Abstract
Free full text
The role of mesenchymal stem cells in liver injury
Associated Data
Abstract
Recently, mesenchymal stem cell (MSC) therapy has been suggested as an effective alternative approach for the treatment of hepatic diseases. MSCs have potential therapeutic value, because they have high self‐renewal ability, are capable of multipotent differentiation, and have low immunogenicity. Furthermore, MSCs have the potential to differentiate into hepatocytes, and the therapeutic value exists in their immune‐modulatory properties and secretion of trophic factors, such as growth factors and cytokines. Moreover, MSCs can suppress inflammatory responses, reduce hepatocyte apoptosis, increase hepatocyte regeneration, regress liver fibrosis, and enhance liver functionality.
1. INTRODUCTION
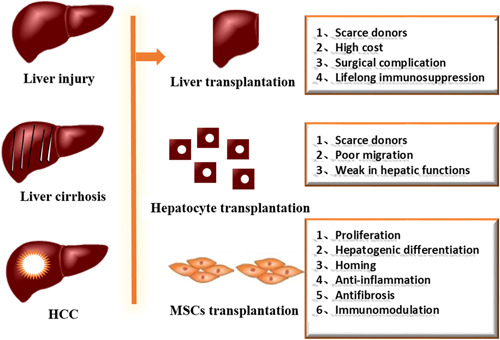
Various stimulators, such as viral hepatitis, alcohol steatohepatitis, nonalcoholic steatohepatitis, and autoimmunity, can cause the occurrence of chronic inflammation of the liver, which leads to injury of the liver, and the activation of inflammatory cells and hepatic stellate cells, finally leading to the occurrence of liver fibrosis (Jorge Matias et al., 2017). With the aggravation of liver fibrosis, the body's dysfunction would eventually develop into cirrhosis, and even liver cancer (Wang et al., 2016) (Figure (Figure1).1). Liver transplantation and hepatocyte transplantation are efficient approaches to managing liver fibrosis. However, due to the lack of liver organs, immune rejection, surgical complications, and other factors, this cannot be applied in clinical practice (Javazon et al., 2001). Therefore, given the increasing number of patients and the limited number of donors, and the increase in morbidity and mortality of liver fibrosis, alternative therapies are needed.
Mesenchymal stem cells (MSCs) have been proposed for the treatment of fibrosis, based on their hepatocyte differentiation and regeneration potential, as well as their immunomodulatory properties. The Mesenchymal and Tissue Stem Cell Committee of the International Society for Cell and Gene Therapy (ISCT) proposed a minimal set of standard criteria to define MSCs, which includes adherence to plastic, specific surface antigen expression, and the potential to differentiate into osteoblasts, adipocytes, and chondrocytes (Ankrum & Karp, 2010; Friedenstein, 1976). It has been generally recognized that MSCs have CD29+, CD44+, CD73+, CD90+, CD105+, CD45−, CD34−, CD14−, CD11b−, and HLA‐DR− (Dominici et al., 2006) (Table (Table1).1). Furthermore, MSCs are being clinically investigated in over 800 clinical trials due to their ease of isolation and propagation, relative safety profile, secretion of paracrine factors, and ability to interact and mitigate immune effector responses (Olsen et al., 2018).
Table 1
Surface marker expression profile of MSCs in humans
| MSC‐positive markers | MSC‐negative markers |
|---|---|
| CD29 | CD45 |
| CD44 | CD34 |
| CD73 | CD14 |
| CD90 | CD11b |
| CD105 | HLA‐DR |
Abbreviation: MSC, mesenchymal stem cell.
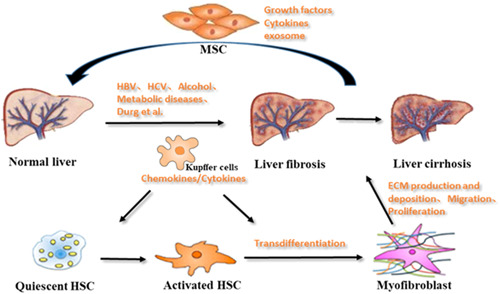
2. THE FORMATION MECHANISM OF LIVER FIBROSIS
Normal liver tissue comprises hepatocytes, endothelial cells, hepatic macrophages, and hepatic stellate cells (HSCs). HSCs are nonparenchymal cells located between hepatocytes and endothelial cells, which store a large amount of vitamin A and lipids (Wake, 1971). Furthermore, HSCs can regulate portal vein pressure and promote material exchange. The number of HSCs accounts for 13% of liver cells. In addition, HSCs are the main extracellular matrix (ECM)‐producing cells in the liver. Notably, several studies have shown that HSCs play a critical role in liver fibrosis. When the liver is exposed to various injuries, quiescent HSCs change into activated HSCs. Activated HSCs specifically express high levels of alpha‐smooth muscle actin (α‐SMA). This is identified by the intense deposition of the ECM, which is transformed into myofibroblast‐like cells (Tsuchida & Friedman, 2017; Zhou et al., 2016). Hence, HSCs are considered as the principal precursor population for myofibroblasts (Figure (Figure2).2). Previously, liver fibrosis was considered to be irreversible. Statistics have shown that cirrhosis is 14th of the most common cause of adult death worldwide, affecting hundreds of millions of people. Until the 1990s, studies have confirmed that liver fibrosis is reversible. Therefore, it is important to take effective antifibrosis treatment measures, which can prevent or even reverse the pathological process of liver fibrosis.
3. THE THERAPEUTIC EFFECT OF MSCs ON DIFFERENT TYPES OF LIVER INJURY
Since 1974, when Friedenstein et al. first isolated and characterized MSCs, MSC‐based therapy has been shown to be safe and effective (Friedenstein, 1974). Nevertheless, many scientists and clinical researchers wanted to improve the success of MSCs in regenerative therapy. MSCs are easily isolated and amenable to culture expansion in vitro. Hence, MSCs are being exploited as an experimental therapy for a variety of human diseases (Jung et al., 2017; A. Huang et al., 2018; Liu et al., 2017). Furthermore, MSCs play several simultaneous roles: limits inflammation through the release of cytokines; aid healing by expressing growth factors; alter host immune responses by secreting immunomodulatory proteins; enhance responses from endogenous repair cells; serve as mature functional cells in some tissues, such as the bone (Galipeau & Stagg, 2013; Gebler et al., 2011). MSCs are presently being clinically investigated in a number of acute and chronic inflammatory diseases. Hence, MSCs are a new and effective therapy to attenuate liver fibrosis due to their immunomodulatory and antifibrotic properties (An et al., 2017; Chang et al., 2017; Huafeng et al., 2017).
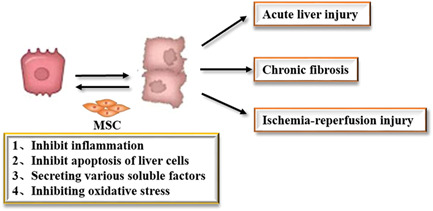
MSCs play an important role in the treatment of liver damage by inhibiting inflammation, inhibiting apoptosis of liver cells, secreting various soluble factors, and inhibiting oxidative stress (Figure (Figure33).
