 10% fetal calf serum. After 24 hours, cells were transfected using lipofectamine LTX with the plasmid coding for the GFAPα (clone RG204548; Origen). To visualize transfected cells, GFAP was fused to green fluorescent protein. Cells were fixed for 24 hours posttransfection with 4% PFA for 10 minutes and then incubated in a saturation buffer (phosphate-buffered saline [PBS]), 0.2% gelatin, 0.1% triton for 1 hour. Cells were then incubated with the patient CSF or serum diluted (1:10 and 1:100, respectively) in saturation buffer for 90 minutes. Cells were subsequently washed in PBS and then incubated with cy3-conjugated anti-human IgG containing DAPI. Bound antibodies were visualized using a fluorescence microscope (Axiophot; Zeiss).
HLA Analysis
Human leukocyte antigen (HLA) genotype imputation with attribute bagging (HIBAG) was used to impute 4-digit HLA alleles in GFAP cases based on available genotype data arrayed on Affymetrix precision medicine research array.8
Statistical Analysis
Summary statistics were reported as frequencies and percentages for categorical variables and as median (range, minimum–maximum) for continuous variables. Differences in HLA carrier frequencies between patients and controls were analyzed by 2-tailed Fisher exact test using R. The Bonferroni method was used to correct for multiple comparisons according to the number of alleles of each locus; corrected p values < 0.05 were considered significant.
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the institutional review board of Hospices Civils de Lyon (19–308). All patients received oral and written information. Samples were stored in Neurobiotec, part of the Hospices Civils de Lyon biobank.
Data Availability
Anonymized data will be shared by request from any qualified investigator for purposes of replicating procedures and results.
Results
Clinical Characteristics of Patients With CSF GFAP-IgG
From May 2017 to April 2020, 52 patients tested positive for anti-GFAP antibody in CSF. Six patients were excluded due to lack of clinical information. Thus, 46 patients were included for analysis, involving 6 patients published in prior reports.9-13 The demographics and clinical characteristics of the 46 patients with CSF GFAP-IgG are summarized in Table 1 and Table 2. Adult and pediatric features are similar.
Table 1
Demographic and Clinical Characteristics of Patients With Anti-GFAP Antibodies
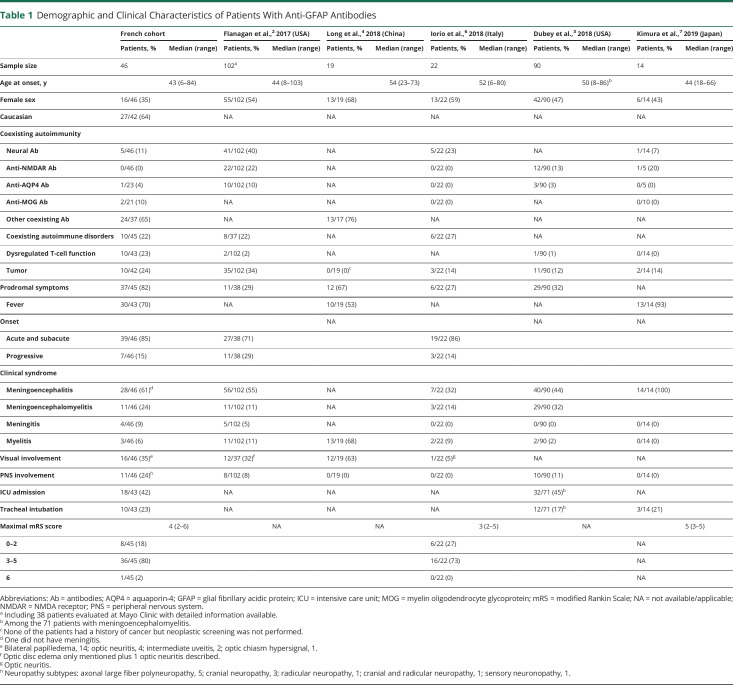
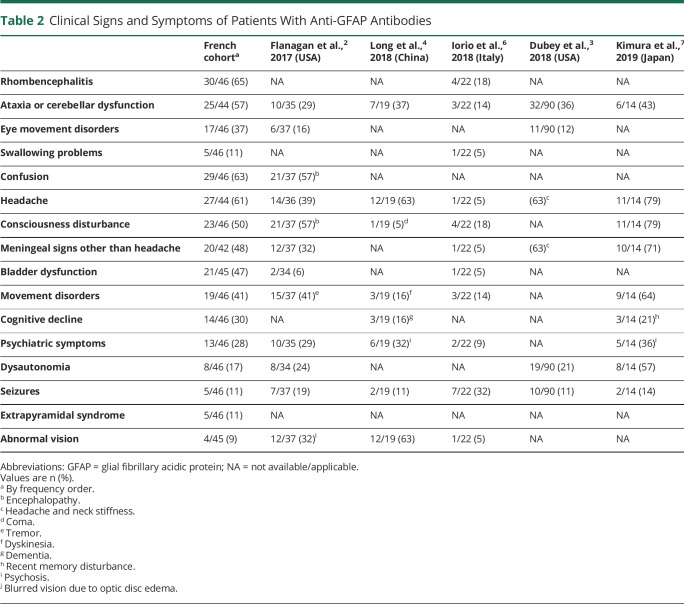
Table 2
Clinical Signs and Symptoms of Patients With Anti-GFAP Antibodies
Demographic Features
The median age at disease onset was 43 years (range 6–84 years); 8 patients were children, with a median age at disease onset of 14 years (range 6–17 years). Thirty patients were male (65%, ratio 1.9:1) and 27 of 42 patients were White (64%). Twenty-eight (61%) patients had coexisting autoimmunity, including coexisting neural (5/46 [11%]) and non-neural autoantibodies (24/37 [65%]) or coexisting autoimmune disorders (10/45 [22%]). Serum ganglioside antibodies were detected in 2 patients, of whom one had coexisting serum myelin-associated glycoprotein–immunoglobulin M, MOG-IgG were detected in serum of 2 patients (2/21 [10%]), and one patient had AQP4-IgG in CSF (1/23 [4%]). None had coexisting CSF onconeuronal antibodies. Serum was evaluated for NMDA receptor (NMDAR)–IgG by IFA (n = 46) and CBA (n = 30), and none was found positive. Non-neural autoantibodies comprised antinuclear, antineutrophil cytoplasmic, antiphospholipid, antithyroid, anti-intrinsic factor and gastric parietal cell, anti-MDA5 antibodies and rheumatoid factor. Ten patients showed one or more coexisting autoimmune disorders: psoriasis (n = 3), thyroiditis (n = 2), ulcerative colitis (n = 2), alopecia areata (n = 2), inflammatory rheumatism (n = 2), and episcleritis (n = 1).
