Abstract
Free full text

Relation of abnormal cardiac stress testing with outcomes in patients undergoing renal transplantation
Abstract
Cardiovascular risk stratification is often performed in patients considered for renal transplantation. In a single center, we sought to examine the association between abnormal stress testing with imaging and post-renal transplant major adverse cardiovascular events (MACE) using multivariable logistic regression. From January 2006 to May 2016 232 patients underwent renal transplantation and 59 (25%) had an abnormal stress test result. Compared to patients with a normal stress test, patients with an abnormal stress test had a higher prevalence of dyslipidemia, diabetes mellitus, obesity, coronary artery disease (CAD), and heart failure. Among those with an abnormal result, 45 (76%) had mild, 10 (17%) moderate, and 4 (7%) severe ischemia. In our cohort, 9 patients (3.9%) had MACE at 30-days post-transplant, 5 of whom had an abnormal stress test. The long-term MACE rate, at a median of 5 years, was 32%. After adjustment, diabetes (OR 2.37, 95% CI 1.12–5.00, p = 0.02), CAD (OR: 3.05, 95% CI 1.30–7.14, p = 0.01) and atrial fibrillation (OR: 5.86, 95% CI 1.86–18.44, p = 0.002) were independently associated with long-term MACE, but an abnormal stress test was not (OR: 0.83, 95% CI 0.37–1.92, p = 0.68). In conclusion, cardiac stress testing was not an independent predictor of long-term MACE among patients undergoing renal transplant.
Introduction
Chronic kidney disease (CKD) represents a significant source of disease burden in the United States, with a prevalence of 14% for CKD stages 1–4 and over 20,000 renal transplants performed every year [1, 2]. Cardiovascular disease (CVD) is the leading cause of morbidity and mortality among patients with CKD and renal transplant [3]. Among older individuals the prevalence of CVD is 2-fold higher (69.9% vs 34.7%) in patients with CKD compared with those without CKD [1]. Furthermore, patients with CKD present with a different clinical profile than the general population; they are often asymptomatic, and conventional cardiac risk factors are less predictive of cardiovascular disease [4]. The American Heart Association/American College of Cardiology (AHA/ACC) expert consensus guidelines in 2012 recommend routine non-invasive cardiac stress testing for renal transplantation candidates irrespective of symptoms or functional status [5]. However, the cardiology and renal-transplant guidelines are not congruous on this topic and it is unclear if pre-operative stress testing is useful for predicting early and/or late major cardiovascular events (MACE) in patients undergoing renal transplant [6]. We therefore aimed to assess the association between cardiac stress testing results and post-transplant MACE at our institution.
Methods
In this retrospective single-center cohort study, we selected patients who underwent renal transplant at Rhode Island Hospital, Providence, RI USA from January 2006 to May 2016. Our inclusion criteria were adults age >18 years who underwent renal transplantation and who had cardiac stress testing with imaging within 24 months prior to transplant. Our primary outcome was post-transplant MACE, defined as all-cause mortality, stroke, myocardial infarction, congestive heart failure, and revascularization, at follow-up including in-hospital events. Endpoints were extracted from the electronic health records and institutional renal transplant database.
We extracted data pertaining to patient’s demographics, co-morbidities, stress testing, cardiac catheterization as well as clinical endpoints. Eligible stress tests included exercise, pharmacologic, and/or combined exercise-pharmacologic nuclear myocardial perfusion or echocardiographic testing. Stress tests were categorized as normal or abnormal and abnormal results were further graded as mild, moderate, or severe ischemia based on the severity and extent of ischemia using standard criteria [7, 8]. Our patient records provided access to stress reports and not the raw quantitative stress data. In our hospital system, for nuclear stress tests image interpretation was performed incorporating visual and quantitative analysis compared to a gender specific normal database using standardized thresholds for severity and extent of ischemia [9]. For stress echo, wall motion index was scored semi-quantitatively using a 17-segment model [10]. For patients who received multiple stress tests within 24 months prior to transplantation, the most recent test report was used for data extraction.
Categorical variables were expressed as percentages, and continuous variables were expressed as means with standard deviations. Except BMI, none of the variables had missing values >5%. Univariate differences in baseline characteristics between normal and abnormal stress test groups were evaluated using chi2 tests for categorical variables and student’s t-test for continuous variables. Univariate and multivariate logistic regression models were used to evaluate the association between abnormal stress test and long-term MACE, but only 30-day MACE rates are reported due to the small number of events. The covariates for multivariate models were selected based on their clinical relevance based on previous studies or those variables with a p<0.1 on the univariate analysis. Associations were examined in a hierarchical model with the following covariates: age, sex, hypertension, diabetes, hyperlipidemia, obesity, current/prior smoking, prior coronary artery disease (CAD), congestive heart failure, atrial fibrillation, and peripheral vascular disease. History of CAD was broadly defined as: known obstructive coronary disease, prior MI, or prior PCI/CABG. Non-obstructive coronary disease by catheterization and coronary CT were not included. All analyses were performed using Stata16.0 (StataCorp, College Station, Texas), and P value < .05 was considered statistically significant. All study procedures were approved by the Lifespan Institutional Review Board at Rhode Island Hospital.
Results
Overall, the patient population was predominantly white, male, and over 45 years old. Of the 539 patients in our renal transplant database, 240 had undergone stress testing with imaging within 24 months prior to transplant and were eligible for the study. Two hundred and twelve (91.4%) participants underwent nuclear myocardial perfusion, and the remainder underwent stress echocardiography.