The characteristics of acute liver injury are rapid onset, rapid disease progression, and its development into acute liver failure. The present treatment is orthotopic liver transplantation. Studies have revealed that MSC transplantation can effectively improve the condition of acute liver injury in mice (Guo et al., 2016; S. Zhang et al., 2018). Furthermore, mice serum alanine transaminase (ALT), alkaline phosphatase, total bilirubin, and albumin levels can be restored to normal levels at four weeks after cell injection. At the same time, MSCs can be located in the liver, and play a certain role in the treatment of abnormal liver function.
Chronic fibrosis is also a type of liver injury. Chronic hepatitis leads to the activation of HSCs, and the conversion into myofibroblasts to produce a large amount of ECM. With the rate of decomposition, collagen fibers would gradually deposit, leading to the occurrence of liver fibrosis. Studies have confirmed by the histopathological observation that the degree of fibrosis can be reduced and that the computed tomography (CT) perfusion scan also revealed a significant hemodynamic improvement after the portal vein transplantation of MSCs to rats with liver fibrosis (Y. Wang et al., 2010). In the mouse liver fibrosis model, it was found that the transplantation of MSCs can significantly reduce the expression of Type I collagen, and at the same time, the number of α‐SMA‐positive HSCs was found to be reduced, indicating that the mechanism of reducing fibrosis may be through the inhibition of the activation of HSCs (B. Huang et al., 2016).
The ischemia‐reperfusion injury caused by liver transplantation is also a type of liver injury. This is mainly caused by transient hepatic portal vein hypertension and the destruction of hepatic sinus endothelial cells, which leads to a prolonged ischemia period, and in turn, leads to severe inflammation after blood reperfusion (Kwan Man et al., 2010). Studies have shown that in the rat model of hepatic ischemia‐reperfusion, the liver regeneration rate of rats transplanted with MSCs was significantly higher, when compared to that of rats in the control group, after 2 days, and that the expression levels of vascular endothelial growth factor (VEGF), tumor necrosis factor‐α (TNF‐α), hepatocyte growth factor (HGF), and other regeneration‐related proteins were also higher when compared to the control group (Seki et al., 2012).
4. REGULATORY MECHANISM OF MSCs IN THE TREATMENT OF LIVER INJURY
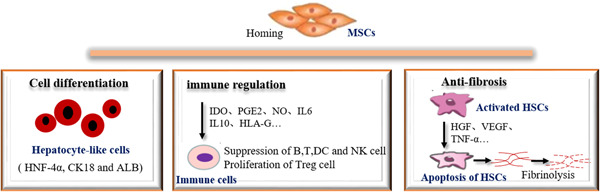
More and more researchers are using stem cells to treat a variety of liver diseases. A large number of studies have confirmed that MSC transplantation can improve liver function, reduce liver fibrosis, and repair liver injury, and the therapeutic mechanism may be realized through the following aspects (Figure (Figure44).
4.1. Differentiation of MSCs
MSCs can be induced to differentiate into hepatocyte‐like cells in vitro, and can express hepatocyte‐specific markers. Seo was the first to report that adipose‐derived stem cells can differentiate into hepatoid cells in vitro, and the expression of hepatocyte‐specific marker albumin was detected by flow cytometry (Seo et al., 2005). Yamamoto reported that hepatoid cells differentiated from adipose‐derived stem cells were very similar to normal hepatocytes in gene expression by cluster analysis. In addition, Agnieszka isolated adipose‐derived stem cells from six patients who were undergoing gastrectomy for gastric cancer. After incubation with HGF and basic fibroblast growth factor (bFGF) in vitro, the adipose‐derived stem cells exhibited a high degree of liver differentiation and were able to express markers of human hepatocytes (Banas et al., 2007). All these indicate that MSCs can be induced to differentiate into hepatocytes in vitro, and express the markers of hepatocytes.
MSCs have the characteristic of homing, that is, they can migrate to certain specific areas, especially tissue focus areas (Karp & Leng Teo, 2009). Many scientists have taken advantage of this feature to directly transplant MSCs in the microenvironment of the liver injury, in which MSCs differentiate into liver cells and repair the damaged liver. Horng reported that in the rat model of liver injury induced by thioacetamide‐transplanted MSCs, the levels of serological indexes ALT, aspartate aminotransferase, albumin, and total bilirubin returned to the normal steady‐state and that the pathological morphology of liver tissues gradually became normal (Harn et al., 2012). Guangfeng et al. reported that adipose‐derived stem cells were transplanted into liver failure models, and did not differentiate into hepatocytes after three days (Guangfeng et al., 2015). Studies have shown that the microenvironment of liver injury is conducive to the migration of transplanted MSCs to the liver. However, it needs to be further confirmed whether uninduced MSCs can differentiate themselves in the liver.
4.2. Immune regulation of MSCs
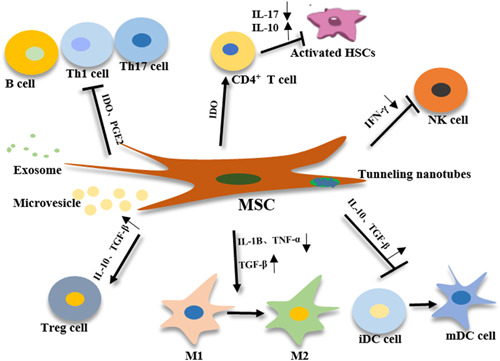
The liver is a very important immune organ in the human body, which contains a large number of immune cells. The immune response is closely correlated to the development of liver diseases (Ahmed, 2018; Devarbhavi & Raj, 2018; Noll et al., 2016). MSCs can maintain homeostasis by secreting soluble factors that interact with cells of the innate and adaptive immune systems, enabling these to participate in the treatment of a variety of liver diseases, without being affected by immune rejection (Michelle et al., 2011; Keating, 2008) (Figure (Figure55).