Ten of 43 patients (23%) had dysregulated T-lymphocyte function conditions: 3 patients had chronic significant lymphopenia of unknown origin (total lymphocytes <0.8 G/L or CD4 T cells <500/mm3); 2 patients had lymphopenia in context of hemopathy; 2 patients had chronic HIV infection, of whom one had CD4 lymphopenia; 2 patients were treated with anti-programmed cell death 1 (anti-PD1) (1 with nivolumab, 1 with pembrolizumab initiated 14 and 3 months before meningoencephalitis onset, respectively); and 1 patient had received anti–tumor necrosis factor therapy for 8 years before anti-GFAP-IgG diagnosis. Of note, one patient had positive CSF JC virus PCR (limit of detection: 50 copies/mL) without HIV infection.
Neoplasms were recorded in 10 of 42 patients (24%). Three patients had a history of neoplasia, preceding neurologic presentation by a median of 7 months (range 7–216 months): ethmoidal anaplastic hemangiopericytoma, metastatic pulmonary hepatoid adenocarcinoma, and metastatic clear cell renal cell carcinoma. Oncologic screening was performed for 39 of 43 remaining patients, including fluorodeoxyglucose (FDG) PET-CT for 25 patients (64%). In 7 patients, neoplasia was detected subsequently to neurologic symptoms (median 2 months, range 1–12 months): ovarian teratoma in 2 patients (mature, n = 1; non specified n = 1) and one each of medullar and meningeal lymphocytic lymphoma, pre-Waldenström state, lung adenocarcinoma, trunk basal cell carcinoma, and papillary renal cell carcinoma. Six patients underwent systematic oncologic screening during follow-up (median 13 months, range 8–36 months) without any neoplasm diagnosed.
Clinical Features
Thirty-nine patients (85%) presented with an acute or subacute onset, 7 patients (15%) with a progressive setup. Thirty-seven of 45 patients (82%) had prodromal symptoms the month preceding neurologic presentation, including fever of unknown origin for 30 of them (30/43 [70%]).
The predominant clinical syndrome was meningoencephalitis (28 patients [61%]), including one patient who did not have clinical and biological meningitis, followed by meningoencephalomyelitis (11 patients [24%]), meningitis (4 patients [9%]), and myelitis (3 patients [6%]). Rhombencephalitis was reported in 30 cases (30/46 [65%]), with cerebellar dysfunction (25/44 [57%]), eye movement disorders (17/46 [37%]), and swallowing problems (5/46 [11%]). Other disease manifestations included confusion (29/46 [63%]), consciousness disturbance (23/46 [50%]), cognitive decline (14/46 [30%]) including primarily dysexecutive disorders, but also memory troubles, psychiatric symptoms (13/46 [28%]), seizures (5/46 [11%]), movement disorders including myoclonus, tremor, dyskinesia, dystonia, and hyperekplexia (19/46 [41%]), parkinsonism including bradykinesia, rigidity, and rest tremor (5/46 [11%]), headache (27/44 [61%]), meningeal signs other than headache (20/42 [48%]), bladder dysfunction (21/45 [47%]), dysautonomia (8/46 [17%]), abnormal vision (4/45 [9%]) including one severe bilateral vision loss, and intractable hiccups without apparent area postrema lesion on MRI (2/46 [4%]).
Visual tract involvement was reported for 16 patients (35%), associated with meningoencephalitis (n = 7), meningoencephalomyelitis (n = 5), meningitis (n = 3), or myelitis (n = 1). Bilateral papilledema was detected in 14 patients. Among them, intracranial hypertension (ICH) was confirmed by lumbar puncture or intracranial pressure monitoring for 3 and suspected for 4 considering clinical symptoms (cephalalgia, diplopia, sixth cranial nerve paresis, and transient visual obscurations) but no measurement of intracranial pressure; 3 patients had optic neuritis (bilateral, n = 2; unilateral, n = 1), 2 had intermediate uveitis, and 2 had isolated bilateral papilledema (symptoms of ICH with normal opening CSF pressure, n = 1; no symptom of ICH, n = 1). One patient had optic neuritis and one patient had optic chiasm hypersignal, each without papilledema.