Among these, 5 were excluded due to missing MACE data as they were lost to follow-up and 3 for inability to obtain stress test reports from an outside facility, resulting in the final cohort of 232 patients (Fig 1). Of note, 24 patients included in our analysis had inconclusive stress results secondary to submaximal heart rate responses. Given that they had otherwise negative stress results, these patients are included with the normal stress test group. In our cohort, 59 (25.4%) patients had an abnormal stress result, and 173 (74.6%) had a normal stress result. Among those with abnormal result, 45 (76.3%) were graded to have mild, 10 (17.0%) moderate, and 4 (6.8%) severe ischemia.
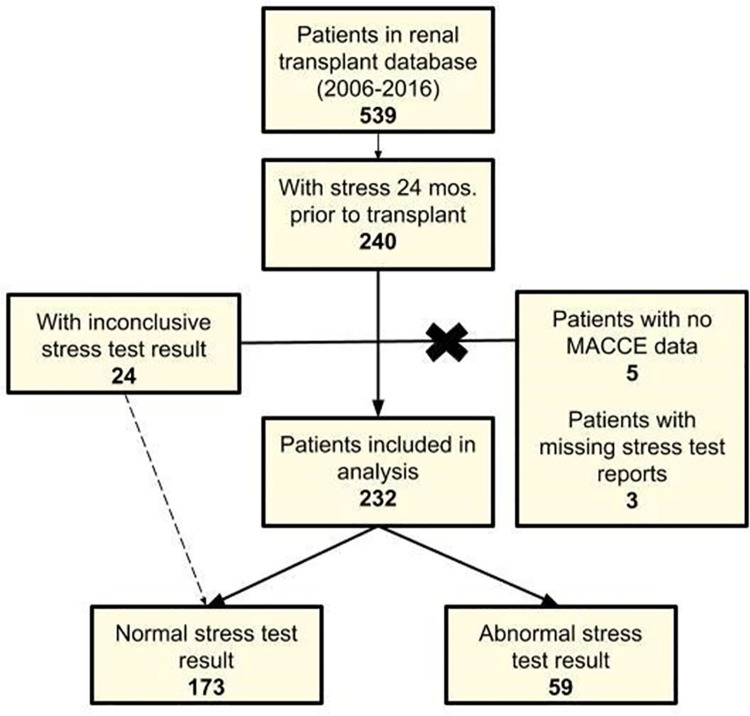
Flowchart detailing patients included in final data analysis from original identification in database of renal transplant patients. Of the 539 patients in the database, 240 met inclusion criteria (demographic and stress test within 24 months prior to transplant). Of these 240 patients, 8 were excluded because of missing MACE outcomes data or stress test report.
Table 1 shows baseline demographic and clinical data stratified by patients with normal vs. abnormal stress test. Compared to patients with normal stress test, patients with abnormal stress test had higher prevalence of dyslipidemia, diabetes mellitus, obesity, CAD, percutaneous coronary intervention and coronary artery bypass surgery, and heart failure. In our cohort of 232 patients, 9 (3.9%) patients had MACE in the 30-day post-transplant period, 5 of which had an abnormal stress test.
Table 1
| Stress Test | p value | ||
|---|---|---|---|
| Normal (n = 173) | Abnormal (n = 59) | ||
| Age, years | 51.2 ± 12.7 | 53.1 ± 11.9 | 0.31 |
| Women, % | 41.4 | 28.8 | 0.10 |
| Race, % | 0.42 | ||
| White | 68.4 | 77.6 | |
| Black | 19.9 | 8.6 | |
| Hispanic | 7.6 | 8.6 | |
| Asian | 2.9 | 3.5 | |
| Other | 1.2 | 1.7 | |
| Hypertension | 97.1 | 94.8 | 0.41 |
| Dyslipidemia | 57.2 | 72.4 | 0.04 |
| Diabetes mellitus | 38.7 | 58.6 | 0.008 |
| BMIa, kg/m2 (n = 169) | 27.3 ± 6.3 | 28.9 ± 7.2 | 0.19 |
| Obese | 49.1 | 64.4 | 0.04 |
| Smoking | 42.1 | 28.6 | 0.07 |
| Prior CADb | 15.7 | 52.6 | <0.001 |
| Prior PCIc | 2.3 | 27.6 | <0.001 |
| Prior CABGd | 3.5 | 10.3 | 0.04 |
| Heart failure | 17.9 | 32.8 | 0.02 |
| Atrial fibrillation | 8.1 | 15.5 | 0.11 |
| PADe | 13.3 | 19 | 0.29 |
| Prior dialysis | 82.6 | 78 | 0.30 |
| Dialysis type | 0.99 | ||
| Hemodialysis | 83.9 | 84.8 | |
| Peritoneal dialysis | 7.0 | 6.5 | |
| Both | 9.1 | 8.7 | |
| Dialysis duration, months (n = 223) | 31.9 ± 40.5 | 26.5 ± 25.2 | 0.36 |
| Prior renal transplant | 17.9 | 13.8 | 0.47 |
| Stress test type | |||
| Pharmacological | 43.9 | 49.1 | 0.17 |
| Exercise | 52.6 | 42.4 | |
| Exercise + Pharmacological | 3.5 | 8.5 | |
aBody mass index.
bCoronary artery disease.
cPercutaneous coronary intervention.
dCoronary artery bypass graft.
ePeripheral artery disease.