Wan et al. reported that adipose‐derived stem cell transplantation can significantly inhibit the proliferation of T lymphocytes, and significantly reduce the acute rejection of rats (Wan et al., 2008). Higashimoto et al. reported that adipose‐derived stem cells can inhibit the activation of T‐cells and macrophages, and inhibit hepatitis (Mami et al., 2013). Mohammadzadeh et al. reported that adipose‐derived stem cells have the ability to regulate the differentiation of Th cell subsets, which can suppress Th1, Th2, and Th17 cells while suppressing T cells, thereby avoiding the liver damage caused by an excessive immune response, and inhibiting the immune rejection after transplantation (Mohammadzadeh Adel et al., 2014). Milosavljevic et al. reported that the concentration of indoleamine 2,3‐dioxygenase (IDO) increased after transplantation of MSCs (Milosavljevic et al., 2018). IDO is a very important immunosuppressive factor, which can reduce Th17 cell infiltration, thereby reducing the production of interleukin (IL)‐17, inhibiting the activation of HSCs, and increasing the proportion of CD4+ T cells, and in turn, induces the production of IL‐10, inhibits related immune pathways, and ultimately, improves fibrosis in mice. This shows that MSCs can directly regulate immune cytokines, and further achieve antifibrotic effects through immunosuppressive effects. Wang et al. reported that MSCs can suppress the growth of interferon (IFN)‐γ+, IL‐6+, and other lymphocytes, and regulate the related pathways of inhibiting pro‐fibrosis in nonalcoholic steatohepatitis mice (Huafeng et al., 2017). In 2019, Luo et al. reported that MSCs can inhibit the activation of M1 pro‐inflammatory macrophages, regulate the immune pathway, promote the activation of M2 anti‐inflammatory macrophages, and increase the expression of metalloproteinase‐13 (Luo et al., 2019). In addition, the activation of HSCs accelerate the rate of fibrinolysis and delay or even block the development of liver fibrosis diseases.
In addition, inflammation‐related pathways caused by immune responses play an important role in the development of liver diseases. In the early development stage of liver fibrosis, hepatocytes and endothelial cells are damaged to release chemokines, recruit macrophages to damaged lesions, and release a series of pro‐inflammatory factors, such as transforming growth factor‐β (TGF‐β), and in turn, activate HSCs, which are further transformed into myofibroblasts, produce a large amount of ECM, and promote the development of fibrosis (J. Zhang et al., 2019). MSCs can secrete various cytokines and growth factors, such as HGF, IL‐6, prostaglandin, TNF‐α, bFGF, and VEGF (Yu et al., 2014; Yuan et al., 2013). After the liver is stimulated by alcohol and viruses, hepatocytes will undergo apoptosis, while the cytokine IL‐6 secreted by MSCs can upregulate the expression of antiapoptotic factors Bcl‐2 and Bcl‐xl during liver repair, inhibiting the further decline of liver cells (Kovalovich et al., 2001). At the same time, MSCs can secrete prostaglandin E, and promote liver cell regeneration and proliferation (Liu et al., 2018). In addition, angiogenesis factors and basic fibroblasts secreted by MSCs can promote the angiogenesis of liver cells, and further reshape the liver microenvironment. Reza et al. transplanted human MSCs into mice with acute liver failure induced by CCl4 (Saidi et al., 2015). It was observed that the levels of ALT improved and the secretion of IL‐6 increased in the serum of mice treated with MSCs. The study conducted by Banas and other studies has confirmed that HGF can be secreted to reduce inflammation in the liver lesion area after MSC transplantation, thereby achieving the purpose of treating fibrosis (Agnieszka et al., 2008). After culturing MSCs of umbilical cord (UC) blood in vitro, An et al. collected the secreted proteome after stimulation under certain conditions and intraperitoneally injected these into a rat model of liver fibrosis (An et al., 2017). It was observed that the degree of fibrosis improved in the rat model. Furthermore, they were also able to prove through in vitro experiments that the milk fat globule epidermal growth factor (EGF) factor 8 (MFGE8) in the proteome secreted by MSCs can inhibit the TGF‐β/Samd signaling pathway, which in turn leads to the inactivation of HSCs (Aimaiti et al., 2017). Yu Saito et al. reported that the VEGF secreted by MSCs can stimulate liver regeneration in ischemia‐reperfusion mice and improve liver injury after coculture with hepatocytes (Saito et al., 2013).
4.3. Antifibrosis effect of MSCs
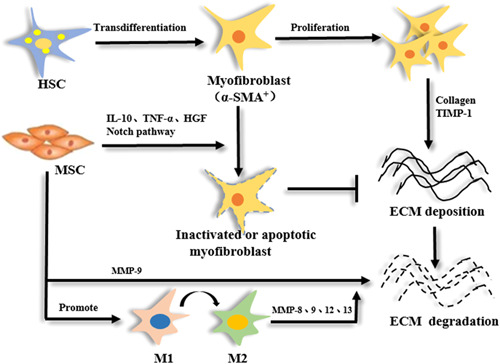
Liver fibrosis is the abnormal proliferation of connective tissues in the liver. The present pathological mechanism is the activation and proliferation of HSCs and the production of α‐SMA. Endogenous or exogenous MSCs can be directed to migrate under the influence of various factors, penetrate vascular endothelial cells, and tend to target tissues and localize survival (Figure (Figure66).
Studies have confirmed that MSCs are directed toward the damaged area of the liver, and in turn, secrete soluble factors, reduce hepatocyte apoptosis, activate activated HSCs, inhibit their activation, and reshape the liver surroundings (Fuxiang et al., 2015). In the process of liver fibrosis, HSCs are activated to proliferate and transform into myofibroblasts to promote the development of fibrosis. The important reason why MSCs can resist fibrosis is that MSCs can inhibit the activation and proliferation of HSCs. Yu et al. reported that after 72 h of coculture with HSCs, adipose‐derived stem cells could inhibit the proliferation and activation of HSCs, and increase the apoptosis of HSCs (Fuxiang et al., 2015). After transplanting MSCs to treat nonalcoholic steatohepatitis, it was found that the expression of Type IV collagen was reduced, the expression of fibroblast TGF‐B was downregulated, and the number of α‐SMA+ cells decreased. Huang et al. confirmed that MSCs can resist fibrosis by downregulating the macrophage inflammatory infiltration and promoting hepatic stellate cell apoptosis after transplanting MSCs to treat liver failure. MSCs can also degrade the ECM by upregulating the expression of matrix metalloprotein‐9 (B. Huang et al., 2016).
h of coculture with HSCs, adipose‐derived stem cells could inhibit the proliferation and activation of HSCs, and increase the apoptosis of HSCs (Fuxiang et al., 2015). After transplanting MSCs to treat nonalcoholic steatohepatitis, it was found that the expression of Type IV collagen was reduced, the expression of fibroblast TGF‐B was downregulated, and the number of α‐SMA+ cells decreased. Huang et al. confirmed that MSCs can resist fibrosis by downregulating the macrophage inflammatory infiltration and promoting hepatic stellate cell apoptosis after transplanting MSCs to treat liver failure. MSCs can also degrade the ECM by upregulating the expression of matrix metalloprotein‐9 (B. Huang et al., 2016).
5. KINETICS OF MSCsS AFTER LIVER TRANSPLANTATION
After MSC enters the body, it does not conform to the typical distribution and metabolic model of traditional drugs. Traditional drugs are passively distributed, but MSC has a feature of chemotaxis to the injured site, and the distribution of MSC in healthy and diseased bodies is different.
