Abstract
Background
Pancreatic cystic lesions (PCLs) are considered a precursor of pancreatic cancer. Needle-based confocal endomicroscopy (nCLE) is an imaging technique that enables visualization of the mucosal layer to a micron resolution. Its application has demonstrated promising results in the distinction of PCLs. This study evaluated the utility of nCLE in patients with indeterminate PCLs undergoing endoscopic ultrasound fine-needle aspiration (EUS-FNA) to distinguish mucinous from non-mucinous lesions.Aim
To evaluate the accuracy of nCLE in indeterminate PCLs undergoing EUS-FNA to distinguish mucinous from non-mucinous lesions.Methods
Patients who required EUS-FNA between 2015 and 2017 were enrolled prospectively. During EUS-FNA, confocal imaging, analyses of the tumor markers carcinoembryonic antigen and amylase, and cytologic examination were conducted. All patients were followed for at least 12 mo and underwent laboratory testing and computed tomography scanning or magnetic resonance imaging. nCLE videos were independently reviewed by 6 observers to reach a final diagnosis (mucinous vs non-mucinous) based on criteria derived from previous studies; if there was disagreement > 20%, a final diagnosis was discussed after consensus re-evaluation. The sensitivity, specificity, and accuracy of nCLE were calculated. Adverse events were recorded.Results
Fifty-nine patients were included in this study. Final diagnoses were derived from surgery in 10 patients, cytology in 13, and imaging and multidisciplinary team review in 36. Three patients were excluded from final diagnosis due to problems with nCLE acquisition. Fifty-six patients were included in the final analysis. The sensitivity, specificity, and accuracy of nCLE were 80% [95% confidence interval (CI): 65-90], 100% (95%CI: 72-100), and 84% (95%CI: 72-93), respectively. Post-procedure acute pancreatitis occurred in 5%.Conclusion
EUS-nCLE performs better than standard EUS-FNA for the diagnosis of indeterminate PCL.Free full text

Needle-based confocal endomicroscopy in the discrimination of mucinous from non-mucinous pancreatic cystic lesions
Abstract
BACKGROUND
Pancreatic cystic lesions (PCLs) are considered a precursor of pancreatic cancer. Needle-based confocal endomicroscopy (nCLE) is an imaging technique that enables visualization of the mucosal layer to a micron resolution. Its application has demonstrated promising results in the distinction of PCLs. This study evaluated the utility of nCLE in patients with indeterminate PCLs undergoing endoscopic ultrasound fine-needle aspiration (EUS-FNA) to distinguish mucinous from non-mucinous lesions.
AIM
To evaluate the accuracy of nCLE in indeterminate PCLs undergoing EUS-FNA to distinguish mucinous from non-mucinous lesions.
METHODS
Patients who required EUS-FNA between 2015 and 2017 were enrolled prospectively. During EUS-FNA, confocal imaging, analyses of the tumor markers carcinoembryonic antigen and amylase, and cytologic examination were conducted. All patients were followed for at least 12 mo and underwent laboratory testing and computed tomography scanning or magnetic resonance imaging. nCLE videos were independently reviewed by 6 observers to reach a final diagnosis (mucinous vs non-mucinous) based on criteria derived from previous studies; if there was disagreement > 20%, a final diagnosis was discussed after consensus re-evaluation. The sensitivity, specificity, and accuracy of nCLE were calculated. Adverse events were recorded.
RESULTS
Fifty-nine patients were included in this study. Final diagnoses were derived from surgery in 10 patients, cytology in 13, and imaging and multidisciplinary team review in 36. Three patients were excluded from final diagnosis due to problems with nCLE acquisition. Fifty-six patients were included in the final analysis. The sensitivity, specificity, and accuracy of nCLE were 80% [95% confidence interval (CI): 65-90], 100% (95%CI: 72-100), and 84% (95%CI: 72-93), respectively. Post-procedure acute pancreatitis occurred in 5%.