Peripheral nervous system (PNS) involvement was found for 11 patients (24%), including axonal large fiber polyneuropathy (n = 5), cranial neuropathy (n = 3), radicular neuropathy (n = 1), one cranial and radicular neuropathy (n = 1), and sensory neuronopathy (n = 1). No PNS involvement was isolated. Eleven patients had an electroneuromyogram, of which 7 were abnormal (large fiber polyneuropathy, n = 5; sensory neuronopathy, n = 1; radicular neuropathy, n = 1). Cranial or radicular involvement was noticed on clinical examination for 4 patients and on MRI examination for the 3 others.
Severity
Most patients presented a maximal mRS score between 3 and 5 (36/45 [80%]), whereas 8 patients had a maximal mRS score between 0 and 2 (18%). One patient died (mRS score 6 [2%]) due to intracranial hypertension, 2 months following anti-GFAP meningoencephalitis onset. Thus, the median mRS score was 4 (range 2–6). Intensive care unit admission was frequent (18/43 [42%]), and 10 patients (23%) needed orotracheal intubation.
Biological Findings of Patients With CSF-GFAP-IgG
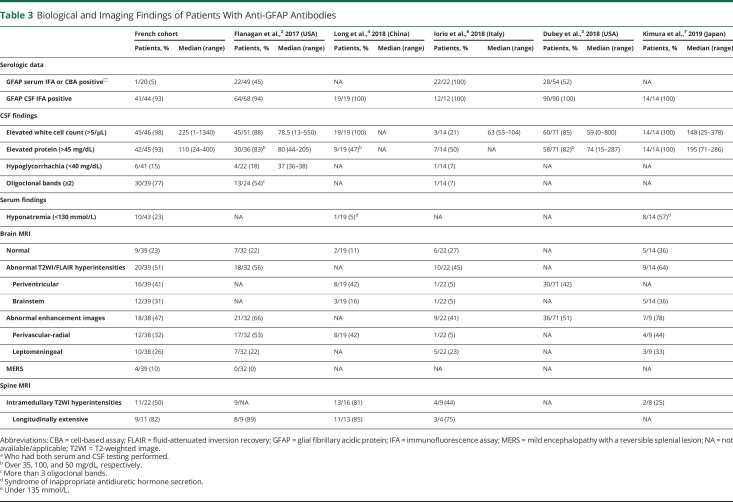
These findings are summarized in Table 3. For CSF anti-GFAP antibody detection, IFA was positive in 41 of the 44 patients (93%) tested. Three patients had negative IFA testing and 2 patients did not have IFA performed. Among 20 patients tested for serum anti-GFAP antibody, only one patient (5%) was positive. Hyponatremia (<130 mmol/L) was found in 10 of 43 patients (23%). Elevated CRP was present for only 1 out of 42 patients (2%) but with sterile infectious research. CSF analysis revealed pleocytosis in 45 patients (98%; median 225 cells/µL, range 1–1,340). Only one patient had neutrophil predominance, while 43 patients had lymphocytic pleocytosis. Elevated protein was detected in 42 of 45 patients (93%; median 110 mg/dL, range 24–400) and 15% (6/41) showed hypoglycorrhachia, normalized at 3 months for 3 patients (no control performed for the 3 others). Inflammatory CSF with 2 or more oligoclonal bands was noticed in 30 of 39 patients (77%). Four additional patients had single supernumerary band in CSF isoelectric focusing and one of them had plasma cells.
Table 3
Biological and Imaging Findings of Patients With Anti-GFAP Antibodies
HLA Typing
DNA was available for 26 patients. There was no statistically significant difference in carrier frequencies between patients and controls for class I (A, B, C) and class II (DRB1, DQB1, DQA1, and DPB1) genes (eTable1, links.lww.com/WNL/B666).
MRI Findings of Patients With CSF-GFAP-IgG
Brain and spinal cord MRI were available for review for 39 and 22 patients, respectively (Table 3). Regarding brain MRI, findings were heterogeneous: 9 (23%) patients had normal imaging, 20 (51%) patients had abnormal T2 hyperintensities, and 18 of 38 (47%) patients had abnormal contrast enhancements. T2 hyperintensities were multifocal and confluent in 41% and 36% of patients, respectively, whereas 10% of patients showed demyelinating lesions. T2-weighted/fluid-attenuated inversion recovery (FLAIR) lesions involved periventricular regions (n = 16 [41%]) and brainstem (n = 12 [31%]) mostly, but thalamus (n = 7 [18%]), internal and external capsule (n = 7 [18%]), basal ganglia (n = 6 [15%]), corona radiata and semiovale centrum (n = 6 [15%]), temporopolar area (n = 5 [13%]), limbic structures (n = 2 [5%]), and hypothalamus (n = 2 [5%]) were also affected. Remarkable patterns were symmetrical FLAIR hyperintensities involving basal ganglia, thalami, internal and external capsules (n = 6 [15%]) and posterior bulbopontic FLAIR hyperintensities suggesting infectious rhomboencephalitis (n = 3 [8%]) (Figure 1). Four (10%) patients had splenial corpus callosum lesions with restricted diffusion as seen in mild encephalopathy/encephalitis with reversible splenial lesion (MERS) syndrome. Lesions spread to anterior corpus callosum and/or bilateral centrum semi-ovale in 2 of them (MERS type II). One patient showed area postrema and optic chiasma involvement. Perivascular-radial gadolinium enhancement was found in 12 of 38 (32%) patients and leptomeningeal enhancement was noted in 10 of 38 (26%) patients. Other enhancement patterns included periependymal (n = 3 [8%]), cranial nerve involvement (n = 3 [8%]), ovoid (n = 2 [5%]), and patchy (n = 1 [3%]) (Figure 2).