On univariate analysis of long-term outcomes, compared to patients with normal stress test, patients with abnormal stress test had significantly higher rates of MACE (27.8% vs 45.8%, odds ratio (OR) 2.20, 95% CI 1.19–1.04, p = 0.01), but comparable all-cause mortality (7.0% vs 15.3%, p = 0.06). There was no difference in long-term MACE with respect to the severity of ischemia. MACE events were 47% (21/45) in patients with mild ischemia, 40% (4/10) with moderate ischemia and 50% (2/4) in severe ischemia group (p = 0.92). On multivariate analysis accounting for clinical risk factors, abnormal stress test was not associated with increased odds of long-term MACE (adjusted OR: 0.83, 95% CI 0.37–1.92, p = 0.68). Of the other clinical risk factors, diabetes (OR 2.37, 95% CI 1.12–5.00, p = 0.02), history of CAD (OR: 3.05, 95% CI 1.30–7.14, p = 0.01) and atrial fibrillation (OR: 5.86, 95% CI 1.86–18.44, p = 0.002) were found to be associated with long-term MACE (Table 2). The results remained consistent when sensitivity analysis was performed combining equivocal/inconclusive stress test results with the abnormal group.
Table 2
| Variables | MACE (OR, 95% confidence interval) | P value |
|---|---|---|
| Abnormal stress test | 0.83 (0.37–1.92) | 0.68 |
| Age | 1.02 (0.99–1.05) | 0.28 |
| Male | 1.27 (0.63–2.54) | 0.50 |
| Hypertension | 1.22 (0.13–11.6) | 0.86 |
| Diabetes | 2.37 (1.12–5.00) | 0.024 |
| Hyperlipidemia | 0.66 (0.30–1.43) | 0.29 |
| Obese (BMI ≥30) | 0.99 (0.52–1.89) | 0.97 |
| Current/prior smoker | 1.10 (0.50–2.05) | 0.98 |
| Coronary artery disease | 3.05 (1.30–7.14) | 0.01 |
| Congestive heart failure | 1.38 (0.61–3.16) | 0.44 |
| Atrial fibrillation | 5.86 (1.86–18.44) | 0.002 |
| Peripheral vascular disease | 1.05 (0.42–2.66) | 0.91 |
MACE: major adverse cardiovascular event, OR: odds ratio, BMI: body mass index.
Among patients with an abnormal stress test, 10 were referred for cardiac catheterization (15.6%). The average interval between stress test and catheterization was 3.9 (±3.4) months.
Five had obstructive coronary artery disease of which 2 underwent percutaneous coronary intervention. In addition, 3 patients with normal stress tests were referred for catheterization for typical angina symptoms, and all (100%) had obstructive CAD.
Discussion
Our retrospective cohort study explored the ability of stress testing to predict MACE in renal transplant patients as well as clinical characteristics independently associated with long-term MACE. The most significant findings from our study were that (1) 30-day MACE rates were low and similar in patients with and without abnormal stress testing and (2) long-term at a median of 5 years, abnormal cardiac stress testing was not independently associated with post-transplant MACE in renal transplant candidates. High risk clinical characteristics that were independently associated with long-term MACE were atrial fibrillation, diabetes mellitus and known CAD.
While used as the primary method for risk stratification, an abnormal stress test did not prevent patients from undergoing renal transplant. This may be related to the fact that stress testing is performed in otherwise suitable transplant candidates and that the majority of abnormal results were graded as mild, with only one quarter classified as moderate or severe. Further, because stress testing was generally performed in our facility as part of a routine pre-operative screening assessment, we have no comprehensive data about patients’ symptom burden at time of stress test. It is plausible that many patients with an abnormal stress test who proceeded to transplant were asymptomatic or had mild symptoms controlled with guideline directed medical therapy. The rate of cardiac catheterization after an abnormal stress test result was very low in our center, since after an abnormal stress test, patients were referred for cardiology consultation, and the risk for transplantation was determined to be acceptable in these largely asymptomatic patients without revascularization. A few patients, however, did undergo revascularization as a result of the work up and went on to transplantation.
In our study, the overall MACE rate was high at median follow-up of 5 years and abnormal stress test was not associated with MACE. The prognostic value of stress test for predicting hard clinical events varies based on patient’s age, clinical and symptom profile. The inability of an abnormal stress test result to predict MACE suggest that conditions other than obstructive CAD, such as atrial fibrillation or microvascular disease, which are not as reliably detected by stress testing, or new plaque rupture, which is inherently unpredictable, may have played a substantial role in prognosis in patients with advanced CKD and renal transplantation.
The 2012 AHA Consensus Statement on Cardiac Disease Evaluation and Management Among Kidney and Liver Transplant Candidates states that “Non-invasive stress testing may be considered in kidney transplant candidates with no active conditions on the basis of the presence of multiple CAD risk factors regardless of functional status (Class IIb, Level of Evidence C)” [5]. The strength of this recommendation has been weakened by its low evidence rating, lack of consensus among professional groups in other specialties, and lack of confirmatory studies since the recommendation was published. Our study supports the growing body of literature that questions the routine use of stress testing as a risk stratifying tool in this patient population. In a propensity-matched study, Dunn et al. [11] examined the utility of stress testing in patients with no active ischemic disease within 18 months of renal transplant. Their results showed no association between performance of stress testing and all-cause mortality, total MI, and fatal MI at 30 days post-transplant, suggesting that stress testing does not have a role in predicting peri-operative events [9]. Our study expands on this data by categorizing the results of the stress testing and extending the follow-up period to several years and supports their results in confirming a lack of association at long-term follow-up. Our 30-day event rates were similar to those reported by Dunn et al. [11]; however, a smaller number of overall events precluded multivariate analysis for 30-day follow-up. As an alternative to stress testing, the study by Park et al. suggests that among relatively young patients with good functional capacity and shorter dialysis duration, transthoracic echocardiography may be as effective as stress testing in predicting ischemic heart disease pre-operatively in this population [12]. Others argue that the poor sensitivity, specificity, and positive predictive value of non-invasive methods make them significantly inferior to coronary angiography, and question whether stress testing should be used at all for low-to-moderate risk patients [13]. The CARP trial [14] has notably demonstrated that cardiac revascularization prior to elective vascular surgery does not affect the rate of long-term mortality or MI, suggesting that invasive methods of risk stratification offer increased risk but limited utility. Majority of patients in our study did not routinely undergo cardiac catheterization after an abnormal stress test and were managed with optimal medical therapy. Recently, in a post-hoc analysis from the ISCHEMIA-CKD trial [15] of 194 patients with chronic coronary syndromes and at least moderate ischemia on stress testing who are listed for renal transplant, an invasive strategy did not improve outcomes compared with conservative medical management.