CONCLUSION
EUS-nCLE performs better than standard EUS-FNA for the diagnosis of indeterminate PCL.
Core Tip: Pancreatic cystic lesions are considered a precursor of pancreatic cancer. Needle-based confocal endomicroscopy is an imaging technique that enables visualization of the mucosal layer to a micron resolution. Endoscopic ultrasound with fine-needle aspiration is the most accurate procedure for identifying pancreatic cystic lesions, as it combines cytology with analysis of intracystic carcinoembryonic antigen level, although its accuracy is low. Needle-based confocal endomicroscopy has demonstrated promising results.
INTRODUCTION
Pancreatic cancer is the 10th most common cancer in men and 9th most common cancer in women. Compared to other cancers, pancreatic cancer has the lowest survival, with a 5-year survival rate of 9% and an estimated 56000 new cases per year according to the Surveillance, Epidemiology, and End Results database[1]. Pancreatic cystic lesions (PCLs) are considered a precursor of pancreatic cancer, as some have malignant potential and therefore should be evaluated carefully. However, other PCLs exhibit benign behavior with no surveillance required[2-4].
Currently, endoscopic ultrasound (EUS) with fine-needle aspiration (FNA) is the most accurate procedure for identifying the nature of a pancreatic cyst, as it combines cytology with analysis of intracystic carcinoembryonic antigen (CEA) level. The specificity, sensitivity, and overall accuracy of CEA in the discrimination of mucinous from non-mucinous is 98%, 48%, and 79%, respectively. However, in the absence of an associated solid component, pancreatic cyst fluid is frequently acellular or paucicellular, with resultant low diagnostic yield[5,6].
Confocal laser endomicroscopy is an innovative imaging technique that enables visualization in real-time, to a micron resolution, of the mucosal layer. Luminal confocal exploration has demonstrated excellent results in distinguishing neoplastic from benign tissue. Needle-based confocal endomicroscopy (nCLE) is a subtype of confocal laser imaging, in which a mini-probe is inserted through a 19-gauge EUS-FNA needle under EUS guidance. The first three clinical trials (total of 126 patients) described the correlation between nCLE and histological features, and established the criteria for characterizing the most frequent type of cysts; however, they did not evaluate the performance of these criteria[7-9]. Moreover, some concerns were raised about the safety of the procedure and interobserver agreement (IOA)[10,11]. Recently, two papers were published evaluating the impact of nCLE on surgical outcome[12,13]; the results were very promising, with some interesting economic consequences for follow-up costs[14].
We present the results of a multicenter prospective study evaluating the diagnostic accuracy of EUS-guided nCLE in differentiating mucinous from non-mucinous PCLs compared to standard of care, by analysis of intracystic CEA and amylase level and/or cytology vs surgical pathology.
MATERIALS AND METHODS
Study design and inclusion criteria
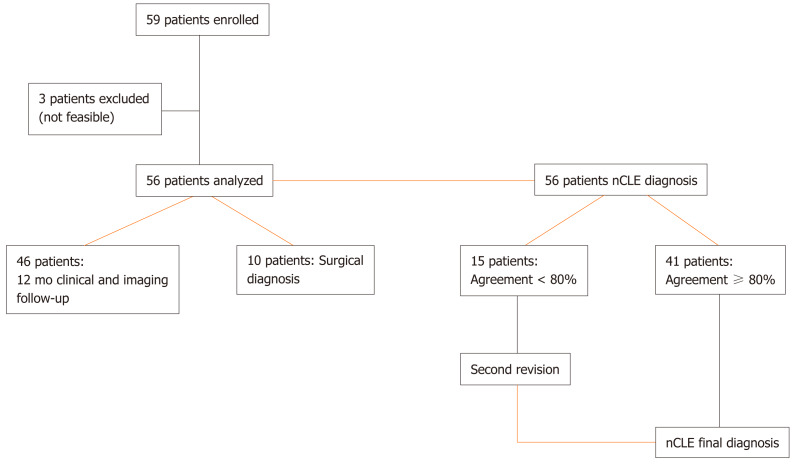
From November 2015 to December 2017, all consecutive patients referred for EUS-FNA for undetermined PCLs were prospectively enrolled and underwent EUS associated with both FNA and nCLE at four centers (AOU-Modena; Ospedale Le Molinette-Torino; Istituto Nazionale Tumori-Milano; Ospedale Maggiore, Crema, Italy). The inclusion criteria were as follows: age > 18 years; ability to provide informed consent; and, had a single undetermined pancreatic cyst > 20 mm without evidence of communication with the main pancreatic duct (PD) in previous imaging investigations. Exclusion criteria were as follows: Known fluorescein allergy; pregnancy; worrisome features or high-risk stigmata according to Fukuoka Guidelines[15]; or, any contraindication to performing EUS (Figure (Figure1).1). The study was carried out in accordance with the Declaration of Helsinki and was approved by the Ethical Committee of Baggiovara Hospital in Modena (Prot. 16/11/2015 prat n 4327; Baggiovara, Italy).