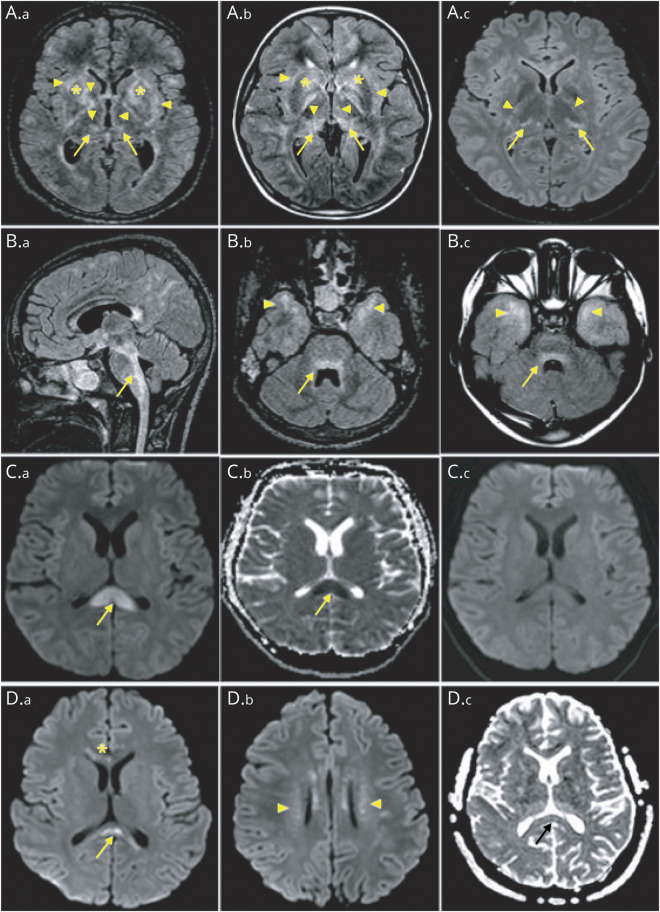
Brain MRI of patients with anti–glial fibrillary acidic protein (GFAP) antibodies showing abnormal hyperintensity lesions on fluid-attenuated inversion recovery images (A.a–B.c) were observed in internal and external capsules (A.a, A.b, and A.c, arrowheads), basal ganglia (A.a and A.b, stars), thalami (A.a, A.b, and A.c, arrows), brainstem (B.a, B.b, and B.c, arrows), and temporal poles (B.b and B.c, arrowheads). Diffusion-weighted images (C.a–D.c) show splenial corpus callosum lesions (C.a and D.a, arrows) with the corresponding reduced diffusion (C.b and D.c, arrows) reversible at 15 days (C.c), involving anterior corpus callosum (D.a, star) and bilateral centrum semiovale (D.b, arrowheads), as respectively seen in mild encephalopathy/encephalitis with reversible splenial lesion type I and type II.
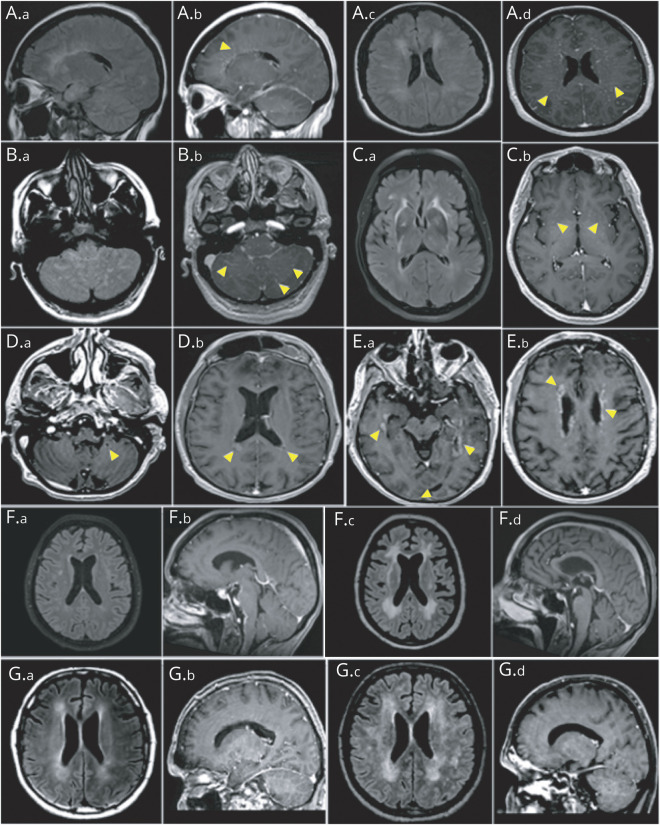
Enhancement patterns (A.a–E.b) and evolution of MRI abnormalities (F.a–G.d) in patients with anti–glial fibrillary acidic protein (GFAP)–associated meningoencephalitis. Brain MRI of patients with anti-GFAP antibodies showing abnormal hyperintensity lesions on fluid-attenuated inversion recovery (FLAIR) images (A.a, A.c, B.a, and C.a), which enhance with a linear radially oriented or punctuate pattern in periventricular areas (A.b, arrowheads), centrum semiovale (A.d, arrowheads), cerebellum (B.b, arrowheads), and basal ganglia (C.b, arrowheads) on postgadolinium T1. Other enhancement patterns included ovoid (D.a and D.b, arrowheads) and periependymal (E.a and E.b, arrowheads). Whereas white matter FLAIR hyperintensities progressed towards extensive leukopathy at 7 months (F.a and F.c), brainstem leptomeningeal contrast enhancement receded 3 months after initial attack treatment (F.b and F.d). In another patient, while periventricular FLAIR hyperintensities expanded (G.a and G.c), periventricular radial enhancement receded at 2 months (G.b and G.d).