There is some literature that supports the use of stress testing, especially among certain higher risk patient populations. Doukky et al. [16] assessed the prognostic utility of the 8 risk factors set forth by the AHA/ACCF consensus statement and the role of noninvasive stress testing in this context. The authors reported that patients with 3–4 risk factors derive the greatest additional prognostic benefit from myocardial perfusion imaging, but stress testing per se was not predictive of post-operative MACE. The Doukky et al. [16] study specifically included 8 risk factors outlined by the AHA/ACCF; our study includes all of these risk factors except left ventricular hypertrophy. The long-term follow-up period was similar (median 4.7 vs 5 years), but our event rate is higher (32.3% vs. 27.1%); however, our MACE included the additional variables of congestive heart failure and revascularization.
Our study has several limitations. This is a retrospective study where data was extracted from review of medical records spanning multiple years and hence subjected to missing or incomplete data as well as variability in documentation. Additionally, we cannot account for patient encounters that occurred outside of our health care facility. Patients’ symptom status was unknown at the time of stress testing, as any testing within the eligible timeframe was assumed to be part of pre-transplant evaluation. Our study population was predominantly limited to white males, warranting further investigation into the risk patterns of other racial/ethnic groups. Finally, eligible patients in our single-center study were derived from a database of patients who successfully received renal transplant, indicating an overall healthier patient population than those with CKD/ESRD who did not undergo transplant. We did not have data available to accurately estimate the number of patients who were removed from consideration of transplantation due to abnormal stress testing results. However, we estimate the number is low due to current practice of cardiology consultation with medical optimization and referral for cardiac catheterization in selected patients that may benefit from percutaneous or surgical revascularization prior to transplantation. Our results therefore may not be generalizable to patients with more significant disease burden.
In conclusion, our results indicate that an abnormal stress test is not predictive of long-term MACE post-renal transplant. Additionally, the high event rate in patients with negative stress tests suggests that we should not be reassured by a normal test result. Our study does not support routine stress testing, particularly in presumably asymptomatic individuals, who are undergoing renal transplantation.
Funding Statement
Dr. Anderson received research funding from Brown University. The funding source played no role in the study design or intepretation.
Data Availability
Data cannot be shared publicly because it was collected for quality purposes and for renal transplant outcome reporting and no patient approval has been obtained for sharing data. Data that is deintified, are available from the Lifespan Biostatistical Core (contact via Jason Machan gro.napsefil@nahcamj) for researchers who meet the criteria for access to confidential data.
References
Decision Letter 0
13 Aug 2021
PONE-D-21-21764
Relation of abnormal cardiac stress testing with outcomes in patients undergoing renal transplantation
PLOS ONE
Dear Dr. Abbott,
Thank you for submitting your manuscript to PLOS ONE. After careful consideration, we feel that it has merit but does not fully meet PLOS ONE’s publication criteria as it currently stands. Therefore, we invite you to submit a revised version of the manuscript that addresses the points raised during the review process. Reviewers felt that the long term outcome data, which is a major asset of the study, could be further developed. The Editor agrees with this sentiment. Inclusion of a survival curves and performance of multivariable analysis of long-term outcome would significantly elevate the impact of the manuscript.
Please submit your revised manuscript by Sep 27 2021 11:59PM. If you will need more time than this to complete your revisions, please reply to this message or contact the journal office at gro.solp@enosolp. When you're ready to submit your revision, log on to https://www.editorialmanager.com/pone/ and select the 'Submissions Needing Revision' folder to locate your manuscript file.
Please include the following items when submitting your revised manuscript:
A rebuttal letter that responds to each point raised by the academic editor and reviewer(s). You should upload this letter as a separate file labeled 'Response to Reviewers'.
A marked-up copy of your manuscript that highlights changes made to the original version. You should upload this as a separate file labeled 'Revised Manuscript with Track Changes'.
An unmarked version of your revised paper without tracked changes. You should upload this as a separate file labeled 'Manuscript'.
If you would like to make changes to your financial disclosure, please include your updated statement in your cover letter. Guidelines for resubmitting your figure files are available below the reviewer comments at the end of this letter.
If applicable, we recommend that you deposit your laboratory protocols in protocols.io to enhance the reproducibility of your results. Protocols.io assigns your protocol its own identifier (DOI) so that it can be cited independently in the future. For instructions see: http://journals.plos.org/plosone/s/submission-guidelines#loc-laboratory-protocols. Additionally, PLOS ONE offers an option for publishing peer-reviewed Lab Protocol articles, which describe protocols hosted on protocols.io. Read more information on sharing protocols at https://plos.org/protocols?utm_medium=editorial-email&utm_source=authorletters&utm_campaign=protocols.
We look forward to receiving your revised manuscript.
Kind regards,
Jeffrey J. Rade, MD
Academic Editor
PLOS ONE
Journal Requirements:
1. When submitting your revision, we need you to address these additional requirements.
Please ensure that your manuscript meets PLOS ONE's style requirements, including those for file naming. The PLOS ONE style templates can be found at
https://journals.plos.org/plosone/s/file?id=wjVg/PLOSOne_formatting_sample_main_body.pdf and
2. Thank you for providing the date(s) when patient medical information was initially recorded. Please also include the date(s) on which your research team accessed the databases/records to obtain the retrospective data used in your study.