Study aims
The primary goal of the study was to determine the accuracy of nCLE in discriminating mucinous from non-mucinous PCLs. The secondary goals were to determine the feasibility of nCLE by evaluating the rate of procedure completion and by rating the ease of the procedure as easy, moderate, or difficult, and to assess the safety of the procedure by recording the immediate and 30-d complication rates (bleeding, infection, perforation, or acute pancreatitis (AP) classified as mild, moderate, or severe according to the European Society of Gastrointestinal Endoscopy guidelines)[16].
Procedures
EUS and EUS-FNA: All EUS procedures were performed by five operators with experience in biliopancreatic EUS (> 200/year) and nCLE (> 15/per operator). Antibiotic prophylaxis was administered 1 h before the procedure and continued for 3 d after[3]. The procedures were performed under deep sedation using a linear array echoendoscope (Olympus®, Tokyo, Japan or Hitachi-Pentax®, Hamburg, Germany) to evaluate the following PCL characteristics: site; morphology; cyst diameter; diameter of the main PD; communication with a duct (main or branch); thickness of the cyst wall; presence of septa and/or wall nodules; and, contrast medium to evaluate the enhancement of any septa or nodule. Once the cyst was visualized, it was punctured from the stomach or duodenum with a 19-gauge needle (ExpectTM; Boston Scientific, Boston, MA, United States) that was preloaded with the AQ-flex 19 miniprobe (Mauna Kea Technologies®, Paris, France). Then 2.5 mL of 10% fluoresceine was intravenously injected, the probe was gently advanced in contact with the cyst wall, and nCLE imaging was performed. After nCLE imaging acquisition, the probe was retrieved from the EUS-FNA needle and the cyst was completely aspirated. The cyst fluid was sent for analysis of CEA and amylase, and cytologic examination.
nCLE classification and diagnosis: Before patient enrollment, 6 investigators received nCLE training to learn technical tips and agreement for imaging interpretation, highlighting the high specificity of nCLE for the diagnosis of serous cystadenoma (SCA), intraductal papillary mucinous neoplasm (IPMN), and mucinous cystic neoplasm (MCN) and for the differentiation of mucinous from non-mucinous lesions, with a 20-video review. The criteria used in this study were derived from previously validated criteria from publications by Napoleon et al[8,9] as well as studies on papillary projections in IPMN[7,17] (Figure (Figure2A),2A), the superficial vascular network in SCA[9] (Figure (Figure2B),2B), MCNs in which the epithelial cyst border appears as a gray band delineated by a thin dark line[9] (Figure (Figure2C),2C), pseudocysts identified by bright gray and black particles[9] (Figure (Figure2D),2D), and cystic pancreatic neuroendocrine tumors (PNETs) characterized by dark irregular clusters of cells surrounded by gray matter[9].