At spinal cord MRI, T2 hyperintensities were observed in 11 (50%) patients, including 10 with clinical related symptoms; 9 of them (82%) had longitudinally extensive spinal cord lesions (3 or more vertebral segments long). Transverse, holocord, centrally located, lateral, and posterior lesions were observed in 8 (73%), 4 (36%), 2 (18%), 2 (18%), and 1 (9%) patients, respectively. Spinal cord lesions were located in the thoracic spinal cord in 9 (82%) patients, in the cervical spinal cord in 8 (73%) patients, and in lumbar spinal cord in only 2 (18%) patients, with multifocal distribution in 3 (27%) patients. Gadolinium enhancement was reported in 5 (45%) patients: leptomeningeal, 4; patchy, 3; and linear, 1 (Figure 3).
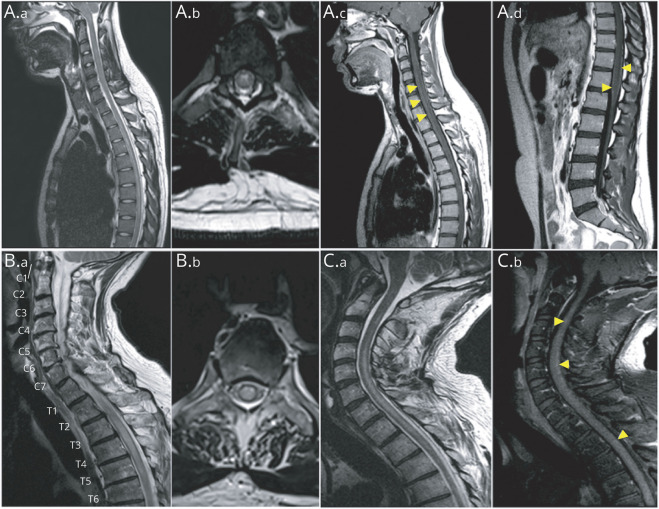
Spine MRI of patients with anti–glial fibrillary acidic protein (GFAP) antibodies show longitudinally extensive (A.a) and most prominent centrally (A.b) abnormal T2 hyperintensity accompanied by central canal enhancement (A.c, arrowheads) and leptomeningeal enhancement (A.d, arrowheads), holocord longitudinally extensive T2 hyperintensity (B.a and B.b), and longitudinally extensive T2 hyperintensity (C.a) accompanied by patchy enhancement (C.b, arrowheads).
Treatment, Outcome, and Follow-up
Treatment responses and outcomes are summarized in Table 4. Adult and pediatric features are similar. The median follow-up duration was 14 months (range 2–74), with 6 (13%) patients lost to follow-up. Thirty-two of 46 patients (70%) were treated with first-line immunotherapy as an attack treatment, including corticosteroids (IV mostly) (n = 27), IV immunoglobulin (IVIg) (n = 20), or plasma exchange (n = 9). Sixteen of them (50%) received 2 or more treatments combined. Significant response was obtained for 28 of 31 patients (90%), with a median period of 2 months (range from 1 week to 9 months) and clinical fluctuations reported in 6 patients before sustained response. Six patients showed improvement after second-line immunotherapy administration (rituximab alone, 3; cyclophosphamide alone, 1; rituximab and cyclophosphamide, 1). One patient died before second-line immunotherapy.