3. We note that the grant information you provided in the ‘Funding Information’ and ‘Financial Disclosure’ sections do not match.
When you resubmit, please ensure that you provide the correct grant numbers for the awards you received for your study in the ‘Funding Information’ section.
4. Thank you for stating the following financial disclosure:
"Dr. Anderson received research funding from Brown University."
Please state what role the funders took in the study. If the funders had no role, please state: "The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript."
If this statement is not correct you must amend it as needed.
Please include this amended Role of Funder statement in your cover letter; we will change the online submission form on your behalf.
5. In your Data Availability statement, you have not specified where the minimal data set underlying the results described in your manuscript can be found. PLOS defines a study's minimal data set as the underlying data used to reach the conclusions drawn in the manuscript and any additional data required to replicate the reported study findings in their entirety. All PLOS journals require that the minimal data set be made fully available. For more information about our data policy, please see http://journals.plos.org/plosone/s/data-availability.
"Upon re-submitting your revised manuscript, please upload your study’s minimal underlying data set as either Supporting Information files or to a stable, public repository and include the relevant URLs, DOIs, or accession numbers within your revised cover letter. For a list of acceptable repositories, please see http://journals.plos.org/plosone/s/data-availability#loc-recommended-repositories. Any potentially identifying patient information must be fully anonymized.
Important: If there are ethical or legal restrictions to sharing your data publicly, please explain these restrictions in detail. Please see our guidelines for more information on what we consider unacceptable restrictions to publicly sharing data: http://journals.plos.org/plosone/s/data-availability#loc-unacceptable-data-access-restrictions. Note that it is not acceptable for the authors to be the sole named individuals responsible for ensuring data access.
We will update your Data Availability statement to reflect the information you provide in your cover letter.
6. Please include captions for your Supporting Information files at the end of your manuscript, and update any in-text citations to match accordingly. Please see our Supporting Information guidelines for more information: http://journals.plos.org/plosone/s/supporting-information.
[Note: HTML markup is below. Please do not edit.]
Reviewers' comments:
Reviewer's Responses to Questions
Comments to the Author
1. Is the manuscript technically sound, and do the data support the conclusions?
The manuscript must describe a technically sound piece of scientific research with data that supports the conclusions. Experiments must have been conducted rigorously, with appropriate controls, replication, and sample sizes. The conclusions must be drawn appropriately based on the data presented.
Reviewer #1: Yes
Reviewer #2: Partly
**********
2. Has the statistical analysis been performed appropriately and rigorously?
Reviewer #1: Yes
Reviewer #2: Yes
**********
3. Have the authors made all data underlying the findings in their manuscript fully available?
The PLOS Data policy requires authors to make all data underlying the findings described in their manuscript fully available without restriction, with rare exception (please refer to the Data Availability Statement in the manuscript PDF file). The data should be provided as part of the manuscript or its supporting information, or deposited to a public repository. For example, in addition to summary statistics, the data points behind means, medians and variance measures should be available. If there are restrictions on publicly sharing data—e.g. participant privacy or use of data from a third party—those must be specified.
Reviewer #1: Yes
Reviewer #2: Yes
**********
4. Is the manuscript presented in an intelligible fashion and written in standard English?
PLOS ONE does not copyedit accepted manuscripts, so the language in submitted articles must be clear, correct, and unambiguous. Any typographical or grammatical errors should be corrected at revision, so please note any specific errors here.
Reviewer #1: Yes
Reviewer #2: Yes
**********
5. Review Comments to the Author
Please use the space provided to explain your answers to the questions above. You may also include additional comments for the author, including concerns about dual publication, research ethics, or publication ethics. (Please upload your review as an attachment if it exceeds 20,000 characters)
Reviewer #1: This is a very interesting an pertinent paper regarding the role of routine stress testing prior to renal transplantation.
The authors have a large cohort of patients that had renal transplantation. They found that the stress test result did not correlate with 30 or 5 year outcomes (MACE). DM, CAD, and AF did correlate with increased risk of adverse events.
This is an important study that adds to a growing body of literature showing that routine stress testing prior to renal transplantation is not helpful. Please see my specific comments below.
1. Study was predominantly limited to white males. This should be mentioned in the limitation section of the discussion.
2. It is unclear to me if the 30 day MACE included events from the index hospitalization during which the transplant was performed. This should be clarified in the methods section.
3. Page 6. Were the patients who had cardiac catheterization included in the analysis?
Reviewer #2: The authors undertook this single center study of patients undergoing renal transplantation. This is a common group of patients to receive routine stress tests, and so this is an important investigation to determine if the stress test results were independently associated with MACE.
The authors found, in multivariable adjusted models, that diabetes, coronary artery disease, and atrial fibrillation were independently associated with 30-day MACE, but abnormal stress test results were not.
Suggestions/considerations for revision:
1) How many people were evaluated for renal transplant at your center and not transplanted due to stress testing results? Or a way to estimate this number?
2) I am wondering how you graded abnormal exercise treadmill tests as mild, moderate, or severe ischemia? You discuss using exercise, pharm, or a combined approach, but don’t discuss the modality (echo, nuclear, treadmill only).
3) I can’t tell if you are looking at MACE at 30 days or a different time frame based on your methods/results. You say 30-day MACE in the abstract but not the body of the paper.
4) Table 2 does not include the covariates adjusted for – I assume it was all the covariates listed in your methods paragraph 3?
5) How did you define prior history of coronary artery disease? Could this include non-obstructive disease seen on cath or coronary CT? I see that you included PCI/prior CABG as a co-variate, but I don’t see that listed in Table 2.