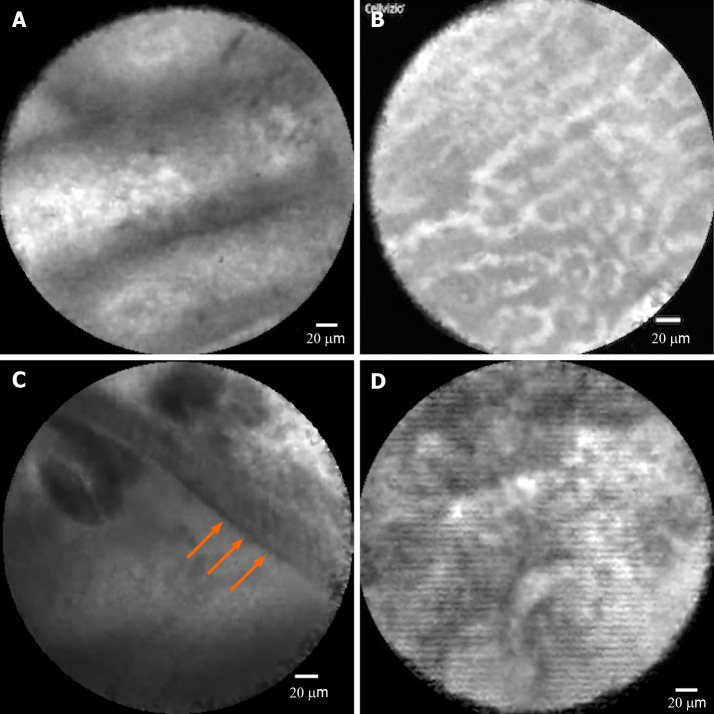
Confocal images of pancreatic cyst subtypes. A: Intraductal papillary mucinous neoplasm, showing papillary projections; B: Serous cystadenoma, showing superficial vascular network; C: Mucinous cystic neoplasm, in which the epithelial cyst border appears as a gray band delineated by a thin dark line; D: Pseudocyst, showing gray and black particles.
After the conclusion of follow-up, all nCLE videos were independently and blindly reviewed by the 6 observers; no clinical or imaging information was provided at this time. After video review, each investigator provided a final diagnosis of mucinous (mucinous cystadenoma or IPMN) or non-mucinous (SCA, pseudocyst, PNET) neoplasia, according to the criteria described above. In cases of disagreement between > 20% of observers, videos were discussed together to reach a final nCLE consensus diagnosis. In the event of persistent disagreement between the investigators, the videos were considered false negatives.
Final diagnosis: The final diagnosis was based on histological analyses of the surgical specimen and/or when FNA results were diagnostic on cell block sections or smears. Otherwise, all patients were followed up at 6 mo with magnetic resonance imaging (MRI) or computed tomography (CT) scan or EUS, and the final diagnosis was based on a consensus of EUS findings plus analysis of CEA level with at least 12 mo follow-up.
IOA
The extent of agreement among raters of nCLE diagnosis was performed with Gwet’s agreement coefficient (AC) [95% confidence interval (CI)]. Gwet’s AC provides a more stable interrater reliability coefficient than Cohen’s kappa. It is also less affected by prevalence and marginal probability than Cohen’s kappa, and therefore should be considered for use with interrater reliability analyses. For all measures of agreement, the following guideline provided by Landis and Koch[19] for the interpretation of kappa was used: < 0.00, poor; 0.00 to 0.20, slight; 0.21 to 0.40, fair; 0.41 to 0.60, moderate; 0.61 to 0.80, substantial; and 0.81 to 1.00, almost perfect[18,19].
Statistical analyses
The categorical variables are expressed as absolute numbers and percentages, while the continuous variables are expressed in the case of normal distribution as mean and standard deviation and relative 95%CI, or in the case of non-normal distribution, as median and interquartile range. The study was approved by the local Ethical Committee of Baggiovara Hospital in Modena (Prot. 4327/2016) and subsequently by the Ethical Committees of all centers involved.