Direct MSCs injection into liver vessels, either through portal vein or hepatic artery, could improve delivery and subsequent engraftment of the cells into the liver. However, this method requires an invasive angiography and may cause serious adverse effects such as bleeding or contrast nephropathy (Mohamadnejad, Namiri, et al., 2007). While intravenous reinfusion is safe and effective in many clinical trials for the treatment of liver fibrosis (Mohamadnejad, Namiri, et al., 2007), many MSCs may be trapped in the lung when they pass through the organ for the first time after peripheral intravenous infusion. Intravenous infusion of 111In‐oxine‐labeled MSCs into the lung through a peripheral vein showed that the radioactivity gradually increased in the liver and spleen during the following hours to days (Gholamrezanezhad et al., 2011). Moreover, some studies had shown that the cells transplanted through a peripheral vein injection can be removed from the lung and transferred into the liver to reverse the acute liver injury induced by CCl4 (Kuo et al., 2008). Therefore, to determine sufficient homing information of the injected MSCs to the target organs, it is necessary to trace MSCs and obtain biological distribution of the cells after different infusion routes.
6. CLINICAL PROGRESS OF MSCs IN THE TREATMENT OF LIVER INJURY
By September 2020, a total of 31 clinical projects of MSCs in the treatment of liver injury diseases have been registered in the Clinical Trial (Table (Table2).2). The researchers are committed to developing new methods to improve the efficiency of liver injury animal models to simulate MSCs in vivo. However, the actual effects need to be evaluated through clinical trials. The model for end‐stage liver disease (MELD) score is widely used in experimental studies and clinical applications to assess the severity of chronic liver disease. Serum levels, total bilirubin, and other indicators are used to assess the prognostic recovery of patients with liver disease.
Table 2
Clinical trials of MSC transplantations with liver fibrosis injury
| NCT Number | Title | Characteristics | Locations |
|---|---|---|---|
| NCT04357600 | Umbilical cord MSC for liver cirrhosis Patient caused by hepatitis B | Phase 1 Phase 2 | Cipto Mangunkusumo hospital, Jakarta, DKI Jakarta, Indonesia |
| NCT04243681 | Combination of autologous MSC and HSC infusion in patients With decompensated cirrhosis | Phase 4 | Asian Institute of Gastroenterology, Hyderabad, Telangana, India |
| NCT03945487 | MSCs treatment for decompensated liver cirrhosis | Phase 2 | Beijing 302 Hospital, Beijing, China |
| NCT03863002 | Safety and efficacy of MSC transplantation for acute‐on‐chronic liver failure | Phase 1 Phase 2 | Tianjin Weikai Bioeng., Ltd., Tianjin, Tianjin, China |
| NCT03838250 | Study to evaluate hepatic artery injection of autologous human bone marrow–derived MSCs in patients with alcoholic LC | Phase 1 | University of Utah, Salt Lake City, Utah, United States |
| NCT03626090 | MSC therapy for liver cirrhosis | Phase 1 Phase 2 |
|
| NCT03460795 | Safety and efficacy study of cotransferring of MSC and regulatory t cells in treating end‐stage liver disease | Phase 1 Phase 2 | Nanjing Medical University, Nanjing, Jiangsu, China |
| NCT03254758 | A Study of ADR‐001 in patients with liver cirrhosis | Phase 1 Phase 2 | Niigata University Medical & Dental Hospital, Niigata, JapanNihon University Itabashi Hospital, Tokyo, Japan |
| NCT02786017 | Injectable Collagen Scaffold™ combined with HUC‐MSCs transplantation for patients with decompensated cirrhosis | The Affiliated Nanjing Drum Tower Hospital of Nanjing University Medical School, Nanjing, Jiangsu, China | |
| NCT02705742 | MSCs transplantation for liver cirrhosis due to HCV hepatitis | Phase 1 Phase 2 | Gulhane Military Medical Academy, Ankara, Turkey |
| NCT02652351 | HUC‐MSCs for hepatic cirrhosis | Phase 1 | The second Affiliated Hospital of University of Soth China, Hengyang, Hunan, China |
| NCT01854125 | Autologous MSC transplantation in cirrhosis patients with refractory ascites | Phase 3 | Jinling Hospital, Nanjing, Jiangsu, China |
| NCT01844063 | Safety and efficacy of diverse MSCs transplantation for liver failure | Phase 1 Phase 2 | Qi Zhang, Guangzhou, Guangdong, China |
| NCT01741090 | The effectiveness and safety for MSC for alcoholic liver cirrhosis | Phase 2 | Yonsei University Wonju College of Medicine Wonju Christian Hospital, Wonju, Kangwondo, Korea, Republic of |
| NCT01728727 | Safety and efficacy of HUC‐derived MSCs for treatment of HBV‐related liver cirrhosis | Phase 1 Phase 2 | Xijing Hospital of Digestive Disease, Xi'an, Shaanxi, China |
| NCT01724398 | Umbilical cord MSCs transplantation combined with plasma exchange for patients with liver failure | Phase 1 Phase 2 | Department of Infectious Diseases, The Third Affliated Hospital of Sun Yat‐sen University, Guangzhou, Guangdong, China |
| NCT01662973 | Umbilical cord MSCs for patients with primary biliary cirrhosis | Phase 1 Phase 2 | Beijing 302 Hospital, Beijing, Beijing, China |
| NCT01573923 | Safety and efficacy study of umbilical MSCs for liver cirrhosis |
| |
| NCT01483248 | Human menstrual blood‐derived MSCs for patients with liver cirrhosis | Phase 1 Phase 2 | the First Affiliated Hospital of Zhejiang University‐IRB, Hangzhou, Zhejiang, China |
| NCT01454336 | Transplantation of autologous MSC in decompensate cirrhotic patients with pioglitazone | Phase 1 | Royan Institute, Tehran, Iran, Islamic Republic of |
| NCT01440309 | Efficacy and safety study of allogenic MSCs for patients with refractory primary biliary cirrhosis | Phase 1 | Peking Union Medical College Hospital, Beijing, China |
| NCT01342250 | HUC MSCs transplantation for patients with decompensated liver cirrhosis | Phase 1 Phase 2 | Shanghai Liver Disease Research Center, the Nanjing Military Command (Shanghai 85 Hospital), Shanghai, Shanghai, China |
| NCT01256138 | Allogene MSCs transplantation in liver for patients with chronic liver diseases through portal vein by ultrasound guiding | Department of Infectious Diseases, 3rd Affiliated Hospital of Sun Yat‐sen University, Guangzhou, Guangdong, China | |
| NCT01256125 | Allogene MSCs transplantation in patients with chronic liver diseases through peripheral vein | Department of Infectious Diseases, 3rd Affiliated Hospital of Sun Yat‐sen University, GuangZhou, Guangdong, China | |
| NCT01224327 | Umbilical cord MSCs infusion via hepatic artery in cirrhosis patients | Phase 1 Phase 2 | Stem Cell Research Center of Medical School Hospital of Qingdao University, Qingdao, Shandong, China |
| NCT01220492 | Umbilical cord MSCs for patients with liver cirrhosis | Phase 1 Phase 2 | Beijing 302 Hospital, Beijing, Beijing, China |
| NCT01218464 | Safety and efficacy of human MSCs for treatment of liver failure | Phase 1 Phase 2 | Beijing 302 Hospital, Beijing, Beijing, China |
| NCT00993941 | BMSC transplantation in liver cirrhosis via portal vein | Phase 2 | Sun Yat‐sen University, Guangzhou, Guangdong, China |
| NCT00976287 | Autologous bone marrow MSCs transplantation via hepatic artery in patients with liver cirrhosis | Phase 2 | |
| NCT00956891 | Therapeutic effects of liver failure patients caused by chronic hepatitis B after autologous MSCs transplantation | The Third Affiliated Hospital Of Sun Yat‐sen University, Guangzhou, Guangdong, China | |
| NCT00476060 | MSC transplantation in decompensated cirrhosis | Phase 2 | Digestive Disease Research Center, Shariati Hospital, Tehran, Iran, Islamic Republic of |
Abbreviations: ADR, adverse drug reaction; BMSC, bone mesenchymal stem cell; HBV, hepatitis B virus; HSC, hepatic stellate cell; HUC, human umbilical cord; LC, liver cirrhosis; MSC, mesenchymal stem cell.