6) You mention sensitivity analysis performed combining equivocal/inconclusive stress tests. Have you looked at other cut-points with the abnormal stress tests, such as those with moderate or severe ischemia only to determine associations with MACE?
7) What about outcomes beyond 30 days? It looks like you have 5 years of follow up data. Did you follow these participants longer to look at associations beyond 30 days?
8) When you present your results, I assume you are presenting odds ratio (2.20) on the last line of page 5, but it is not labelled.
9) Participants who undergo pharmacologic testing because of inability to exercise/use a treadmill are associated with higher adverse outcomes that those undergoing exercise testing. Do you specifically stratify those undergoing pharm vs. exercise testing?
10) I am not sure your conclusion “particularly in asymptomatic individuals” is supported by your study, since you don’t know what symptoms these patients had. I know that is inferred based on the data you present, but probably best to keep that out of your last line. Or qualify to say “presumably asymptomatic”
Minor typos to correct – you go between MACE and MACCE throughout the paper, with MACCE seen in the abstract, on page 6, and Figure 1. First line of page 6 – “and but comparable all-cause mortality” Second line page 6 “with respect of severity of ischemia”. Page 10 – “abnormal stress test, ,”.
**********
6. PLOS authors have the option to publish the peer review history of their article (what does this mean?). If published, this will include your full peer review and any attached files.
If you choose “no”, your identity will remain anonymous but your review may still be made public.
Do you want your identity to be public for this peer review? For information about this choice, including consent withdrawal, please see our Privacy Policy.
Reviewer #1: Yes: Timothy P. Fitzgibbons
Reviewer #2: No
[NOTE: If reviewer comments were submitted as an attachment file, they will be attached to this email and accessible via the submission site. Please log into your account, locate the manuscript record, and check for the action link "View Attachments". If this link does not appear, there are no attachment files.]
While revising your submission, please upload your figure files to the Preflight Analysis and Conversion Engine (PACE) digital diagnostic tool, https://pacev2.apexcovantage.com/. PACE helps ensure that figures meet PLOS requirements. To use PACE, you must first register as a user. Registration is free. Then, login and navigate to the UPLOAD tab, where you will find detailed instructions on how to use the tool. If you encounter any issues or have any questions when using PACE, please email PLOS at gro.solp@serugif. Please note that Supporting Information files do not need this step.
Author response to Decision Letter 0
14 Oct 2021
Editors’ comments:
Reviewers felt that the long-term outcome data, which is a major asset of the study, could be further developed. The Editor agrees with this sentiment. Inclusion of a survival curves and performance of multivariable analysis of long-term outcome would significantly elevate the impact of the manuscript
We are glad that the editors find the long-term outcomes of our study to be of importance. The 30 day and long term (median follow up 5 years) MACE rates and multivariable analysis for the long-term outcomes are now presented. No adjusted outcomes for 30-day events are presented due to the small number of events. We were unable to include survival curves as we do not have the exact date of events. The renal transplant dataset at our institution tracks events over time periods, which include a range of time.
Reviewer 1 comments:
1. Study was predominantly limited to white males. This should be mentioned in the limitation section of the discussion.
Thank you for your comment. We have updated the limitation section to include the following: “Our study population was predominantly limited to white males, warranting further investigation into the risk patterns and utility of stress testing in other racial/ethnic groups.”
2. It is unclear to me if the 30-day MACE included events from the index hospitalization during which the transplant was performed. This should be clarified in the methods section.
Yes, all events from the time of transplant, including in-hospital were recorded in the renal transplant dataset. This detail was added to the methods.
3. Page 6. Were the patients who had cardiac catheterization included in the analysis?
Yes, in our cohort 10 patients with abnormal stress testing underwent cardiac catheterization and 3 had revascularization with PCI prior to transplantation. Patients that had abnormal stress testing that did not go on to transplantation, however, were not captured in this analysis. This was acknowledged in response to one of the comments from reviewer 2 and the manuscript updated accordingly.
Reviewer 2 comments:
1) How many people were evaluated for renal transplant at your center and not transplanted due to stress testing results? Or a way to estimate this number?
Our patient population was selected from a database that only included patients who successfully underwent transplant, and the data dates to 2006. Unfortunately, we do not have data available to accurately estimate those patients who were not captured in our target population due to stress testing results. In review with our renal transplant team, they estimate very few patients were excluded from transplantation based on stress testing results. The rationale is that stress testing is performed in otherwise suitable candidates and if abnormal cardiology consultation obtained and the patient is medically managed or referred for cardiac catheterization. If PCI or CABG is required, patients are generally relisted for transplantation 6 months after revascularization. A situation where stress testing uncovers CAD is advanced and not amenable to revascularization is rare at our center. “We did not have data available to accurately estimate the number of patients who were removed from consideration of transplantation due to abnormal stress testing results. However, we estimate the number is low due to current practice of cardiology consultation with medical optimization and referral for cardiac catheterization in selected patients that may benefit from percutaneous or surgical revascularization prior to transplantation.”
2) I am wondering how you graded abnormal exercise treadmill tests as mild, moderate, or severe ischemia? You discuss using exercise, pharm, or a combined approach, but don’t discuss the modality (echo, nuclear, treadmill only).
Thank you for pointing out that the stress testing modality was not presented. The stress tests were nuclear myocardial perfusion imaging studies (91.4%) and echo (8.6%). In addition to indicating the stress testing modality we added the following details regarding grading abnormality to the methods. “Our patient records provided access to stress reports and not the raw quantitative stress data. In our hospital system, for nuclear stress tests image interpretation was performed incorporating visual and quantitative analysis compared to a gender specific normal database, using standardized thresholds for severity and extent of ischemia. For stress echo wall motion index was scored semi-quantitatively using 17 segment model.” References for stress test interpretation and reporting were added.