Table 4
Treatment, Outcome and Follow-up of Patients With Anti-GFAP Antibodiesa
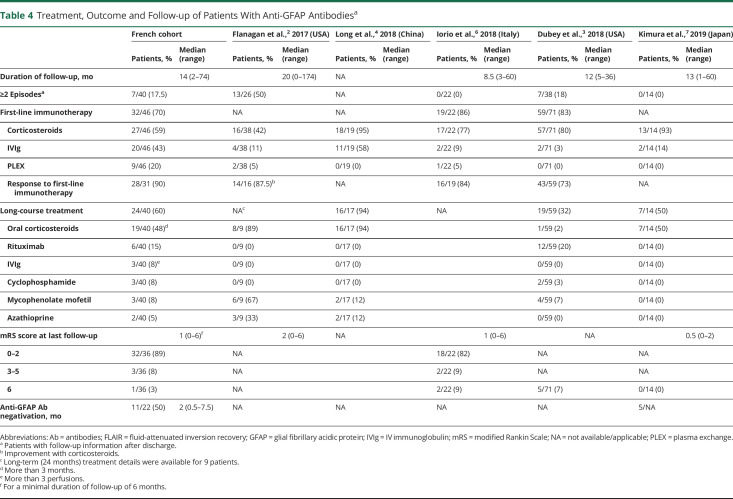
Maintenance therapy was dispensed to 24/40 patients (60%), including oral corticosteroids (over >3 months, median duration of 7 months) in 19 patients (48%), rituximab in 6 patients (15%), IVIg (>3 perfusions) in 3 patients (8%), cyclophosphamide in 3 patients (8%), mycophenolate mofetil in 3 patients (8%), and azathioprine in 2 patients (5%). mRS score at last known date for a minimal follow-up duration of 6 months was available for 36 patients, with a median of 1 (range 0–6). Most patients showed an mRS score between 0 and 2 (32/36 [89%]), while 3 had an mRS score between 3 and 5 (8%). One patient died of status epilepticus 12 months following anti-GFAP meningoencephalitis diagnosis; he was known to have epilepsy due to cerebral metastases of renal carcinoma prior to anti-GFAP disease. Thirty-three patients (82.5%) had a monophasic course; 7 patients (17.5%) had 2 or more episodes. New episodes occurred at a median period of 3 months (range 2–12), during steroid tapering or at steroid stop for 5/7. We identified 3 patients with a different clinical presentation, 3 patients with worsening or recurrence of previous symptoms, and one patient with both. Only one patient had more than 2 episodes (6 in total). One patient had intractable vomiting as first attack, with concomitant anti-AQP4-Ab positive serostatus.
Eleven of 22 patients (50%) showed negative conversion of CSF anti-GFAP antibodies at a median of 2 months (range 0.5–7.5 months), including 5 patients in IFA and CBA, 5 in CBA without IFA control, and 1 in IFA without CBA control. They persisted at 4 years follow-up in one patient. Brain MRI control in 11 of 12 patients revealed contrast enhancement regression between 1 and 7 months (median period 6 months), while periventricular lesions worsening was not rare (36%), sometimes progressing to extensive leukopathy (Figure 2). Splenial lesion suggestive of MERS syndrome improved partially in 2 patients and totally in the 2 others. Median time for spinal contrast enhancement regression, evaluated in 4 patients, was 5 months (range 2–6 months).
Discussion
Our study identified 46 patients with CSF GFAPα antibodies, which represents the second largest cohort worldwide using a multimodal approach, including clinical, biological, and radiologic data. With a median follow-up duration of 14 months for 40 patients, it represents the largest cohort to report outcome and follow-up data. We assessed dynamics of antibodies and MRI, which was poorly reported until now.
We confirm that CBA on CSF is an accurate method for GFAP-IgG detection. In our study, IFA was negative in some patients. As IFA analysis is partly subjective, clinicians should insist for CBA testing when CSF IFA is negative if clinical, biological, and MRI presentation strongly suggests GFAP autoimmunity. Although its sensitivity and specificity appear to be inferior to CBA, interest in IFA remains for simultaneous coexisting antibodies detection. We did not study isoforms other than GFAPα, as precedent studies showed dominant reactivity with this variant (100% sensitivity vs 81% and 54% for ε and κ isoforms, respectively).2,3,6 By contrast to previous reports, anti-GFAP-IgG positivity in serum was rare in our cohort. However, in previous reports when both serum and CSF were tested, none of the patients with Ab detected only in serum had meningoencephalomyelitis phenotype.2,3,6 Poor sensitivity of serum testing may be due to antibody intrathecal synthesis, whereas its poor specificity might explain clinical heterogeneity of GFAP autoimmunity reported before.2,3,6 Progressive multifocal leukoencephalopathy (PML) was suspected for our patient with positive CSF JC virus PCR. Hence, it is possible that JC virus triggered GFAP autoimmunity or that JC virus toxicity towards astrocytes simply unmasked GFAP expression, inducing anti-GFAP antibodies secretion without its own clinical consequences. As GFAP antibody has already been reported in the setting of cerebral tumors (astrocytoma), the exact mechanism of GFAP autoimmunity in the setting of direct CNS injuries is a matter of debate.2 Thus, it is recommended to look for an alternative neurologic disorder in cases with atypical phenotype or poor response to immunotherapy.