Clinical trials have shown that in a study of 45 chronic hepatitis B patients with decompensated liver cirrhosis (LC), there was a significant reduction in the volume of ascites in patients treated with UC‐MSC transfusion when compared to controls (Zheng et al., 2012). In addition, UC‐MSC therapy also significantly improved liver function, as indicated by the increase in serum albumin levels, decrease in total serum bilirubin levels, and decrease in the sodium model for MELD scores. A total of 43 acute‐on‐chronic liver failure (ACLF) patients were associated with hepatitis B virus (HBV) infection in the clinical trial conducted by Ming Shi et al. (2012). The UC‐MSC transfusions significantly increased the survival rates in ACLF patients, reduced the MELD scores, increased the serum albumin, cholinesterase and prothrombin activity, and increased the platelet count. Furthermore, the serum total bilirubin and alanine aminotransferase levels significantly decreased after the UC‐MSC transfusions. From 2010 to 2013, 110 patients with HBV‐related acute‐on‐chronic liver failure were enrolled in this open‐label, nonblinded randomized controlled study (Bing‐Liang et al., 2017). The experimental group (n =
= 56) was infused with 1.0–10.0
56) was infused with 1.0–10.0 ×
× 105
105 cells/kg of allogeneic bone marrow–derived MSCs weekly, for 4 weeks, and were followed up for 24 weeks. Compared with the control group, the allogeneic bone marrow‐derived MSC treatment markedly improved the clinical laboratory measurements, including the serum total bilirubin and MELD scores. It is noteworthy that to better interpret the safety and effectiveness of MSCs, further research is needed, and patients with liver fibrosis induced by different factors should be recruited. Although in recent years, MSC has had many advantages in the treatment of various diseases, its clinical application still needs further in‐depth research. In addition, there are still some problems to be solved in MSC transplantation, such as the appropriate timing of MSC transplantation, injection dose, and optimization of the culture program. However, it remains unclear how the survival rate, colonization rate, and safety of MSCs could be improved in vivo, and how these play its effectiveness on this basis. Hence, further clinical trials and multidisciplinary consultations are needed to clarify these.
cells/kg of allogeneic bone marrow–derived MSCs weekly, for 4 weeks, and were followed up for 24 weeks. Compared with the control group, the allogeneic bone marrow‐derived MSC treatment markedly improved the clinical laboratory measurements, including the serum total bilirubin and MELD scores. It is noteworthy that to better interpret the safety and effectiveness of MSCs, further research is needed, and patients with liver fibrosis induced by different factors should be recruited. Although in recent years, MSC has had many advantages in the treatment of various diseases, its clinical application still needs further in‐depth research. In addition, there are still some problems to be solved in MSC transplantation, such as the appropriate timing of MSC transplantation, injection dose, and optimization of the culture program. However, it remains unclear how the survival rate, colonization rate, and safety of MSCs could be improved in vivo, and how these play its effectiveness on this basis. Hence, further clinical trials and multidisciplinary consultations are needed to clarify these.
7. CONCLUSIONS
In recent years, more and more scholars have used MSCs transplantation for the treatment of liver fibrosis, and they have shown certain antifibrosis effects in vivo and in vitro. At present, to maximize the efficacy of MSCs transplantation, many scholars focus on the combined application of MSCs with other antifibrotic molecules and drugs. The combination of MSCs and other antiliver fibrosis methods may produce better anti‐fibrosis effects, which is the future development direction of MSCs therapy.
At present, the regulatory effect of MSCs transplantation has triggered extensive research on the occurrence and development of liver fibrosis, but it still has limitations. The existing studies on MSCs and liver fibrosis are mostly in vitro and animal experiments, and more clinical studies are still needed to confirm their efficacy. In addition, as pluripotent stem cells with multidirectional differentiation potential, MSCs transplantation can also induce adverse reactions in the body, and more in‐depth discussions are needed on the side effects of its treatment. Therefore, the potential regulatory mechanism and molecular targets of MSCs used in liver fibrosis still need to be further explored and studied to provide reasonable and effective theoretical support for MSCs transplantation in the clinical treatment of liver fibrosis.