RESULTS
Baseline patient characteristics
From November 2015 to December 2017 a total of 59 patients were referred for EUS-FNA of PCLs, and were prospectively enrolled in the study to undergo EUS-guided FNA and nCLE during the same session. Patient demographics and PCL features are listed in Table Table1.1. The mean patient age was 64-year-old, and 41 patients were female (70%). The majority of patients at the time of EUS were asymptomatic (n = 45; 76%); a history of AP was identified in 3 (5%) and concurrent symptoms potentially attributable to PCL were reported in 11 (19%), all of whom had abdominal pain. Previous cross-sectional abdominal imaging reports for PCL evaluation were available in all cases (n = 33 CT, n = 43 MRI).
Table 1
Patients demographics and pancreatic cystic lesions features
|
Characteristic
|
Enrolled, n (%)
|
| Patients, n | 59 |
| Age | 64 ± 13 |
| Sex, female | 41 (70) |
| Clinical presentation | |
| Asymptomatic | 45 (76) |
| Abdominal pain | 11 (19) |
| Pancreatitis | 3 (5) |
| Site of lesion | |
| Head | 13 (22) |
| Uncinate process | 8 (13) |
| Neck | 6 (10) |
| Body | 26 (45) |
| Tail | 6 (10) |
| Cyst diameter mm | 32 (22-45) |
| Morphology | |
| Unilocular macrocyst | 31 (52) |
| Multilocular microcyst | 27 (46) |
| Microcyst | 1 (2) |
| Main pancreatic duct diameter > 3 mm | 5 (8) |
| Communication with a duct | 1 (2) |
| Cyst wall diameter > 1 mm | 20 (34) |
| Septa and/or wall nodules | 35 (59) |
| CEA > 192 ng/mL | 21 (35) |
| Amylases ≥ 50 UI/L | 53 (90) |
CEA: Carcinoembryonic antigen.
The PCLs were distributed as follows: head of pancreas in 13 patients (22%); uncinate process in 8 (13%); neck in 6 (10%); body in 26 (45%); and tail in 6 (10%). The median cyst size was 32 mm (range: 22-45 mm). The majority of lesions were multilocular (n = 27, 46%). The main PD communication was considered exclusion criteria if found during CT or MRI. However, in 1 case, a communication was detected by EUS. No PD dilation (≥ 5 mm) was identified. Solid components or intramural nodules were present in 3 patients (5%). Intracystic CEA was available in 53 cases (95%), with a level > 192 ng/mL in 28 patients (47%) and < 5 ng/mL in 14 cases (24%).
Final diagnosis
Final diagnosis was made of 11 mucinous cystadenomas, 34 branch-duct IPMNs, 13 SCAs, and 1 cystadenocarcinoma (Table (Table2).2). Final diagnosis was derived from surgery in 10 patients (17%), cytology in 13 patients (22%), and a team discussion of the review of all CT/MRI/EUS images and intracystic CEA level in the remaining cases.
Table 2
Final diagnosis
|
Final diagnosis
|
n
(%)
|
| Serous cystoadenoma | 13 (22) |
| Cystoadenocarcinoma | 1 (2) |
| Branch-duct IPMN | 34 (58) |
| Mucinous cystoadenoma | 11 (18) |
IPMN: Intraductal papillary mucinous neoplasm.
Feasibility
The procedure was technically feasible in 56 patients; therefore, the feasibility rate was 95%, with a rating of easy in 48 patients (82%), moderately difficult in 7 patients (11%), and difficult in 4 patients (7%). The median nCLE scanning time was 3 min and did not exceed 4 min in any case.
Comparison of CEA and nCLE
The analysis of “intention to treat” showed sensitivity, specificity, and accuracy for diagnosing mucinous lesions and intracystic CEA > 192 ng/mL of 58% (95%CI: 43-72), 100% (95%CI: 73-100), and 67% (95%CI: 53-78), respectively. The sensitivity, specificity, and accuracy of nCLE were 80% (95%CI: 65-90), 100% (95%CI: 72-100), and 84% (95%CI: 72-92), respectively, in distinguishing mucinous from non-mucinous lesions (Table (Table33).