Our study confirmed subacute meningoencephalitis with or without myelitis as the predominant clinical syndrome. Of interest, brainstem symptoms (including cerebellar ataxia, eye movement disorders, and swallowing troubles) were the most common in our cohort. Along with brainstem involvement on MRI, we suggest that rhombencephalitis may be a typical presentation of GFAP autoimmunity. This is consistent with GFAP expression by Bergmann glia cells of the cerebellum.14 Apart from meningoencephalitis, we found other clinical presentation, including parkinsonism, which was not described in the previous studies. This clinical feature was supported by MRI, with basal ganglia T2 hyperintensities found in similar proportion, as already noted by Kimura et al.7 Bilateral optic disc edema was not rare in our cohort, but its underlying mechanism was heterogeneous. It included intracranial hypertension and neuropapillitis. As previously reported,15 we found 2 cases of associated intermediate uveitis. As GFAP is expressed in the retina by astrocytes and Müller cells,16 especially around the optic nerve head, we suppose that optic disc edema in GFAP autoimmunity can be due to isolated papillitis, involving the breakdown of the retina–blood barrier and contiguous inflammation up to the vitreous. Finally, we found frequent PNS involvement, as described recently.17 This included axonal large fiber polyneuropathy for about half of the cases and cranial or radicular neuropathies associated with meningoencephalomyelitis. We also identified a genuine paraneoplastic sensitive neuronopathy with meningitis and a symptomatic case of multiple involvement of the cranial nerves. In these 2 cases, anti-GFAP antibodies could result from a nonspecific immune system response to PNS injury, as GFAP is expressed by Schwann cells and satellite glial cells of the dorsal root and the cranial nerve ganglia (trigeminal especially).18
Dysregulated T-lymphocyte function, including HIV infection, and immune checkpoint inhibitor (ICI) treatment was not rare in our cohort. Autoimmune diseases have been reported as more frequent during HIV infection than in the general population and it has been reported that HIV primo-infection can elicit a T-cell–mediated immune response in CNS.19-23 Some cases of limbic encephalitis with coexisting antineuronal antibodies related to controlled HIV or primo-infection suggest a postinfectious immune process for these coexisting syndromes.24-28 ICIs have become an essential therapeutic weapon against cancer, by inhibiting the regulatory interactions that limit T cell cytotoxicity to tumors.29 Since they were used, diverse immune-related adverse events (irAEs) have been reported, including various neurologic complications: Guillain-Barré syndrome, myasthenia gravis, hypophysitis, aseptic meningitis, meningoradiculitis, transverse myelitis, and immune encephalitis.30-36 The mechanisms leading to irAEs are likely similar to those promoting antitumor responses and involve expansion of the T-cell repertoire.37 Thus, by disrupting the immune system machinery, HIV infection and ICI therapy may arouse a cytotoxic T-cell response and trigger GFAP autoimmunity. Another hypothesis may be an increased susceptibility to infections. Indeed, we noted frequent infectious prodromal symptoms and lymphopenia, which supports the hypothesis that GFAP autoimmunity could be triggered by infections. Finally, findings of GFAP autoimmunity associated with MERS lesions are also in favor of an infectious trigger. MERS is a rare clinico-radiologic entity, mostly reported in Asian children, associating a mild encephalopathy (including behavioral changes, altered consciousness, and seizures) and a reversible MRI lesion in the splenium of the corpus callosum. Its prognosis is good with spontaneous clinical and radiologic improvement within days or weeks. Though the pathogenesis is unknown, MERS has been linked to numerous infectious agents, either bacterial or viral.38,39
According to Fang et al.1 more than one-third of GFAP autoimmune meningoencephalomyelitis cases are associated with neoplasia, sometimes with a demonstrated paraneoplastic mechanism. Similar to prior reports, diverse neoplasms were diagnosed prospectively in our study.2,3,7 However, heterogeneity of neoplasm types may not suggest systematic paraneoplastic association. In contrast to previous cohorts, we found no coexisting onconeuronal antibodies, including CSF NMDA-R-IgG, which was the most reported.2,3,7 Three patients had associated MOG-IgG or AQP4-IgG. Association with anti-MOG Ab has only been described in one Chinese patient with coexisting AQP4-IgG.40 In both of our patients, simultaneous PNS involvement appeared unusual for MOG-associated disease. As for AQP4-IgG, they were detected in CSF only, and then tested negative 2 years later. We can hypothesize that our patients may not have bona fide MOG and AQP4-Ab-associated disorders, and hence question overlapping syndromes in GFAP autoimmunity.
We found a dramatic response to immunotherapy and good midterm outcome, contrasting with the initial severity, and confirmed steroid responsiveness. In this present study, monophasic course was much more frequent than initially reported.2 New episodes occurred during steroid tapering or at steroid stop for 5 of these 7 patients. We identified 2 patients with worsening identical symptoms 2 months after the last episode. Thus, definition of relapse may not be uniform between the different cohorts, mixing rebounds, genuine relapses, and effect of steroids. Moreover, half of our relapsing patients presented a different clinical syndrome, and exhaustive reassessment including MRI, lumbar puncture, and GFAP-IgG was not systematically done, so we cannot exclude another causal neurologic condition. Long-course treatment, which was dispensed to two-thirds of our patients, might have been successful in preventing relapses. Nevertheless, the relapsing rate was similar in the 2nd Mayo Clinic cohort, whereas one-third of patients had received maintenance therapy.3 Finally, we cannot exclude that some patients would relapse afterwards despite a median follow-up >1 year.
The main limitation of this study is that anti-GFAP-Ab testing was performed in patients registered in 2 French referral centers, causing a possible selection bias. However, clinical syndromes were similar to Mayo Clinic cohorts, in which thousands of samples were tested. It is also limited by its retrospective design, but we did not include patients with excessive missing data.