Notes
Sun H., Shi C., Ye Z., Yao B., Li C., Wang X., & Qian Q. (2022). The role of mesenchymal stem cells in liver injury. Cell Biology International, 46, 501–511. 10.1002/cbin.11725 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- Agnieszka B., Takumi T., Yusuke Y., & Takeshita (2008). Rapid hepatic fate specification of adipose‐derived stem cells and their therapeutic potential for liver failure. Journal of Gastroenterology and Hepatology, 24(1), 70–77. 10.1111/j.1440-1746.2008.05496.x [Abstract] [CrossRef] [Google Scholar]
- Ahmed A. A.‐Q. (2018). The correlation between hepatitis B virus precore/core mutations and the progression of severe liver disease. Frontiers in Cellular and Infection Microbiology, 8, 355. 10.3389/fcimb.2018.00355 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Aimaiti Y., Xin J., Wei W., Chen Z., & Li D. (2017). TGF‐β1 signaling regulates mouse hepatic stellate cell differentiation via the Jagged1/Notch pathway. Life Sciences, 192, 221–230. 10.1016/j.lfs.2017.11.018 [Abstract] [CrossRef] [Google Scholar]
- An S. Y., Jang Y. J., Lim H. J., Han J., Lee J., Lee G., & Do B. R. (2017). Milk fat globule‐EGF factor 8, secreted by mesenchymal stem cells, protects against liver fibrosis in mice. Gastroenterology, 152(5), 1174–1186. 10.1053/j.gastro.2016.12.003 [Abstract] [CrossRef] [Google Scholar]
- Ankrum J., & Karp J. M. (2010). Mesenchymal stem cell therapy: Two steps forward, one step back. Trends in Molecular Medicine, 16(5), 203–209. 10.1016/j.molmed.2010.02.005 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Banas A., Teratani T., Yamamoto Y., Tokuhara M., T., & Ochiya (2007). Adipose tissue‐derived mesenchymal stem cells as a source of human. Hepatocytes, 46(1), 219–228. 10.1002/hep.21704 [Abstract] [CrossRef] [Google Scholar]
- Bing‐Liang L., Jun‐Feng C., Wei‐Hong Q., & Xie (2017). Allogeneic bone marrow–derived mesenchymal stromal cells for hepatitis B virus related acute on chronic liver failure: A randomized controlled trial. Hepatology, 66(1), 209–219. 10.1002/hep.29189 [Abstract] [CrossRef] [Google Scholar]
- Chang N., Jingjing G., Lei X., Zhongxin Z., Xianghui D., Lei T., & Liying L. (2017). HuR mediates motility of human bone marrow‐derived mesenchymal stem cells triggered by sphingosine 1‐phosphate in liver fibrosis. Journal of Molecular Medicine, 95(1), 69–82. 10.1007/s00109-016-1460-x [Abstract] [CrossRef] [Google Scholar]
- Devarbhavi H., & Raj S. (2018). Drug‐induced liver injury with skin reactions: Drugs and host risk factors, clinical phenotypes and prognosis. Liver International, 39(5), 802–811. 10.1111/liv.14004 [Abstract] [CrossRef] [Google Scholar]
- Dominici M., Le Blanc K., Mueller I., Slaper‐Cortenbach I., Marini F., Krause D., & Horwitz E. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 8(4), 315–317. 10.1080/14653240600855905 [Abstract] [CrossRef] [Google Scholar]
- Friedenstein A. J. (1974). Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Experimental Hematology, 2(2), 83–92. [Abstract] [Google Scholar]
- Friedenstein A. J. (1976). Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Experimental Hematology, 4(5), 267–274. [Abstract] [Google Scholar]
- Fuxiang Y., Shiqiang J., Longfeng S., Li W., Shengchu Z., Chunlei D., & Qiyu Z. (2015). Adipose‐derived mesenchymal stem cells inhibit activation of hepatic stellate cells in vitro and ameliorate rat liver fibrosis in vivo. Journal of the Formosan Medical Association, 114(2), 130–138. 10.1016/j.jfma.2012.12.002 [Abstract] [CrossRef] [Google Scholar]
- Galipeau J., & Stagg J. (2013). Mechanisms of immune modulation by mesenchymal stromal cells and clinical translation. Current Molecular Medicine, 13(5), 856–867. 10.2174/1566524011313050016 [Abstract] [CrossRef] [Google Scholar]
- Gebler A., Zabel O., & Seliger B. (2011). The immunomodulatory capacity of mesenchymal stem cells. Trends in Molecular Medicine, 18(2), 128–134. 10.1016/j.molmed.2011.10.004 [Abstract] [CrossRef] [Google Scholar]
- Gholamrezanezhad A., Mirpour S., Bagheri M., Mohamadnejad M., Alimoghaddam K., Abdolahzadeh L., & Malekzadeh R. (2011). In vivo tracking of 111In‐oxine labeled mesenchymal stem cells following infusion in patients with advanced cirrhosis. Nuclear Medicine & Biology, 38(9), 961–967. 10.1016/j.nucmedbio.2011.03.008 [Abstract] [CrossRef] [Google Scholar]
- Guangfeng C., Yinpeng J., Xiujuan S., Yu Q., Yushan Z., Mingliang C., & Xiaoqing L. (2015). Adipose‐derived stem cell‐based treatment for acute liver failure. Stem Cell Research & Therapy, 6(1), 40. 10.1186/s13287-015-0040-2 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Harn H. J., Lin S. Z., Hung S. H., & Chiou T. W. (2012). Adipose‐derived stem cells can abrogate chemical‐induced liver fibrosis and facilitate recovery of liver function. Cell Transplantation, 21(12), 2753–2764. 10.3727/096368912X652959 [Abstract] [CrossRef] [Google Scholar]
- Huafeng W., Dong W., Luhong Y., Yanxia W., Junli J., Dongchen N., & Chengfang L. (2017). Compact bone‐derived mesenchymal stem cells attenuate nonalcoholic steatohepatitis in a mouse model by modulation of CD4 cells differentiation. International Immunopharmacology, 42, 67–73. 10.1016/j.intimp.2016.11.012 [Abstract] [CrossRef] [Google Scholar]
- Huang A., Danni L., Xin Q., Zhiwei Y., Hongmei C., Kaiyue Z., & Yuebing W. (2018). Self‐assembled GFFYK peptide hydrogel enhances the therapeutic efficacy of mesenchymal stem cells in a mouse hindlimb ischemia model. Acta Biomaterialia, 85, 94–105. 10.1016/j.actbio.2018.12.015 [Abstract] [CrossRef] [Google Scholar]
- Huang B., Cheng X., Wang H., Huang W., Hu Z. laG., & Zhang R. (2016). Mesenchymal stem cells and their secreted molecules predominantly ameliorate fulminant hepatic failure and chronic liver fibrosis in mice respectively. Journal of Translational Medicine, 14(1), 1–12. 10.1186/s12967-016-0792-1. http://doi [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Javazon E. H., Colter D. C., & Schwarz E. J. (2001). Rat marrow stromal cells are more sensitive to plating density and expand more rapidly from single‐cell‐derived colonies than human marrow stromal. Cells. Stem cell, 19(3), 219–225. 10.1634/stemcells.19-3-219 [Abstract] [CrossRef] [Google Scholar]