Table 3
Diagnostic yield of carcinoembryonic antigen and needle-based confocal laser endomicroscopy in mucinous vs non-mucinous lesions
|
|
Sensitivity (%)
|
Specificity (%)
|
Accuracy (%)
|
| CEA > 192 ng/mL | 58.0 | 100.0 | 67.0 |
| nCLE mucinous vs non-mucinous | 80.0 | 100.0 | 84.0 |
CEA: Carcinoembryonic antigen; nCLE: Needle-based confocal laser endomicroscopy.
IOA
IOA for nCLE diagnosis was 0.76 (range: 0.65-0.86). In 15 cases (26%), there was disagreement in more than 20% of the observers, so a second revision was necessary. After the second review, the sensitivity, specificity, and accuracy were calculated for 56 patients in whom nCLE was technically feasible.
Adverse events
Six adverse events (10%) were registered: 2 cases of self-limited intracystic bleeding (in 1 SCA and 1 IPMN); 3 cases of AP (in 3 IPMNs); and 1 case of abdominal pain (in 1 IPMN). AP was classified as interstitial edematous pancreatitis according to Atlanta classification[20] and required patient hospitalization; none developed infected pancreatic necrosis or walled-off necrosis.
DISCUSSION
PCLs are a heterogeneous family of lesions; some show benign behavior and others have unequivocal malignant potential and thus are considered a precursor of pancreatic cancer. The increased use of cross-sectional imaging, CT and MRI, has increased the reporting of incidental PCLs by up to 45%[2]. A key element of optimal clinical management of PCLs is identification of the small minority of cysts with early invasive cancer or high-grade dysplasia, and possibly the prediction of patients who will develop them in the future. A major challenge is that commonly used diagnostic tools, such as CT, MRI, and EUS-FNA cytology, and intracystic CEA analysis have suboptimal sensitivities and specificities for identifying patients at high risk, especially in cases of overlapping EUS features or borderline CEA intracystic level[5].
Recently a new technique, nCLE, has demonstrated promising results in visualization of the epithelial lining of the cyst wall, and consequently in the distinction of cyst type with accuracy and specificity that has not previously been described in PCLs. However, only limited studies on this technique with limited patients are available from three select centers: one from Europe[8] and two from the United States[7,11]. Consequently, optimal results could be related to the selected cases more than to the technique’s performance.
The strength of our study was that the performance of nCLE was evaluated in four different centers with high EUS volume, by experts with previous experience in confocal endomicroscopy imaging, in a non-selected group of patients referred for EUS-FNA for undetermined PCLs without PD communication as determined by previous imaging. We also excluded worrisome features and high-risk stigmata as well as solid masses to avoid biased study results. The diagnostic yield of confocal endomicroscopy in our study has been optimal with a specificity of 100%. In a clinical setting, these data confirm the potential of this technique to classify PCLs as high and low risk of progression, and consequently, to modulate the surveillance program for these patients.
The feasibility of EUS-guided nCLE has been a subject of debate due to the use of a large needle[7]. This study showed that the feasibility of the technique is excellent in experienced hands. Our study also confirmed the safety of nCLE; indeed, the rate of post-procedure AP was slightly higher (5%) than that described by Palazzo et al[14] but was lower than that in another report[15]. The cases of AP were mild, and none evolved to walled-off necrosis. We postulated that prolonged examination of the cyst wall could be related to an increased risk of bleeding or debris that could enhance the risk of AP; however, this was not statistically significant.
At the time of study onset, data derived from the two recently published papers by Napoleon et al[12] and Krishna et al[13] were not available; therefore, the performance of this technique is still considered under investigation. Our results support the recently published data, showing the potential of nCLE to be used in selected patients in a clinical setting as proposed by Napoleon et al[12], to evaluate multiple PCLs before surgery in order to guide partial vs total pancreatectomy, or to assess single lesions in young women where, in case of SCA, surveillance could be discontinued.