We have updated the results section to include the above statement. “Two hundred and twelve (91.4%) participants underwent nuclear perfusion scan; the remainder underwent stress echo.”
3) I can’t tell if you are looking at MACE at 30 days or a different time frame based on your methods/results. You say 30-day MACE in the abstract but not the body of the paper.
We apologize that the primary outcome data presentation was not clear and that we erroneously reported adjusted 30 day rather than long tern MACE rates in the abstract. In our study we report MACE at 30 days post-transplant, as well as long-term follow up at a median (IQR) follow-up duration of 5 (3-7) years. There were only 9 events at 30-day MACE, therefore we report only the event rates of these outcomes between normal and abnormal stress test result. MACE at long term follow-up were analyzed with multivariate analysis.
We have updated the abstract to reflect the correct data and added to the methods section “Univariate and multivariate logistic regression models were used to evaluate the association between abnormal stress test and long-term MACE, but only 30-day MACE rates are reported due to the small number of events.” Our results section includes the following, which has been revised to include the number of patients at 30-day MACE with abnormal stress test: “In our cohort of 232 patients, 9 (3.9%) patients had MACE in the 30-day post-transplant period, 5 of whom had an abnormal stress test. Seventy-five (32.3%) patients had MACE at a median (IQR) follow up duration of 5 (3-7) years.”
4) Table 2 does not include the covariates adjusted for – I assume it was all the covariates listed in your methods paragraph 3?
Thank you for pointing out this omission. Variables with a p<0.1 on the univariate analysis (Table 1) or deemed clinically significant (age, AF, PVD) were included. To avoid collinearity, prior CAD but not prior PCI/CABG was included in the model. This detail was added to the methods.
5) How did you define prior history of coronary artery disease? Could this include non-obstructive disease seen on cath or coronary CT? I see that you included PCI/prior CABG as a co-variate, but I don’t see that listed in Table 2.
Prior PCI/CABG was included in the univariate analysis, but not used in regression analysis due to inclusion of CAD in the model; consequently ‘Prior PCI/CABG’ is intentionally absent from Table 2. History of CAD was broadly defined as: known obstructive coronary disease, prior MI, or prior PCI/CABG. Non-obstructive coronary disease seen on catheterization and coronary CT findings were not included.
We have removed “prior history of revascularization (PCI or CABG)” from the list of covariates in the methods section.
Additionally, we have updated the Methods section by defining CAD.
6) You mention sensitivity analysis performed combining equivocal/inconclusive stress tests. Have you looked at other cut-points with the abnormal stress tests, such as those with moderate or severe ischemia only to determine associations with MACE?
Due to the small sample size of patients with moderate to severe ischemia we were underpowered to analyze this subgroup separately. The severity of ischemia, however, was not significantly associated with unadjusted rates of long-term MACE. In the results “There was no difference in long term MACE with respect to the severity of ischemia. MACE events were 47% (21/45) in patients with mild ischemia, 40% (4/10) with moderate ischemia and 50% (2/4) in severe ischemia group (p=0.92)”. Also, in the limitations we acknowledge that results may not apply to patients with greater burden of disease than our population.
7) What about outcomes beyond 30 days? It looks like you have 5 years of follow up data. Did you follow these participants longer to look at associations beyond 30 days?
Sorry if this was unclear (see response to comment 3). In our study we examine MACE at 30 days post-transplant, as well as long-term follow up at a median (IQR) follow-up duration of 5 (3-7) years. The abstract, methods and results were reviewed to assure this was clear. We removed reference to MACCE as the endpoints were MACE at 30 days and long term.
8) When you present your results, I assume you are presenting odds ratio (2.20) on the last line of page 5, but it is not labelled.
Thank you for pointing out this omission, we have added that 2.2 refers to the odds ratio (OR) on univariate logistic regression.
9) Participants who undergo pharmacologic testing because of inability to exercise/use a treadmill are associated with higher adverse outcomes that those undergoing exercise testing. Do you specifically stratify those undergoing pharm vs. exercise testing?
While we acknowledge that inability to perform exercise is a potential predictor or poor outcome, we did not perform analysis stratified by type of stress testing to avoid overanalyzing the data and inability to control for confounding in choice of stress modality.
10) I am not sure your conclusion “particularly in asymptomatic individuals” is supported by your study, since you don’t know what symptoms, these patients had. I know that is inferred based on the data you present, but probably best to keep that out of your last line. Or qualify to say “presumably asymptomatic”
We have updated the manuscript to the following: “Our study doesn’t support routine stress testing, particularly in presumably asymptomatic individuals, who are undergoing renal transplantation.”
Minor typos to correct – you go between MACE and MACCE throughout the paper, with MACCE seen in the abstract, on page 6, and Figure 1. First line of page 6 – “and but comparable all-cause mortality” Second line page 6 “with respect of severity of ischemia”. Page 10 – “abnormal stress test,”.
We thank the reviewer for taking the time to provide such detailed comments. We recognize the inconsistences and grammatical errors and have corrected them.
Attachment
Submitted filename: PLOSresponsestoreviewersFINAL.docx
Decision Letter 1
16 Nov 2021
Relation of abnormal cardiac stress testing with outcomes in patients undergoing renal transplantation
PONE-D-21-21764R1
Dear Dr. Abbott,
We’re pleased to inform you that your manuscript has been judged scientifically suitable for publication and will be formally accepted for publication once it meets all outstanding technical requirements.
Within one week, you’ll receive an e-mail detailing the required amendments. When these have been addressed, you’ll receive a formal acceptance letter and your manuscript will be scheduled for publication.