- Jorge Matias C., Jun Y., Myoung‐Kuk J., Geum‐Youn G., Silvia A., Lexing Y., & Robert F. S. (2017). MicroRNA‐21 and dicer are dispensable for hepatic stellate cell activation and the development of liver fibrosis. Hepatology, 67(6), 2414–2429. 10.1002/hep.29627. http://doi [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Jung J. H., Fu X., & Yang P. C. (2017). Exosomes generated from iPSC‐derivatives: New direction for stem cell therapy in human heart diseases. Circulation Research, 120(2), 407–417. 10.1161/CIRCRESAHA.116.309307 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Karp J. M., & Leng Teo G. S. (2009). Mesenchymal stem cell homing: The devil is in the details. Cell Stem Cell, 4(3), 206–216. 10.1016/j.stem.2009.02.001 [Abstract] [CrossRef] [Google Scholar]
- Keating A. (2008). How do mesenchymal stromal cells suppress t cells. Cell Stem Cell, 2(2), 0–108. 10.1016/j.stem.2008.01.007 [Abstract] [CrossRef] [Google Scholar]
- Kovalovich K., Li W., DeAngelis R., Greenbaum L. E., Ciliberto G., & Taub R. (2001). Interleukin‐6 protects against Fas‐mediated death by establishing a critical level of anti‐apoptotic hepatic proteins FLIP, Bcl‐2, and Bcl‐xL. Journal of Biological Chemistry, 276(28), 26605–26613. 10.1074/jbc.M100740200 [Abstract] [CrossRef] [Google Scholar]
- Kuo T. K., Hung S. P., Chuang C. H., Chen C. T., Shih Y. R., Fang S. C., & Lee O. K. (2008). Stem cell therapy for liver disease: Parameters governing the success of using bone marrow mesenchymal stem cells. Gastroenterology, 134, 2111–2121. 10.1053/j.gastro.2008.03.015 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Kwan Man K. C. S., Kevin T. P. N., Jiang W. X., Dong Y. G., Chris K. W. S., & Chung Mau L. (2010). Molecular signature linked to acute phase injury and tumor invasiveness in small‐for‐size liver grafts. Annals of Surgery, 251(6), 1154–1161. 10.1097/SLA.0b013e3181d96e3d [Abstract] [CrossRef] [Google Scholar]
- Liu Y., Ren H., Wang J., Yang F., Li J., Zhou Y., & Shi X. (2018). Prostaglandin E2 secreted by mesenchymal stem cells protects against acute liver failure via enhancing hepatocyte proliferation. The FASEB Journal, 33(2), 2514–2525. 10.1096/fj.201801349RR [Abstract] [CrossRef] [Google Scholar]
- Luo X.‐Y., Meng X.‐J., Cao D.‐C., Wang W., Zhou K., Li L., & Ping W. (2019). Transplantation of bone marrow mesenchymal stromal cells attenuates liver fibrosis in mice by regulating macrophage subtypes. Stem Cell Research & Therapy, 10(1). 10.1186/s13287-018-1122-8 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Mami H., Yoshio S., Masayuki Takamura S. U., Alessandro Nasti K. Y., & Shuichi K. (2013). Adiposetissue derived stromal stem cell therapy in murine ConA‐derived hepatitis is dependent on myeloid‐lineage and CD4+ T‐cell suppression. European Journal of Immunology, 43(11), 2956–2968. 10.1002/eji.201343531 [Abstract] [CrossRef] [Google Scholar]
- Michelle M. D., Ritter T., Ceredig R., & Matthew D. G. (2011). Mesenchymal stem cell effects on T‐cell effector pathways. Stem Cell Research & Therapy, 2(4), 34. 10.1186/scrt75 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Milosavljevic N., Gazdic M., Simovic Markovic B., Arsenijevic A., Nurkovic J., Dolicanin Z., & Volarevic V. (2018). Mesenchymal stem cells attenuate liver fibrosis by suppressing Th17 cells–An experimental study. Transplant International, 31, 102–115. 10.1111/tri.13023 [Abstract] [CrossRef] [Google Scholar]
- Mohamadnejad M., Namiri M., Bagheri M., Masiha Hashemi S., Ghanaati H., Zare Mehrjardi N., & Baharvand H. (2007). Phase 1 human trial of autologous bone marrow‐hematopoietic stem cell transplantation in patients with decompensated cirrhosis. World Journal of Gastroenterology, 13, 3359–3363. 10.3748/wjg.v13.i24.3359 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Mohammadzadeh Adel A. P. A., Somayeh Shahrokhi S. M. H., Sadegh Lotf Alah Moradi M. S., et al. (2014). Immunomodulatory effects of adipose‐derived mesenchymal stem cells on the gene expression of major transcription factors of T cell subsets. International Immunopharmacology, 20(2), 316–321. 10.1016/j.intimp.2014.03.003 [Abstract] [CrossRef] [Google Scholar]
- Noll J., Helk E., Fehling H., Bernin H., Marggraff C., Jacobs T., & Ittrich H. (2016). IL‐23 prevents IL‐13‐dependent tissue repair associated with Ly6C(lo) monocytes in Entamoeba histolytica‐induced liver damage. Journal of Hepatology, 64(5), 1147–1157. 10.1016/j.jhep.2016.01.013 [Abstract] [CrossRef] [Google Scholar]
- Olsen T. R., Ng K. S., Lock L. T., Ahsan T., & Rowley J. A. (2018). Peak MSC—Are we there yet. Frontiers in Medicine, 5, 178. 10.3389/fmed.2018.00178 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Saidi R., Rajeshkumar R., Shariftabrizi A., Zimmerman A., & Walter O. (2015). Human adipose‐derived mesenchymal stem cells promote liver regeneration. Journal of Investigative Surgery, 28(6), 303–308. 10.3109/08941939.2015.1006379 [Abstract] [CrossRef] [Google Scholar]
- Saito Y., Shimada M., Utsunomiya T., Ikemoto T., Yamada S., Morine Y., & Iwahashi S. (2013). The protective effect of adipose‐derived stem cells against liver injury by trophic molecules. Journal of Surgical Research, 180(1), 162–168. 10.1016/j.jss.2012.10.009 [Abstract] [CrossRef] [Google Scholar]
- Seki T., Yokoyama Y., Nagasaki H., Kokuryo T., & Nagino M. (2012). Adipose tissue‐derived mesenchymal stem cell transplantation promotes hepatic regeneration after hepatic ischemia‐reperfusion and subsequent hepatectomy in rats. Journal of Surgical Research, 178(1), 63–70. 10.1016/j.jss.2012.02.014 [Abstract] [CrossRef] [Google Scholar]
- Seo M. J., Suh S. Y., Bae Y. C., & Jung J. S. (2005). Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochemical and Biophysical Research Communications, 328(1), 0–264. 10.1016/j.bbrc.2004.12.158 [Abstract] [CrossRef] [Google Scholar]
- Shi M., Zhang Z., Xu R., Lin H., Fu J., Zou Z., & Wang F. S. (2012). Human mesenchymal stem cell transfusion is safe and improves liver function in acute‐on‐chronic liver failure patients. Stem Cells Translational Medicine, 1(10), 725–731. 10.5966/sctm.2012-0034 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Tsuchida T., & Friedman S. L. (2017). Mechanisms of hepatic stellate cell activation. Nature Reviews Gastroenterology Hepatology, 14(7), 397–411. 10.1038/nrgastro.2017.38 [Abstract] [CrossRef] [Google Scholar]
- Wake K. (1971). “Sternzellen” in the liver: Perisinusoidal cells with special reference to storage of vitamin A. American Journal of Anatomy, 132(4), 429–462. 10.1002/aja.1001320404 [Abstract] [CrossRef] [Google Scholar]