The limitation of our study was that it was conducted in a limited study population; thus, only small numbers of final surgical diagnoses were available. This has been frequently described in PCL studies due to the surveillance approach suggested by various international guidelines, even in lesions with a high risk of progression (mucinous cystadenoma and IPMN > 3 cm)[21].
CONCLUSION
In conclusion, a few years after the first publication on nCLE in PCLs[7], this study confirms that the diagnostic yield of EUS-guided nCLE is higher than any available technique for PCL characterization, and as such is a valuable tool in PCL management.
ARTICLE HIGHLIGHTS
Research background
Some pancreatic cystic lesions (PCLs) have unequivocal malignant potential, but the precise determination of the risk of progression with endoscopic ultrasound (EUS), fine-needle aspiration (FNA), analysis of carcinoembryonic antigen (CEA) level, and cytology is still challenging. Among the novel tools for assessing PCLs, needle-based confocal endomicroscopy (nCLE) has been identified as one of the most sensitive, but some concerns have been raised about its safety and reproducibility.
Research motivation
The first clinical trials published described a correlation between nCLE and histological features, and established the criteria for characterizing the most frequent type of cysts. However, no multicenter prospective studies have been performed at the time of study conception to evaluate the safety of the procedure and interobserver agreement (IOA).
Research objectives
The purpose of this multicenter prospective study was to evaluate the diagnostic accuracy of EUS-guided nCLE to differentiate mucinous from non-mucinous in PCLs compared to standard of care, by analysis of intracystic CEA and amylase level and/or cytology vs surgical pathology.
Research methods
The strength of the study is its observational design in high-volume centers compared to the single-center studies previously published. All nCLE videos were independently reviewed by 6 observers blind to clinical or imaging information; each investigator provided a final diagnosis, and if the disagreement between reviewers was > 20%, videos were discussed together in order to reach a final nCLE consensus diagnosis. In the event of persistent disagreement among investigators, the videos were considered false negatives.
Research results
A total of 59 patients were enrolled in this study to receive EUS-FNA and nCLE. The procedure was technically feasible in 95% of patients; nCLE sensitivity, specificity, and accuracy for the diagnosis of mucinous lesions were 80% [95% confidence interval (CI): 65-90], 100% (95%CI: 72-100), and 84% (95%CI: 72-92), respectively, and for distinguishing mucinous from non-mucinous lesions compared to intracystic CEA > 192 ng/mL were 58% (95%CI: 43-72), 100% (95%CI: 73-100), and 67% (95%CI: 53-78), respectively. IOA for nCLE diagnosis was 0.76, and 10% of adverse events were recorded.
Research conclusions
Our study confirmed the feasibility of nCLE and its excellent performance in the discrimination of mucinous vs non-mucinous lesions. This new finding confirms the possibility of an accurate pre-operative diagnosis. The strength of the study was the multicenter, prospective observational design and the selection of a study group of real undetermined pancreatic cysts without pancreatic duct communication and free of worrisome features; this was also a weakness due to the low number of cases with surgical/histological diagnosis. The excellent performance of nCLE opens various possible scenarios for the management of undetermined PCLs.
Research perspectives
Future research should include fine-needle biopsies with biopsy forceps to improve pathological diagnosis without surgery.
Footnotes
Institutional review board statement: The study was carried out in accordance with the Declaration of Helsinki and was approved by Ethical Committee of Baggiovara Hospital in Modena (Prot. 16/11/2015 prat n 4327).
Informed consent statement: All patients received written information about the study with results and possible complications. They all provide informed consent, as a negation of a written informed consent resulted in exclusion of the patient from the study.
Conflict-of-interest statement: The authors declare no conflicts of interest.
STROBE statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Manuscript source: Invited manuscript
Peer-review started: February 23, 2021
First decision: June 17, 2021
Article in press: September 6, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Krishna SG, Rathnaswami A, Wang WQ S-Editor: Gao CC L-Editor: A P-Editor: Zhang YL
Contributor Information
Helga Bertani, Gastroenterology and Digestive Endoscopy Unit, Azienda Ospedaliero Universitaria Policlinico di Modena, Modena 41124, Italy. [email protected].