An invoice for payment will follow shortly after the formal acceptance. To ensure an efficient process, please log into Editorial Manager at http://www.editorialmanager.com/pone/, click the 'Update My Information' link at the top of the page, and double check that your user information is up-to-date. If you have any billing related questions, please contact our Author Billing department directly at gro.solp@gnillibrohtua.
If your institution or institutions have a press office, please notify them about your upcoming paper to help maximize its impact. If they’ll be preparing press materials, please inform our press team as soon as possible -- no later than 48 hours after receiving the formal acceptance. Your manuscript will remain under strict press embargo until 2 pm Eastern Time on the date of publication. For more information, please contact gro.solp@sserpeno.
Kind regards,
Jeffrey J. Rade, MD
Academic Editor
PLOS ONE
Additional Editor Comments (optional):
Reviewers' comments:
Reviewer's Responses to Questions
Comments to the Author
1. If the authors have adequately addressed your comments raised in a previous round of review and you feel that this manuscript is now acceptable for publication, you may indicate that here to bypass the “Comments to the Author” section, enter your conflict of interest statement in the “Confidential to Editor” section, and submit your "Accept" recommendation.
Reviewer #1: All comments have been addressed
Reviewer #2: All comments have been addressed
**********
2. Is the manuscript technically sound, and do the data support the conclusions?
The manuscript must describe a technically sound piece of scientific research with data that supports the conclusions. Experiments must have been conducted rigorously, with appropriate controls, replication, and sample sizes. The conclusions must be drawn appropriately based on the data presented.
Reviewer #1: Yes
Reviewer #2: Yes
**********
3. Has the statistical analysis been performed appropriately and rigorously?
Reviewer #1: Yes
Reviewer #2: Yes
**********
4. Have the authors made all data underlying the findings in their manuscript fully available?
The PLOS Data policy requires authors to make all data underlying the findings described in their manuscript fully available without restriction, with rare exception (please refer to the Data Availability Statement in the manuscript PDF file). The data should be provided as part of the manuscript or its supporting information, or deposited to a public repository. For example, in addition to summary statistics, the data points behind means, medians and variance measures should be available. If there are restrictions on publicly sharing data—e.g. participant privacy or use of data from a third party—those must be specified.
Reviewer #1: Yes
Reviewer #2: Yes
**********
5. Is the manuscript presented in an intelligible fashion and written in standard English?
PLOS ONE does not copyedit accepted manuscripts, so the language in submitted articles must be clear, correct, and unambiguous. Any typographical or grammatical errors should be corrected at revision, so please note any specific errors here.
Reviewer #1: Yes
Reviewer #2: Yes
**********
6. Review Comments to the Author
Please use the space provided to explain your answers to the questions above. You may also include additional comments for the author, including concerns about dual publication, research ethics, or publication ethics. (Please upload your review as an attachment if it exceeds 20,000 characters)
Reviewer #1: All my concerns have been addressed. Paper is very well written, succinct and clear. Discussion is excellent.
Reviewer #2: Thank you for your thoughtful revisions. My last comment would be interpreting your results in the context of the Ischemia study.
**********
7. PLOS authors have the option to publish the peer review history of their article (what does this mean?). If published, this will include your full peer review and any attached files.
If you choose “no”, your identity will remain anonymous but your review may still be made public.
Do you want your identity to be public for this peer review? For information about this choice, including consent withdrawal, please see our Privacy Policy.
Reviewer #1: Yes: Timothy P. Fitzgibbons MD PhD
Reviewer #2: No
Acceptance letter
22 Nov 2021
PONE-D-21-21764R1
Relation of abnormal cardiac stress testing with outcomes in patients undergoing renal transplantation
Dear Dr. Abbott:
I'm pleased to inform you that your manuscript has been deemed suitable for publication in PLOS ONE. Congratulations! Your manuscript is now with our production department.
If your institution or institutions have a press office, please let them know about your upcoming paper now to help maximize its impact. If they'll be preparing press materials, please inform our press team within the next 48 hours. Your manuscript will remain under strict press embargo until 2 pm Eastern Time on the date of publication. For more information please contact gro.solp@sserpeno.
If we can help with anything else, please email us at gro.solp@enosolp.
Thank you for submitting your work to PLOS ONE and supporting open access.
Kind regards,
PLOS ONE Editorial Office Staff
on behalf of
Dr. Jeffrey J. Rade
Academic Editor
PLOS ONE
Articles from PLOS ONE are provided here courtesy of PLOS
Full text links
Read article at publisher's site: https://doi.org/10.1371/journal.pone.0260718
Read article for free, from open access legal sources, via Unpaywall:
https://journals.plos.org/plosone/article/file?id=10.1371/journal.pone.0260718&type=printable
Citations & impact
This article has not been cited yet.
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/137511060
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Thallium stress testing does not predict cardiovascular risk in diabetic patients with end-stage renal disease undergoing cadaveric renal transplantation.
Am J Med, 90(5):563-570, 01 May 1991
Cited by: 35 articles | PMID: 2029013
Cardiovascular Events after New-Onset Atrial Fibrillation in Adults with CKD: Results from the Chronic Renal Insufficiency Cohort (CRIC) Study.
J Am Soc Nephrol, 29(12):2859-2869, 30 Oct 2018
Cited by: 21 articles | PMID: 30377231 | PMCID: PMC6287862
Screening for significant coronary artery disease in high-risk renal transplant candidates.
Coron Artery Dis, 18(7):553-558, 01 Nov 2007
Cited by: 34 articles | PMID: 17925609
Pre-Operative Cardiovascular Testing and Post-Renal Transplant Clinical Outcomes.
Cardiovasc Revasc Med, 20(7):588-593, 17 Apr 2019
Cited by: 1 article | PMID: 31097384
Funding
Funders who supported this work.