We confirm on a large scale that GFAP autoimmunity is a relatively homogeneous condition, mainly associated with a monophasic acute/subacute meningoencephalomyelitis with prodromal symptoms, for which tumors and T-cell dysfunction seem to be frequent triggers. Our study expends the clinical spectrum to less frequent presentation, including PNS involvement and acute parkinsonism. With a median follow-up duration of >1 year, we found that the majority of patients followed a monophasic course with a good outcome. However, some cases experienced a more severe relapsing condition. Thus, future identification of factors associated with risk of relapse or disability is needed to monitor immunotherapy.6,7 Our findings on a high rate of GFAP-IgG negative conversion raise the question whether GFAP antibody persistency at a defined period could predict the risk of sequelae or relapse.
Acknowledgment
The authors thank NeuroBioTec Hospices Civils de Lyon BRC (France, AC-2013-1867, NFS96-900) for banking sera and CSF samples.
Glossary
| Ab | antibodies |
| AQP4 | aquaporin-4 |
| CBA | cell-based assay |
| CRP | C-reactive protein |
| FLAIR | fluid-attenuated inversion recovery |
| GFAP | glial fibrillary acidic protein |
| HEK293 | human embryonic kidney 293 |
| HLA | human leukocyte antigen |
| ICH | intracranial hypertension |
| ICI | immune checkpoint inhibitor |
| IFA | immunofluorescence assay |
| IgG | immunoglobulin G |
| irAE | immune-related adverse event |
| IVIg | IV immunoglobulin |
| MERS | mild encephalopathy/encephalitis with reversible splenial lesion |
| MOG | myelin oligodendrocyte glycoprotein |
| mRS | modified Rankin Scale |
| PBS | phosphate-buffered saline |
| PFA | paraformaldehyde |
| PML | progressive multifocal leukoencephalopathy |
| PNS | peripheral nervous system |
Study Funding
The authors report no targeted funding. This study is supported by FRM (Fondation pour la Recherche Médicale) DQ20170336751. This work has been developed within the BETPSY project, which is supported by a public grant overseen by the French National Research Agency (ANR), as part of the second “Investissements d'Avenir” program (reference ANR-18-RHUS-0012). S.M.-C. is supported by a research grant from Fundación Alfonso Martín Escudero (Spain).
Disclosure
The authors have no conflicts of interest to disclose. Go to Neurology.org/N for full disclosures.
References
Articles from Neurology are provided here courtesy of American Academy of Neurology
Full text links
Read article at publisher's site: https://doi.org/10.1212/wnl.0000000000013087
Read article for free, from open access legal sources, via Unpaywall:
https://n.neurology.org/content/neurology/98/6/e653.full.pdf
HAL Open Archive
https://hal-u-picardie.archives-ouvertes.fr/hal-03578197
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/118263643
Article citations
Autoimmune Encephalitis and Paraneoplastic Neurologic Syndromes: A Nationwide Study on Epidemiology and Antibody Testing Performance.
Neurol Neuroimmunol Neuroinflamm, 11(6):e200318, 28 Oct 2024
Cited by: 0 articles | PMID: 39467237 | PMCID: PMC11521097
Unveiling GFAP Astrocytopathy: Insights from Case Studies and a Comprehensive Review of the Literature.
Antibodies (Basel), 13(4):79, 25 Sep 2024
Cited by: 0 articles | PMID: 39449321 | PMCID: PMC11503365
Review Free full text in Europe PMC
Pediatric MOG-Ab-Associated Encephalitis: Supporting Early Recognition and Treatment.
Neurol Neuroimmunol Neuroinflamm, 11(6):e200323, 11 Oct 2024
Cited by: 0 articles | PMID: 39393046 | PMCID: PMC11488826
Glial Fibrillary Acidic Protein Astrocytopathy: Review of Pathogenesis, Imaging Features, and Radiographic Mimics.
AJNR Am J Neuroradiol, 45(10):1394-1402, 03 Oct 2024
Cited by: 2 articles | PMID: 38844367
Review
Clinical characteristics of overlapping syndrome in patients with GFAP-IgG and MOG-IgG: a case series of 8 patients and literature review.
J Neurol, 271(10):6811-6821, 27 Aug 2024
Cited by: 0 articles | PMID: 39190107
Review
Go to all (44) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Clinical and immunological characteristics of the spectrum of GFAP autoimmunity: a case series of 22 patients.
J Neurol Neurosurg Psychiatry, 89(2):138-146, 26 Sep 2017
Cited by: 81 articles | PMID: 28951498
Clinical characteristics of autoimmune glial fibrillary acidic protein (GFAP) astrocytopathy in children: A case series of 16 patients.
J Neuroimmunol, 382:578176, 07 Aug 2023
Cited by: 0 articles | PMID: 37572437
Review
Autoimmune Glial Fibrillary Acidic Protein Astrocytopathy: A Novel Meningoencephalomyelitis.
JAMA Neurol, 73(11):1297-1307, 01 Nov 2016
Cited by: 215 articles | PMID: 27618707
Clinical Manifestation, Auxiliary Examination Features, and Prognosis of GFAP Autoimmunity: A Chinese Cohort Study.
Brain Sci, 12(12):1662, 03 Dec 2022
Cited by: 4 articles | PMID: 36552122 | PMCID: PMC9775969

 *
*