Raffaele Pezzilli, Department of Internal Medicine, Policlinico S.Orsola Malpighi, Bologna 40121, Italy.
Flavia Pigò, Gastroenterology and Digestive Endoscopy Unit, Azienda Ospedaliero Universitaria Policlinico di Modena, Modena 41124, Italy.
Mauro Bruno, Gastroenterology and Digestive Endoscopy Unit, AOU Città della Salute e della Scienza, University of Turin, Turin 10100, Italy.
Claudio De Angelis, Gastroenterology and Digestive Endoscopy Unit, AOU Città della Salute e della Scienza, University of Turin, Turin 10100, Italy.
Guido Manfredi, Gastroenterology and Digestive Endoscopy Department, Ospedale Maggiore, Crema 26013, Italy.
Gabriele Delconte, Department of Diagnostic Endoscopy and Endoscopic Surgery, Istituto Nazionale Tumori, Milano 20019, Italy.
Rita Conigliaro, Gastroenterology and Digestive Endoscopy Unit, Azienda Ospedaliero Universitaria Policlinico di Modena, Modena 41124, Italy.
Elisabetta Buscarini, Gastroenterology and Digestive Endoscopy Department, Ospedale Maggiore, Crema 26013, Italy.
Data sharing statement
The dataset is available. For more information please contact [email protected]. We can provide the anonymized version.
References
Articles from World Journal of Gastrointestinal Endoscopy are provided here courtesy of Baishideng Publishing Group Inc
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/128412881
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.4253/wjge.v13.i11.555
Article citations
Using Endoscopy in the Diagnosis of Pancreato-Biliary Cancers.
Cancers (Basel), 15(13):3385, 28 Jun 2023
Cited by: 0 articles | PMID: 37444495 | PMCID: PMC10340478
Review Free full text in Europe PMC
Pancreatic Incidentaloma.
J Clin Med, 11(16):4648, 09 Aug 2022
Cited by: 4 articles | PMID: 36012893 | PMCID: PMC9409921
Review Free full text in Europe PMC
Accuracy and agreement of a large panel of endosonographers for endomicroscopy-guided virtual biopsy of pancreatic cystic lesions.
Pancreatology, 22(7):994-1002, 31 Aug 2022
Cited by: 11 articles | PMID: 36089484 | PMCID: PMC10548449
Application of Artificial Intelligence in the Management of Pancreatic Cystic Lesions.
Biomimetics (Basel), 7(2):79, 14 Jun 2022
Cited by: 6 articles | PMID: 35735595 | PMCID: PMC9221027
Review Free full text in Europe PMC
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Diagnosis of Pancreatic Cystic Lesions by Virtual Slicing: Comparison of Diagnostic Potential of Needle-Based Confocal Laser Endomicroscopy versus Endoscopic Ultrasound-Guided Fine-Needle Aspiration.
J Pathol Inform, 10:34, 13 Nov 2019
Cited by: 6 articles | PMID: 31799020 | PMCID: PMC6883479
Needle-based confocal laser endomicroscopy of pancreatic cystic lesions: a prospective multicenter validation study in patients with definite diagnosis.
Endoscopy, 51(9):825-835, 22 Oct 2018
Cited by: 41 articles | PMID: 30347425
Endoscopic Ultrasound-Guided Confocal Laser Endomicroscopy Increases Accuracy of Differentiation of Pancreatic Cystic Lesions.
Clin Gastroenterol Hepatol, 18(2):432-440.e6, 18 Jun 2019
Cited by: 42 articles | PMID: 31220640
The Role of Endoscopic Ultrasound in the Diagnosis of Cystic Lesions of the Pancreas.
Visc Med, 34(3):192-196, 08 Jun 2018
Cited by: 5 articles | PMID: 30140684 | PMCID: PMC6103354
Review Free full text in Europe PMC