- Wan C. D., Cheng R., Wang H., & Liu T. (2008). Immunomodulatory effects of mesenchymal stem cells derived from adipose tissues in a rat orthotopic liver transplantation model. Hepatobiliary & pancreatic diseases international: HBPD INT, 7(1), 29–33. [Abstract] [Google Scholar]
- Wang P., Koyama Y., Liu X., Xu J., Ma H. Y., Liang S., & Kisseleva T. (2016). Promising therapy candidates for liver fibrosis. Frontiers in Physiology, 7, 47. 10.3389/fphys.2016.00047 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Wang Y., Lian F., & Tan G. S. (2010). Effects of portal vein transplantation of allogeneic adipose stem cells on fibrotic liver in rats. Chin J Pathologic Sci, 26(11), 2197–2201. 10.3969/j.issn.1000-4718 [CrossRef] [Google Scholar]
- Yu B., Zhang X., & Li X. (2014). Exosomes derived from mesenchymal stem cells. Inter J Mol Sci, 15(3), 4142–4157. 10.3390/ijms15034142 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Yuan S., Jiang T., Sun L., Zheng R., Ahat N., & Zhang Y. (2013). The role of bone marrow mesenchymal stem cells in the treatment of acute liver failure. BioMed Research International, 2013, 1–9. 10.1155/2013/251846 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zhang J., Han C., Ungerleider N., Chen W., Song K., Wang Y., & Tong W. (2019). A novel TGF‐β and H19 signaling axis in tumor‐initiating hepatocytes that regulates hepatic carcinogenesis. Hepatology, 69, 1549–1563. 10.1002/hep.30153 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zhang S., Zhu Z., Wang Y., Liu S., Zhao C., Guan W., & Zhao Y. (2018). Therapeutic potential of Bama miniature pig adipose stem cells induced hepatocytes in a mouse model with acute liver failure. Cytotechnology, 70(4), 1131–1141. 10.1007/s10616-018-0201-0 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zheng Z., Hu L., Ming S., Ruonan X., Junliang F., Jiyun L., & Fu‐Sheng W. (2012). Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. Journal of Gastroenterology and Hepatology, 27(Suppl 2), 112–120. 10.1111/j.1440-1746.2011.07024.x [Abstract] [CrossRef] [Google Scholar]
- Zhou C., York S. R., Chen J. Y., Pondick J. V., Motola D. L., Chung R. T., & Mullen A. C. (2016). Long noncoding RNAs expressed in human hepatic stellate cells form networks with extracellular matrix proteins. Genome Medicine, 8(1), 31. 10.1186/s13073-016-0285-0 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1002/cbin.11725
Read article for free, from open access legal sources, via Unpaywall:
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1002/cbin.11725
Citations & impact
Impact metrics
Citations of article over time
Article citations
Adipose-derived mesenchymal stem cells inhibit hepatic stellate cells activation to alleviate liver fibrosis via Hippo pathway.
Stem Cell Res Ther, 15(1):378, 24 Oct 2024
Cited by: 0 articles | PMID: 39449061 | PMCID: PMC11515333
Precision Nanomedicine with Bio-Inspired Nanosystems: Recent Trends and Challenges in Mesenchymal Stem Cells Membrane-Coated Bioengineered Nanocarriers in Targeted Nanotherapeutics.
J Xenobiot, 14(3):827-872, 24 Jun 2024
Cited by: 1 article | PMID: 39051343 | PMCID: PMC11270309
Review Free full text in Europe PMC
Research progress in stem cell therapy for Wilson disease.
Regen Ther, 27:73-82, 15 Mar 2024
Cited by: 1 article | PMID: 38525238 | PMCID: PMC10959646
Review Free full text in Europe PMC
Recent Advances in Mesenchymal Stem/Stromal Cell-Based Therapy for Alcohol-Associated Liver Disease and Non-alcoholic Fatty Liver Disease.
Stem Cells Transl Med, 13(2):107-115, 01 Feb 2024
Cited by: 4 articles | PMID: 38016185 | PMCID: PMC10872699
Review Free full text in Europe PMC
Multiple Dimensions of using Mesenchymal Stem Cells for Treating Liver Diseases: From Bench to Beside.
Stem Cell Rev Rep, 19(7):2192-2224, 27 Jul 2023
Cited by: 4 articles | PMID: 37498509
Review
Go to all (8) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
Clinical Trials (Showing 31 of 31)
- (1 citation) ClinicalTrials.gov - NCT01728727
- (1 citation) ClinicalTrials.gov - NCT01454336
- (1 citation) ClinicalTrials.gov - NCT01854125
- (1 citation) ClinicalTrials.gov - NCT03863002
- (1 citation) ClinicalTrials.gov - NCT03945487
- (1 citation) ClinicalTrials.gov - NCT01220492
- (1 citation) ClinicalTrials.gov - NCT01224327
- (1 citation) ClinicalTrials.gov - NCT02786017
- (1 citation) ClinicalTrials.gov - NCT01440309
- (1 citation) ClinicalTrials.gov - NCT01256138
- (1 citation) ClinicalTrials.gov - NCT02705742
- (1 citation) ClinicalTrials.gov - NCT03460795
- (1 citation) ClinicalTrials.gov - NCT01844063
- (1 citation) ClinicalTrials.gov - NCT01342250
- (1 citation) ClinicalTrials.gov - NCT01741090
- (1 citation) ClinicalTrials.gov - NCT01724398
- (1 citation) ClinicalTrials.gov - NCT04357600
- (1 citation) ClinicalTrials.gov - NCT01483248
- (1 citation) ClinicalTrials.gov - NCT03254758
- (1 citation) ClinicalTrials.gov - NCT00476060
- (1 citation) ClinicalTrials.gov - NCT02652351
- (1 citation) ClinicalTrials.gov - NCT00956891
- (1 citation) ClinicalTrials.gov - NCT01662973
- (1 citation) ClinicalTrials.gov - NCT01256125
- (1 citation) ClinicalTrials.gov - NCT03838250
- (1 citation) ClinicalTrials.gov - NCT00976287
- (1 citation) ClinicalTrials.gov - NCT01573923
- (1 citation) ClinicalTrials.gov - NCT00993941
- (1 citation) ClinicalTrials.gov - NCT01218464
- (1 citation) ClinicalTrials.gov - NCT04243681
- (1 citation) ClinicalTrials.gov - NCT03626090
Show less
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Mesenchymal stem cell therapy for liver fibrosis.
Korean J Intern Med, 30(5):580-589, 27 Aug 2015
Cited by: 109 articles | PMID: 26354051 | PMCID: PMC4578027
Review Free full text in Europe PMC
Strategies to improve the efficiency of mesenchymal stem cell transplantation for reversal of liver fibrosis.
J Cell Mol Med, 23(3):1657-1670, 11 Jan 2019
Cited by: 37 articles | PMID: 30635966 | PMCID: PMC6378173
Review Free full text in Europe PMC
Therapeutic implications of mesenchymal stem cells in liver injury.
J Biomed Biotechnol, 2011:860578, 20 Dec 2011
Cited by: 47 articles | PMID: 22228987 | PMCID: PMC3250695
Review Free full text in Europe PMC
Mesenchymal stem cell therapy for cirrhosis: Present and future perspectives.
World J Gastroenterol, 21(36):10253-10261, 01 Sep 2015
Cited by: 33 articles | PMID: 26420953 | PMCID: PMC4579873
Review Free full text in Europe PMC

 1
and
1
and